Introduction
The incidence of liver disease has increased rapidly
in recent years. Liver transplantation is an efficient method to
treat end-stage liver disease. However, ischemia-reperfusion injury
(IRI) of the transplanted liver is inevitable. In addition, the
risk of developing an alloimmune response necessitates lifelong
oral immunosuppressant treatment, which induces varying degrees of
graft fibrosis and thus further affects long-term graft survival
(1,2). It has been proposed that the primary
factors leading to the development of liver fibrosis following
transplantation include the duration of IRI, donor age, the
hepatitis virus genotype of recipients and immunosuppressant
therapy. Among these, developing an alloimmune response following
transplantation is considered to be the primary factor leading to
the development of liver fibrosis; however, the specific mechanism
through which this occurs remains unclear (3).
Accumulating evidence suggests that
epithelial-mesenchymal transition (EMT) leads to tumor metastasis
and the occurrence of cancer, including hepatocellular carcinoma
(4,5). EMT is a chronic process accompanied
by the loss of cell-cell junctions in epithelial cells. The
principal characteristic of liver fibrosis is the excessive
synthesis of extracellular matrix (ECM) components, predominantly
collagen. As the degradation rate of collagen is relatively slow,
this leads to a dynamic imbalance and excessive ECM deposition in
the liver. This is a recognized mechanism for the development of
liver fibrosis (6). However,
various other factors are known to be involved in the development
of liver fibrosis, including underlying pathological histology,
cytology, cytokines and intracellular signal transduction systems
(7–9). Active hepatic stellate cells are the
principal source of ECM production during liver fibrosis (10). Growth factors and factors involved
in regulating vascular function, and lipid levels are additionally
involved in the formation and development of this condition. Among
them, transforming growth factor-β1 (TGF-β1), a key fibrogenic
cytokine, is reported to be one of the most important cytokines in
liver fibrosis (11,12).
Emodin is one of the effective ingredients of the
Chinese medicine rhubarb. It has been reported that emodin has a
number of pharmacological effects, including antiviral,
antibacterial, immunoregulatory and antioxidant functions (13,14).
Studies have demonstrated that emodin is able to induce apoptosis
via EMT suppression and caspase-dependent signaling (15–17).
It has additionally been reported that emodin may alleviate
pancreatitis and suppress lung fibrosis by inhibiting TGF-β1
signaling in rats (18,19). Further studies have reported that
emodin may reduce CCL4-induced liver fibrosis in rats;
however, the optimal dosage and the specific mechanism involved
requires further investigation (20). The present study aimed to further
analyze the role of emodin in alleviating CCL4-induced
liver fibrosis by inhibiting EMT and the TGF-β1 signaling
pathway.
Materials and methods
Animals
A total of 50 adult Sprague-Dawley male rats (6–8
weeks) weighing 240–260 g, were purchased from Shanghai SLAC
Laboratory Animal Co., Ltd. (Shanghai, China). Rats were housed in
an a room at 22±0.5°C with a relative humidity of 60±2% in a 12 h
light/dark cycle. in Huashan Hospital affiliated to Fudan
University (Shanghai, China), with free access to food and water.
All procedures were approved by the Bioethics Committee of Huashan
Hospital affiliated to Fudan University.
Reagents and antibodies
Emodin was purchased from Shanghai Future Industrial
Limited by Share, Ltd. (Shanghai, China). CCl4 was
obtained from Beijing BeiHua Fine Chemicals Co., Ltd. (Beijing,
China). Olive oil was purchased from Beyotime Institute of
Biotechnology (Haimen, China). TRIzol reagent, superscript II
reverse transcriptase and random primer oligonucleotides were
purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The Absolute QPCR SYBR® Green premix was
purchased from Takara Bio, Inc. (Otsu, Japan). The sequences of
primers used in the present study are presented in Table I. The antibodies against mothers
against decapentaplegic homolog 2 (Smad2; 1:1,000; cat. no. 5339;
CST Biological Reagents Co., Ltd., Shanghai, China), Smad3
(1:1,000; cat. no. 9513; CST Biological Reagents Co., Ltd.),
phosphorylated (p)-Smad2 (1:1,000; cat. no. 3108; CST Biological
Reagents Co., Ltd.), p-Smad3 (1:1,000; cat. no. 9520; CST
Biological Reagents Co., Ltd.), E-cadherin (1:1,000; cat. no.
14472; CST Biological Reagents Co., Ltd.), vimentin (1:1,000; cat.
no. 5741; CST Biological Reagents Co., Ltd.), TGF-b1 (1:1,000; cat.
no. 3711; CST Biological Reagents Co., Ltd.) and GAPDH (1:1,000;
cat. no. 5174; CST Biological Reagents Co., Ltd.) and fibronectin
(1:500; cat. no. ab2413; Abcam, Cambridge, UK). An ELISA kit to
detect TGF-β1 (cat. no. F3766) was purchased from Westang
Technology Ltd. (Shanghai, China).
 | Table I.Assessment of liver fibrosis. |
Table I.
Assessment of liver fibrosis.
| Grade | Steatosis
criteria | Fibrosis
criteria |
|---|
| Grade 0 | No hepatocytes were
involved | No fibrosis |
| Grade 1 | <30% of
hepatocytes were involved | Portal fibrosis
without septa |
| Grade 2 | 30 to 50% of
hepatocytes were involved | Portal fibrosis
with a few septa |
| Grade 3 | 51 to 75% of
hepatocytes were involved | Numerous septa
without cirrhosis |
| Grade 4 | >75% of
hepatocytes were involved | Cirrhosis |
Animal model
Rats were randomly divided into five groups: Normal
control (n=10), which were injected subcutaneously with olive oil
and administered oral sodium carboxymethylcellulose (CMC; Emodin
can be dissolved to form suspension of CMC); CCl4 group
(n=10), which were injected subcutaneously with 2 ml/kg 40%
CCl4 (a mixture of pure CCl4 and sterile
olive oil) twice a week for 12 weeks and administered oral sodium
CMC; and the emodin treatment groups, which were subcutaneously
injected with 2 ml/kg 40% CCl4 twice a week for 12 weeks
followed by administration of emodin dissolved in sodium CMC, at
10, 20 or 40 mg/kg once daily for 12 weeks (n=10 for each
concentration). The rat survival rate in each group was determined.
At the end of the treatment period all the rats were sacrificed,
and blood and liver tissue were harvested for the following
experiments.
Liver function test
Peripheral blood was centrifuged at 2,000 × g for 10
min at 4°C. The serum was used to measure the levels of alanine
aminotransferase (ALT) and aspartate aminotransferase (AST) using a
biochemical analyzer (7060; Hitachi, Ltd., Tokyo, Japan). In
addition, commercial kits from Westang (Shanghai, China) were
applied to determine alkaline phosphatase (ALP; cat. no. F15216)
and γ-glutamyl transpeptidase (GGT; cat. no. F15120) levels.
Histological study
Liver tissue was embedded with paraffin following
10% formalin fixation at 4°C for 48 h and sliced to a thickness of
~5 µm. Hematoxylin and eosin (H&E; 3 min each), Sirius red (10
min) and Masson's Trichrome stainings (1 h) were performed
post-dewaxing at room temperature. According to the criteria of the
Chinese Medical Association Committee of Fatty Liver Disease
(21) and Nouchi et al
(22), the extent of liver
cirrhosis in sections stained with H&E was determined in the
present study (22,23). Steatosis was graded on the basis of
the extent of parenchyma involved, as described in Table I. The stage of liver fibrosis was
graded using the METAVIR five-point scale, additionally described
in Table I (24). Collagen accumulation was quantified
following Sirius red and Masson's Trichrome staining in 10 randomly
selected areas per sample, at a magnification of ×200 with the
image analysis software Image-Pro Plus (version 6.1; Media
Cybernetics, Inc., Rockville, MD, USA) under a light microscope
(Olympus, Japan). The percentage of the section that was positively
stained for interstitial collagen was quantified using Image-Pro
Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). These
photos were collaged using Photoshop software 6.0 (Adobe Systems,
Inc., San Jose, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from liver tissues using
TRIzol reagent (Invitrogen, Thermo Fisher Scientific, Inc.). A
total of 1 µg total RNA was reverse transcribed into cDNA using a
RevertAid™ First Strand cDNA Synthesis kit (Fermentas; Thermo
Fisher Scientific, Inc.). RT-qPCR was performed using the
SYBR® Premix Ex Taq kit (Takara Bio, Inc.) in a
MasterCycler RealPlex4 system (Eppendorf, Hamburg, Germany). The
thermocycling conditions consisted of: 30 sec at 95°C, followed by
40 cycles (5 sec at 95°C, 30 sec at 55°C and 60 sec at 72°C). The
primers used are listed in Table
II. The expression of mRNA was normalized to GAPDH expression
using the 2−ΔΔCq method (25).
 | Table II.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
(5′→3′) |
|---|
| GAPDH | F:
AGGTCGGTGTGAACGGATTT |
|
| R:
GGGGTCGTTGATGGCAACA |
| Collagen I | F:
GAGAGAGCATGACCGATGGA |
|
| R:
CGTGCTGTAGGTGAATCGAC |
| Collagen III | F:
CTGGTCCTGTTGGTCCATCT |
|
| R:
ACCTTTGTCACCTCGTGGAC |
| Slug | F:
GAGCATTTGCAGACAGGTCA |
|
| R:
ACAGCAGCCAGATTCCTCAT |
| Snail | F:
ACAGCAGCCAGATTCCTCAT |
|
| R:
GTCACGTTCTTCCGCTTCTC |
| TWIST1 | F:
ATGCGGAAGACAGAAAATGG |
|
| R:
GTCACGTTCTTCCGCTTCTC |
| ZEB1 | F:
GGAGTCCGCAGTCTTACGAG |
|
| R:
TCTGGAGGACCTGGTAGAGG |
| TGF-β1 | F:
CTTTGTACAACAGCACCCGC |
|
| R:
CGGGTGACTTCTTTGGCGTA |
ELISA analysis
Liver tissue was collected and processed according
to the manufacturer's protocols of the ELISA kit. The grinding
fluid or standard were added to the plate, which was subsequently
placed at 37°C for 40 min following mixing. Primary antibody
working fluid, enzyme conjugate and TMB solution were added
sequentially subsequent to washing the plate. The absorbance was
measured at 450 nm using a microplate reader.
Western blotting
Samples of liver tissues were treated with protein
extraction reagent (Beyotime Institute of Biotechnology) and was
centrifuged at 12,000 × g at 4°C for 20 min to obtain the
supernatant according to the manufacturer's protocols. Protein
concentration was subsequently quantified with a bicinchoninic acid
protein assay. Proteins (20 µg/lane) were subjected to 10% SDS-PAGE
prior to transfer onto polyvinylidene fluoride membranes. Following
this, membranes were blocked with 5% skim milk for 1 h at room
temperature and incubated with primary antibodies at 4°C for 12 h.
Peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:10,000; cat. no. 111-035-003; Jackson ImmunoResearch) was
incubated with the membranes at room temperature for 2 h. Bands
were visualized with an enhanced chemiluminescent substrate kit
(Amersham Pharmacia). The expression of E-cadherin, vimentin,
fibronectin, TGF-β1, Smad2, Smad3, p-Smad2 and p-Smad3 was
quantified by normalizing to GAPDH using Image-Pro Plus, version
6.0 (Media Cybernetics, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed by SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Statistical analysis was performed using Student's t-test, or
one-way analysis of variance followed by the Student-Newman-Keuls
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Treatment with emodin alleviates
CCl4-induced death and improves liver function
The chemical structure of emodin is presented in
Fig. 1A. Subcutaneous injection of
CCl4 reduced the survival rate of rats to 30%, although
administration of emodin at doses of 10, 20 and 40 mg/kg was
reported to increase the survival rates at week 12 to 60, 70 and
80%, respectively (Fig. 1B). In
order to examine the protective role of emodin, a range of liver
function tests were performed in the present study, including
measuring ALT, AST, ALP and γ-GGT levels in the peripheral blood
serum of animals from each group. The results of the present study
demonstrated that the liver function index was significantly
reduced following stimulation with CCl4 compared with in
the control group (ALT: 28.09±5.86 vs. 96.07±5.37 U/l; AST:
47.59±5.45 vs. 141.07±7.08 U/l; ALP: 200.72±16.13 vs. 500.02±15.71
U/l and γ-GGT: 20.23±1.16 vs. 88.09±4.55 U/l). Conversely,
following treatment with emodin, the liver function index increased
in a dose-dependent manner, particularly with a dose of 40 mg/kg
(ALT: 96.07±5.37 vs. 45.29±3.22 U/l; AST: 141.07±7.08 vs.
64.15±3.73 U/l; ALP: 500.02±15.71 vs. 291.48±13.74 U/l and γ-GGT:
88.09±4.55 vs. 28.42±1.58 U/l; Fig.
1C-F).
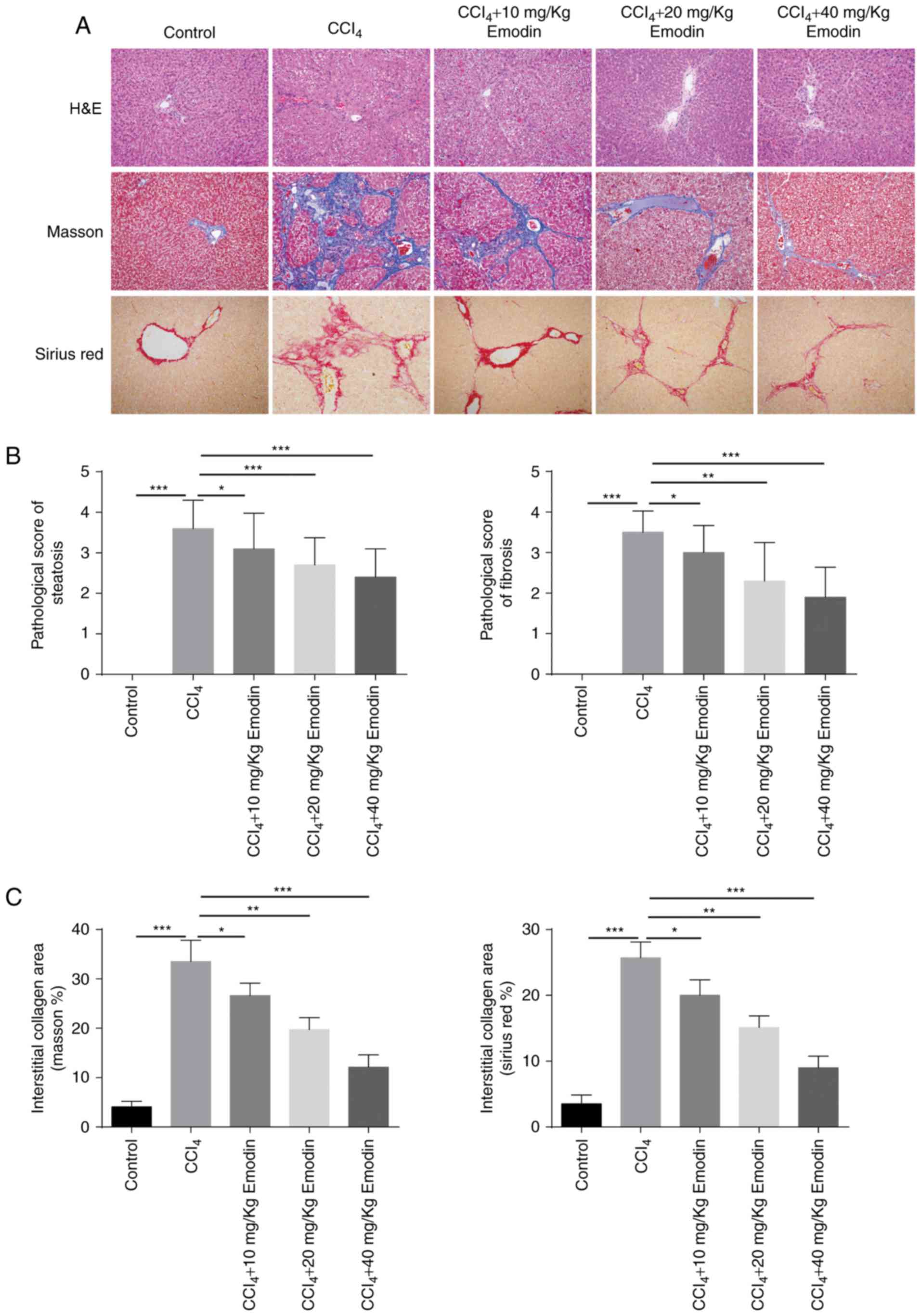
Emodin notably attenuates the
formation of fatty liver and fibrosis, and reduces the deposition
of collagen
Histological analysis notably revealed the structure
of the liver lobule in the control group and demonstrated that
there were no apparent hepatic cell lesions, no dilation or
congestion in the liver sinusoid, and no inflammatory cell
infiltration or fibroplasia in the portal area. However, in the
CCl4 group, the lobular structure was disordered, and
fat degeneration and necrosis in liver cells, and infiltration of
inflammatory cells, were observed. In addition, liver lobes were
divided by collagen fibers and a large amount of collagen
deposition was observed in the portal area. Following treatment
with emodin, the structure of the liver lobule was observed to be
largely complete, no necrotic lesions were detected, and
infiltration of inflammatory cells and fibrotic hyperplasia were
rare in the portal area. Among all treatment groups, the lightest
extent of liver injury was detected in animals administered 40
mg/kg emodin (Fig. 2A and B).
Masson's Trichrome and Sirius staining revealed a significant
increase in collagen deposition in rats treated with
CCl4 compared with the control group (Masson's
Trichrome: 4.1±1.1 vs. 33.5±4.32%; Sirius: 3.5±1.35 vs.
25.7±2.41%). Following treatment with emodin at various
concentrations, collagen deposition was reduced, with the most
significant effect observed in the 40 mg/kg group (Masson's
Trichome: 33.5±4.32 vs. 12.1±2.51%; Sirius: 25.7±2.41 vs. 9±1.76%;
Fig. 2A and C).
Emodin reduces CCl4-induced
EMT in rat liver tissue
EMT is an important factor during the process of
liver fibrosis. In the present study, western blot analysis and
RT-qPCR were employed to detect markers of EMT. Western blotting
data revealed that the expression levels of the mesenchymal markers
vimentin and fibronectin were upregulated following CCl4
administration. Conversely, the epithelial marker E-cadherin was
highly expressed in the control group and downregulated following
CCl4 administration. These results suggest that
CCl4 stimulation may induce liver fibrosis in rats.
Compared with the CCl4 group, emodin decreased the
expression of vimentin and fibronectin, and increased the
expression of E-cadherin, with most marked effects noted in the 40
mg/kg treatment group (Fig. 3A).
This was consistent with the results of the RT-qPCR, investigating
the expression of the principal transcriptional repressors of
E-cadherin: Snail family transcriptional repressor (Snail) 2
(Slug), Snail, twist-related protein 1 (TWIST1) and zinc finger
E-box-binding homeobox 1 (ZEB1), and the expression of collagen
types I and III. The results of the present study revealed that
Slug, Snail, TWIST1, ZEB1, collagen I and collagen III mRNA
expression levels were increased in the CCl4 group.
Emodin reduced the expression of all of these EMT-associated
markers in a dose-dependent manner, with the most significant
effect noted in the 40 mg/kg treatment group. These results
therefore indicated that emodin may be able to inhibit
CCl4-induced EMT in the livers of rats (Fig. 3B).
 | Figure 3.Emodin inhibits
CCl4-induced epithelial-mesenchymal transition in rat
liver tissue. (A) Representative western blotting images
demonstrating the expression of E-cadherin, fibronectin and
vimentin in liver tissues from the control, CCl4 and
CCl4 + emodin groups. (B) mRNA levels of Slug, Snail,
TWIST1, ZEB1, collagen I and III, and SMA were analyzed by reverse
transcription-quantitative polymerase chain reaction. Data are
presented as mean ± standard deviation, n=6. *P<0.05;
**P<0.01, ***|P<0.001. Slug, snail family transcriptional
repressor 2; Snail, snail family transcriptional repressor; TWIST1,
twist-related protein 1; ZEB1, zinc finger E-box binding homeobox
1. |
Emodin reduces CCl4-induced
TGF-β1 production and the expression of p-Smad2 and Smad3
TGF-β1 serves a critical role in the course of EMT.
In order to understand whether TGF-β1 was involved in the
emodin-mediated reduction in EMT in the present study, TGF-β1,
p-Smad2 and p-Smad3 expression was investigated following treatment
with 40 mg/kg emodin (Fig. 4).
ELISA results confirmed that the content of liver TGF-β1 increased
in CCl4 compared with in the control group (87.98±4.47
vs. 321.97±17.44 pg/ml). Following treatment with emodin, the
levels of TGF-β1 decreased significantly (321.97±17.44 vs.
118.72±12.18 ng/ml; Fig. 4A and
C). This was in accordance with the findings of the RT-qPCR and
western blotting. In order to investigate whether TGF-β1 signaling
was inhibited following treatment with emodin, the expression
levels of p-Smad2 and p-Smad3 were examined. The results of the
present study indicated that CCl4 induced the
phosphorylation of Smad2 and 3, which was significantly attenuated
by 40 mg/kg emodin (Fig. 4A and
C).
Discussion
Liver fibrosis is a common pathological
characteristic of the majority of chronic liver diseases. Following
liver transplantation, the occurrence of varying degrees of
fibrosis is inevitable (26). It
characterized by the loss of the normal liver architecture due to
structural abnormalities in the nodules. At present, there is a
lack of effective clinical treatment for liver fibrosis.
Traditional Chinese medicine and natural medicine may offer a
promising alternative.
Emodin is an active monomer extracted from the
traditional Chinese medicines knotweed and rhubarb root. Emodin has
been reported to exert anti-inflammatory, antitumor, antiviral and
immunomodulatory effects (27).
Previous studies have reported that emodin additionally exhibits an
antifibrotic effect in a rat lung fibrosis model, in addition to in
heart tissue and other organs (28–30).
It has been reported that emodin has a protective effect in liver
fibrosis (20). However, its
specific mechanism and the optimal dose for antifibrotic treatment
in the liver require further investigation. In the present study,
CCl4 was employed to induce liver fibrosis in rats.
Emodin was administered at doses of 10, 20 and 40 mg/kg, and the
effects on rat survival and liver function were observed.
The results of the present study demonstrated an
increase in survival rates at doses of 20 and 40 mg/kg. ALT, AST,
ALP and γ-GGT enzymes are released from liver cells into the serum
when cell damage or necrosis occurs, with the levels of the enzymes
reflecting the amount and type of liver damage (31,32).
The biochemical index recorded in the present study suggested that
liver function was severely damaged in the CCl4 group.
Histological examination confirmed the appearance of degenerated
and necrotic liver cells in this group. Masson's Trichrome and
Sirius red staining revealed abnormal collagen deposition and a
significant increase in the liver fibrosis index. These data
indicated that treatment with CCl4 may successfully
induce liver fibrosis in rats, and that CCl4-induced
liver damage may be prevented by administering emodin via a
subcutaneous injection. Indices of liver damage, including ALT,
AST, ALP and γ-GGT, were significantly reduced following treatment
with emodin and liver cell degeneration and necrosis, and collagen
deposition, were ameliorated. The most significant effects were
observed following administration of the 40 mg/kg dose. To the best
of our knowledge, the present study was the first to demonstrate
that emodin may reduce the progression of liver fibrosis via the
suppression of EMT and TGF-β signaling, as demonstrated by animal
survival rates, together with biochemical, molecular and
histological data.
There is accumulating evidence that during damage to
hepatic epithelial cells, including hepatocytes and cholangiocytes,
phenotypic markers are lost, including N-and E-cadherin; hepatic
epithelial cells subsequently transform into fibroblasts,
myofibroblasts or mesenchymal cells, with the concomitant
expression of markers, including vimentin and α-smooth muscle actin
(33–35). This transformation is associated
with the progression of liver fibrosis. It has been reported that
within a rat model of lung fibrosis, emodin may significantly
ameliorate bleomycin-induced EMT (19). In the present study, the expression
levels of E-cadherin were significantly lower and the expression of
vimentin was significantly higher in the CCl4 group
compared with the control, suggesting that CCl4
stimulation may induce EMT in liver cells, which subsequently adopt
a mesenchymal cell phenotype. Following treatment with emodin, the
expression of E-cadherin was increased and the expression of
vimentin was decreased, indicating that CCl4-induced EMT
is suppressed by emodin. Furthermore, the most significant effects
were observed with the treatment at 40 mg/kg.
TGF-β1 signaling via Smad molecules is a
well-documented mechanism of liver fibrosis (36). It serves a critical role in the
initiation of fibrosis and the activation of hepatic stellate cell
transformation. Activation of Smad2 is reported to be involved in
the regulation of cell proliferation, transformation, synthesis,
secretion and apoptosis caused by TGF-β1 (37). Smad signaling molecules are
considered to be some of the most important intracellular TGF-β1
receptor kinase substrates (38,39).
Among them, Smad2 and Smad3 belong to the family of regulatory
Smads, which combine with serine/threonine receptors. In mammals,
TGF-β1 transmits intracellular signals via Smad2 and Smad3
phosphorylation. In the process of liver fibrosis, TGF-β1 activates
Smad2/3, and subsequently phosphorylates Smad2/3 and Smad1/5/8 to
form a heteropolymer with Smad4. Finally, Smad 4 translocates to
the nucleus to regulate gene transcription by interacting directly
with DNA or via coenzyme factors, including Snail, Slug or Twist1
(40,41). Furthermore, Smad4 may inhibit the
expression of epithelial genes and promote mesenchymal gene
expression (42). Thus, the
inhibition of Smad2/3 may block the TGF-β1 signaling pathway. In
the present study, the expression of TGF-β1 and phosphorylation
Smad2/3 were significantly higher in the liver of animals of the
CCl4 group compared with the control group, indicating
that this may be a mechanism for CCl4-induced rat liver
fibrosis. Additionally, the present study demonstrated that
CCl4 induced the production of TGF-β1 and
phosphorylation of Smad2/3, which was suppressed via treatment with
40 mg/kg emodin.
Collectively, the results of the present study
suggested that the mechanism for emodin-mediated suppression of
CCl4-induced liver fibrosis may function via the
inhibition of EMT and TGF-β1/Smad signaling. This suggests that
emodin may be a potential novel therapeutic agent for the clinical
prevention of liver fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Nature Science Foundation of China (grant nos. 81400675
and 81603406).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
FL performed the western blot analysis and wrote the
manuscript. JZ performed the morphological staining experiments of
the liver and analyzed the experimental data. JQ and GW performed
the ELISA an revised the manuscript. ZM designed the experiment and
funded this research. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Bioethics
Committee of Huashan Hospital affiliated with Fudan University
(Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Terrault NA: Hepatitis C therapy before
and after liver transplantation. Liver Transpl. 14 Suppl 2:S58–S66.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu H, Jayakumar S, Traboulsi M and Lee
SS: Cirrhotic cardiomyopathy: Implications for liver
transplantation. Liver Transpl. 23:826–835. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gane E: The natural history and outcome of
liver transplantation in hepatitis C virus-infected recipients.
Liver Transpl. 9:S28–S34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao J, Liu J, Long J, Fu J, Huang L, Li J,
Liu C, Zhang X and Yan Y: microRNA-23b suppresses
epithelial-mesenchymal transition (EMT) and metastasis in
hepatocellular carcinoma via targeting Pyk2. Biomed Pharmacother.
89:642–650. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Z, Sun B, Zhao X, Zhang Y, Gu Q, Liang
X, Dong X and Zhao N: The expression and functional significance of
Runx2 in hepatocellular carcinoma: Its role in vasculogenic mimicry
and epithelial-mesenchymal transition. Int J Mol Sci. 18:pii: E500.
2017. View Article : Google Scholar
|
|
6
|
Xie G and Diehl AM: Evidence for and
against epithelial-to-mesenchymal transition in the liver. Am J
Physiol Gastrointest Liver Physiol. 305:G881–G890. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kattaia AA, El-Baset Abd SA, Mohamed EM,
Abdul-Maksou RS and Elfakharany YM: Molecular mechanisms underlying
histological and biochemical changes induced by nitrate in rat
liver and the efficacy of S-Allylcysteine. Ultrastruct Pathol.
41:10–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Yang X, Jing Y, Zhang S, Zong C,
Jiang J, Sun K, Li R, Gao L, Zhao X, et al: Contribution and
mobilization of mesenchymal stem cells in a mouse model of carbon
tetrachloride-induced liver fibrosis. Sci Rep. 5:177622015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Praneenararat S, Chamroonkul N, Sripongpun
P, Kanngurn S, Jarumanokul R and Piratvisuth T: HBV DNA level could
predict significant liver fibrosis in HBeAg negative chronic
hepatitis B patients with biopsy indication. BMC Gastroenterol.
14:2182014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar S, Wang J, Shanmukhappa SK and
Gandhi CR: Toll-like receptor 4-independent carbon
tetrachloride-induced fibrosis and lipopolysaccharide-induced acute
liver injury in mice: Role of hepatic stellate cells. Am J Pathol.
187:1356–1367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Eischeid AN and Chen XM: Col1A1
production and apoptotic resistance in TGF-β1-induced
epithelial-to-mesenchymal transition-like phenotype of 603B cells.
PLoS One. 7:e513712012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Shen RW, Han B, Li Z, Xiong L,
Zhang FY, Cong BB and Zhang B: Notch signaling mediated by
TGF-β/Smad pathway in concanavalin A-induced liver fibrosis in
rats. World J Gastroenterol. 23:2330–2336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu JD, Liu S, Wang W, Li LS, Li XF, Li Y,
Guo H, Ji T, Feng XY, Hou XL, et al: Emodin induces chloride
secretion in rat distal colon through activation of mast cells and
enteric neurons. Br J Pharmacol. 165:197–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin HD, Li KT, Duan QQ, Chen Q, Tian S,
Chu ESM and Bai DQ: The effect of aloe-emodin-induced photodynamic
activity on the apoptosis of human gastric cancer cells: A pilot
study. Oncol Lett. 13:3431–3436. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen T, Zheng LY, Xiao W, Gui D, Wang X
and Wang N: Emodin ameliorates high glucose induced-podocyte
epithelial-mesenchymal transition in-vitro and in-vivo. Cell
Physiol Biochem. 35:1425–1436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Wu X, Chen M, Duan W, Sun L, Yan M
and Zhang L: Emodin induces apoptosis through caspase 3-dependent
pathway in HK-2 cells. Toxicology. 231:120–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Way TD, Huang JT, Chou CH, Huang CH, Yang
MH and Ho CT: Emodin represses TWIST1-induced
epithelial-mesenchymal transitions in head and neck squamous cell
carcinoma cells by inhibiting the β-catenin and Akt pathways. Eur J
Cancer. 50:366–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang CH, Gao ZQ, Ye B, Cai JT, Xie CG,
Qian KD and Du Q: Effect of emodin on pancreatic fibrosis in rats.
World J Gastroenterol. 13:378–382. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guan R, Wang X, Zhao X, Song N, Zhu J,
Wang J, Wang J, Xia C, Chen Y, Zhu D and Shen L: Emodin ameliorates
bleomycin-induced pulmonary fibrosis in rats by suppressing
epithelial-mesenchymal transition and fibroblast activation. Sci
Rep. 6:356962016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong MX, Jia Y, Zhang YB, Li CC, Geng YT,
Zhou L, Li XY, Liu JC and Niu YC: Emodin protects rat liver from
CCl(4)-induced fibrogenesis via inhibition of hepatic stellate
cells activation. World J Gastroenterol. 15:4753–4762. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Expert Committee on the Diagnosis and
Management of Fatty Liver Disease, Chinese Medical Association:
Recommendation for standardization of diagnosis and treatment of
fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 21:652–655.
2013.(In Chinese). PubMed/NCBI
|
|
22
|
Nouchi T, Worner TM, Sato S and Lieber CS:
Serum procollagen type III N-terminal peptides and laminin P1
peptide in alcoholic liver disease. Alcohol Clin Exp Res.
11:287–291. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zeng MD, Fan JG, Lu LG, Li YM, Chen CW,
Wang BY and Mao YM: Chinese National Consensus Workshop on
Nonalcoholic Fatty Liver Disease: Guidelines for the diagnosis and
treatment of nonalcoholic fatty liver diseases. J Dig Dis.
9:108–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim KM, Choi WB, Park SH, Yu E, Lee SG,
Lim YS, Lee HC, Chung YH, Lee YS and Suh DJ: Diagnosis of hepatic
steatosis and fibrosis by transient elastography in asymptomatic
healthy individuals: A prospective study of living related
potential liver donors. J Gastroenterol. 42:382–388. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li F, Miao L, Sun H, Zhang Y, Bao X and
Zhang D: Establishment of a new acute-on-chronic liver failure
model. Acta Pharm Sin B. 7:326–333. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monisha BA, Kumar N and Tiku AB: Emodin
and its role in chronic diseases. Adv Exp Med Biol. 928:47–73.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen Q, Pang L, Huang S, Lei W and Huang
D: Effects of emodin and irbesartan on ventricular fibrosis in
Goldblatt hypertensive rats. Pharmazie. 69:374–378. 2014.PubMed/NCBI
|
|
29
|
Gao R, Chen R, Cao Y, Wang Y, Song K,
Zhang Y and Yang J: Emodin suppresses TGF-β1-induced
epithelial-mesenchymal transition in alveolar epithelial cells
through Notch signaling pathway. Toxicol Appl Pharmacol. 318:1–7.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guan R, Zhao X, Wang X, Song N, Guo Y, Yan
X, Jiang L, Cheng W and Shen L: Emodin alleviates bleomycin-induced
pulmonary fibrosis in rats. Toxicol Lett. 262:161–172. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Woo YS, Lee KH, Lee KT, Lee JK, Kim JM,
Kwon CHD, Joh JW, Kang D and Cho J: Postoperative changes of liver
enzymes can distinguish between biliary stricture and graft
rejection after living donor liver transplantation: A longitudinal
study. Medicine (Baltimore). 96:e68922017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu CT, Yang J, Huang SM, Feng L and Li ZJ:
Analysis of islet beta cell functions and their correlations with
liver dysfunction in patients with neonatal intrahepatic
cholestasis caused by citrin deficiency (NICCD). Medicine
(Baltimore). 96:e86382017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li TZ, Kim SM, Hur W, Choi JE, Kim JH,
Hong SW, Lee EB, Lee JH and Yoon SK: Elk-3 contributes to the
progression of liver fibrosis by regulating the
epithelial-mesenchymal transition. Gut Liver. 11:102–111. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao YL, Zhu RT and Sun YL:
Epithelial-mesenchymal transition in liver fibrosis. Biomed Rep.
4:269–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li L, Sun W, Wu T, Lu R and Shi B: Caffeic
acid phenethyl ester attenuates lipopolysaccharide-stimulated
proinflammatory responses in human gingival fibroblasts via NF-κB
and PI3K/Akt signaling pathway. Eur J Pharmacol. 794:61–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hayashida T: Integrins modulate cellular
fibrogenesis at multiple levels; Regulation of TGF-β signaling.
Endocr Metab Immune Disord Drug Targets. 10:302–319. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li L, Lin M, Li L, Wang R, Zhang C, Qi G,
Xu M, Rong R and Zhu T: Renal telocytes contribute to the repair of
ischemically injured renal tubules. J Cell Mol Med. 18:1144–1156.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Park SA, Kim MJ, Park SY, Kim JS, Lee SJ,
Woo HA, Kim DK, Nam JS and Sheen YY: EW-7197 inhibits hepatic,
renal, and pulmonary fibrosis by blocking TGF-β/Smad and ROS
signaling. Cell Mol Life Sci. 72:2023–2039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Santibañez JF, Quintanilla M and Bernabeu
C: TGF-β/TGF-β receptor system and its role in physiological and
pathological conditions. Clin Sci (Lond). 121:233–251. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Blank U and Karlsson S: The role of Smad
signaling in hematopoiesis and translational hematology. Leukemia.
25:1379–1388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD pathway and its regulation in hepatic fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hu B, Gharaee-Kermani M, Wu Z and Phan SH:
Essential role of MeCP2 in the regulation of myofibroblast
differentiation during pulmonary fibrosis. Am J Pathol.
178:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|