Introduction
Atopic dermatitis (AD) is a common chronic
inflammatory skin disease that affects ~20% of children worldwide,
and the prevalence of AD increases rapidly every year (1). AD is characterized by chronic
recurrence of skin inflammation, epidermal barrier dysfunction,
IgE-mediated sensitization, edema, and thickened epidermis
(2). Although various genetic and
environmental factors have been reported to contribute to the
pathogenesis and development of AD, the precise cause of AD has not
yet been determined (3). Current
treatments for AD include topical ointments or systemic oral
administration of steroids and antihistamines to decrease
inflammatory damage and itching (4). Steroids are widely used to treat AD
because they alleviate atopic symptoms, while functioning as
anti-inflammatory agents and promoting cell proliferation and
immunosuppression. However, prolonged treatment with steroids has
side effects such as the development of drug tolerance, endocrine
abnormalities, increased susceptibility to infections, metabolic
abnormalities, and skin atrophy that leads to the cracking of skin
and bleeding (5). Therefore, there
is a growing interest in AD treatments using natural materials that
have fewer side effects. Natural compounds and natural extracts of
various herbs have been reported as potential medicines to prevent
and treat inflammatory skin diseases.
Wheat (Triticum sp.) is an important crop
worldwide. The young grass of Triticum aestivum, called
wheatgrass, is richer in nutrients such as vitamins, minerals, and
proteins than the mature cereal plant (6). T. aestivum is used as a health
food supplement in the form of tablets, juice, powder, and fresh
produce. Many papers report that T. aestivum has anticancer,
anti-inflammation, antioxidant (7,8), and
therapeutic effects in diseases such as diabetes, colitis,
allergies, and heart diseases (9–11).
In previous studies, we found that T. aestivum sprouts are
effective in treating several diseases, such as diabetes (12), obesity (13,14),
liver injury (15,16), and cancer (17). Thus, T. aestivum sprouts
represent a potential remedy for these diseases. Furthermore, the
dichloromethane fraction of T. aestivum ameliorated allergic
reaction by inhibition of Th2 cell differentiation in mice
(18). AD is mediated by and
related to allergic disease (2).
However, the influence of T. aestivum on allergy-mediated
inflammation is not clearly understood. In addition, the specific
effects of T. aestivum sprouts in AD are not yet known.
In the present study, considering their various
biological effects, we evaluated the effects of T. aestivum
sprouts in AD. We report the anti-atopic effects of a 70% ethanol
extract of T. aestivum sprouts (TAEE) in vitro and
in vivo.
Materials and methods
Preparation of TAEE
T. aestivum Lamarck was supplied by the
National Institute of Crop Science (Jeonbuk, Korea). After
germination, the seeds were grown in organic sterile peat moss at a
constant temperature (average 20±2°C). The T. aestivum
sprouts were harvested at 2 weeks after germination, lyophilized,
and laboratory-scale pulverized. The pulverized T. aestivum
sprouts (30 g) were ultrasonically extracted with 70% EtOH for 1 h
and then filtered. After evaporation on a rotary vacuum evaporator
(N-000; EYELA, Tokyo, Japan), the TAEE was obtained. For subsequent
experiments, the TAEE was stored at 4°C and protected from light
until immediately before the experiment. The TAEE was dissolved in
purified water for use in subsequent experiments.
Cell culture
Human keratinocytes (HaCaT cells) were obtained from
the Korean cell line bank (Seoul, Korea). Cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Lonza, Walkersville, MD,
USA), containing 10% fetal bovine serum (FBS; Biotechnics Research,
CA, USA), 100 units/ml of penicillin, and 100 µg/ml of streptomycin
(both Welgene, Seoul, Korea) at 37°C in a humidified 5%
CO2 air atmosphere.
Cell Counting kit-8 (CCK-8) assay
The HaCaT cell proliferation rate was evaluated
using a CCK-8 (Dojindo, Kumamoto, Japan), according to the
manufacturer's instructions. Briefly, HaCaT cells were seeded at
5×103 cells/well in 96-well plates. After incubation for
24 h, the cells were treated with different TAEE concentrations
(0–400 µg/ml), and then incubated for another 24 or 48 h. The cells
were washed with phosphate-buffered saline (PBS), the CCK-8
solution was added, and the cells were incubation for 1.5 h. The
absorbance of cells was measured at 450 nm using a microplate
reader (Synergy HTX Multi-Mode Reader; BioTek, Winooski, VT,
USA).
Animals and treatment
Female BALB/c mice (4 weeks old) were purchased from
Samtako Bio Korea (Osan, Korea). To induce AD-like skin lesions in
mice, 2,4-dinitrochlorobenzene (DNCB) was used. The mice were
divided into five groups: i) Normal control group, not treated with
DNCB; ii) AD group, treated with DNCB; iii) TAEE 100 group, treated
with DNCB and administered 100 mg/kg TAEE p.o.; iv) TAEE 200 group,
treated with DNCB and administered 200 mg/kg TAEE p.o.; and v) dexa
group, treated with DNCB and administered 1 mg/kg dexamethasone
p.o. After the mice were acclimatized in the facility for one week,
the dorsal skin hairs of the mice were removed using an electronic
clipper and hair removal cream, and the skin was allowed to heal
for 24 h. A 1% (w/v) DNCB solution was prepared with an acetone and
olive oil mixture (4:1, v/v), and the solution was applied to the
back of the mice once a day for 3 days from the start of the
experiment. Afterwards, a 0.5% DNCB solution was applied once every
2 for 10 days. TAEE or dexamethasone were administered for 10 days
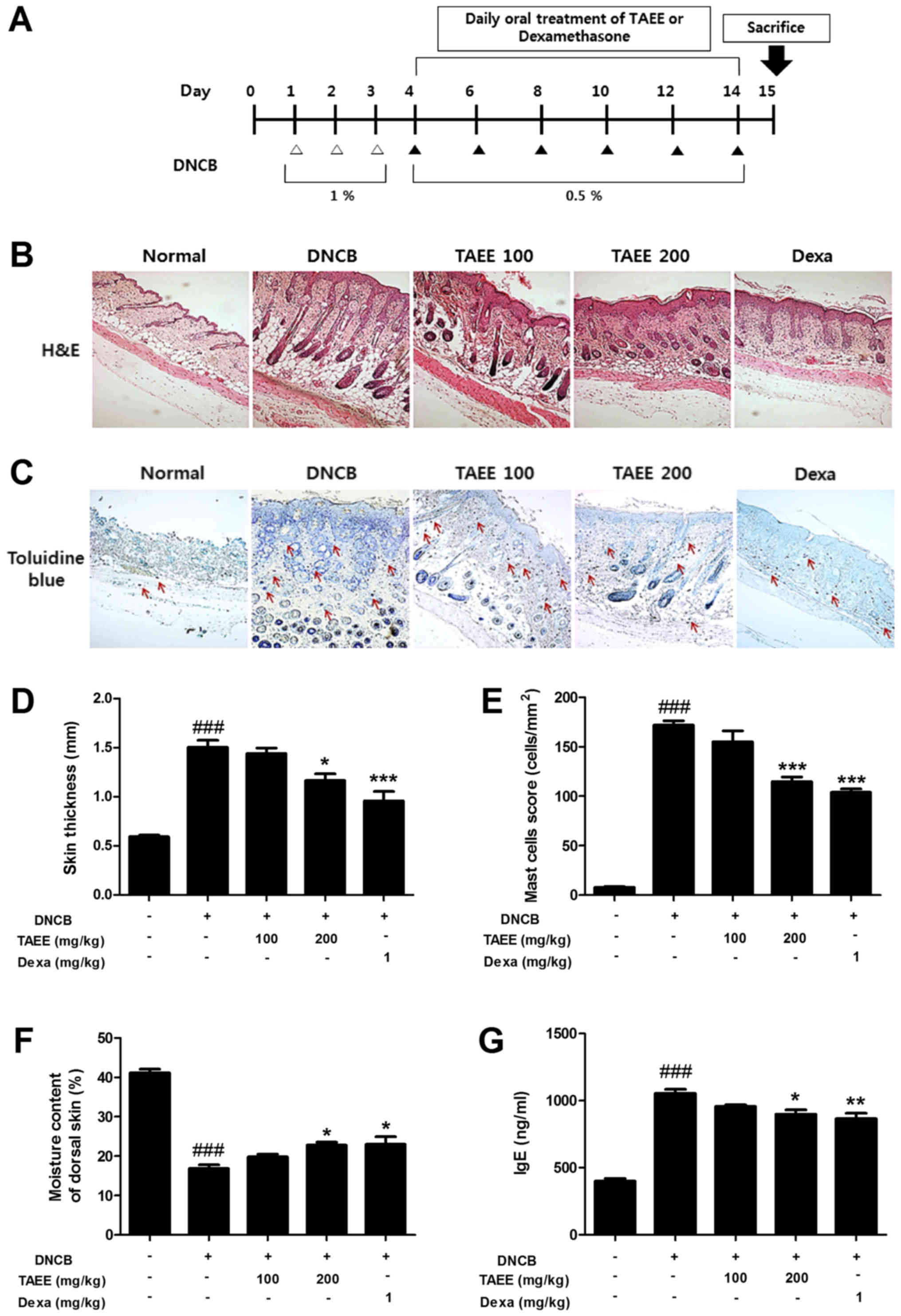
from day 4 to day 14 of the experiment. The design of the
experiment is summarized in Fig.
1A. The research was conducted in accordance with the ethical
regulations of the Animal Experiment Ethics Committee of Chonbuk
National University and with their approval (approval no. CBNU
2017-0002).
Histological analysis
Dorsal skin of the mice was sampled, fixed in 4%
formaldehyde solution at room temperature for 24 h, and embedded in
paraffin. Each paraffin block was serially sectioned into five 4-µm
sections (n=5). Each tissue section was deparaffinized with xylene
and stained with hematoxylin for 1 min and with eosin for 3 min.
Other section was stained with toluidine blue for determining the
number of mast cells. After staining, each tissue section was
dehydrated, sealed with mounting solution, and examined under an
optical microscope (CX21; Olympus, Tokyo, Japan).
Analysis of dorsal skin moisture
content
The moisture content of the dorsal skin was analyzed
using the TS-skin diagnosis system (Aram Huvis Co., Ltd., Seongnam,
Korea), which measures the moisture content (%) based on the
electrical capacitance of the skin surface. According to the
manufacturer's instructions, three different regions of the dorsal
skin were measured for 10 sec each.
Serum IgE measurement
Blood was collected from the mice using 23 G
syringes and centrifuged at 3,000 rpm for 10 min to separate the
serum. Total serum IgE was analyzed using sandwich enzyme-linked
immunosorbent assay (ELISA), performed using a mouse IgE ELISA kit
(BD Biosciences, San Jose, CA, USA). After incubation overnight at
4°C with 250 µl of diluted capture antibodies in 0.1 M sodium
carbonate (pH 9.5), 200 µl of assay diluent was added to each well
and blocked for 1 h at room temperature. The serum and serially
diluted standard solutions were dispensed at 100 µl per well and
allowed to react at room temperature for 2 h. Diluted detection
antibodies and streptavidin-horseradish peroxidase (HRP; 100 µl)
were then added into each well and incubated at room temperature
for 1 h. Between each step, the wells were washed with 0.05%
PBS-Tween-20. After the final wash, 100 µl of the substrate
solution was dispensed and allowed to react for 30 min in the dark.
To stop the reaction, 50 µl of 2 N H2SO4 was
added to each well, and the absorbance was measured at 450 nm using
a microplate reader (Synergy HTX Multi-Mode Reader; BioTek).
RNA extraction
Cells were seeded in 6-well plates at a
concentration of 1×105 cells/ml. After overnight
incubation, the cells were pretreated with TAEE for 2 h and
incubated with 10 ng/ml tumor necrosis factor α (TNF-α) and 10
ng/ml interferon γ (IFN-γ; ProSpec-Tany TechnoGene, Rehovot,
Israel) for 6 h. In addition, the dorsal skin tissue was cut at the
end of the experiment. One milliliter of TRIzol solution (Ambion,
Austin, TX, USA) was added to each well to extract the total RNA.
The RNA was mixed with 0.2 ml of chloroform and centrifuged at
12,000 rpm at 4°C. The supernatant was collected, mixed with 0.5 ml
of 2-propanol, and centrifuged at 12,000 rpm for 10 min, after
which the RNA pellets were dried. The dried RNA pellets were
dissolved in RNase-free water.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was quantified using spectrophotometry,
after which cDNA was synthesized using 2 µg of total RNA and a
PrimeScript™ II 1st strand cDNA synthesis kit (Takara Bio Inc.,
Otsu, Japan). RT-PCR was performed using a Real-Time™ PCR System
with SYBR-Green PCR Master Mix (both Applied Biosystems, Foster
City, CA, USA). The PCR conditions were as follows: 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), was simultaneously measured for normalization. The
nucleotide sequences of the primers used are shown in Table I.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Sense | Anti-sense | BPs |
|---|
| hRANTES |
CGCTGTCATCCTCATTGCTA |
GCACTTGCCACTGGTGTAGA | 148 |
| hMDC |
TGCCGTGATTACGTCCGTTAC |
AAGGCCACGGTCATCAGAGTAG | 201 |
| hIP-10 |
TTGCTGCCTTATCTTTCTGACTC |
ATGGCCTTCGATTCTGGATT | 222 |
| mRANTES |
TGCCCACGTCAAGGAGTATTTC |
AACCCACTTCTTCTCTGGGTTG | 112 |
| mMDC |
GTGGCTCTCGTCCTTCTTGC |
GGACAGTTTATGGAGTAGCTT | 249 |
| mIP-10 |
CTGAGTGGGACTCAAGGGAT |
TCGTGGCAATGATCTCAACAC | 151 |
| hSOCS-1 |
TTTTTCGCCCTTAGCGTGA |
AGCAGCTCGAAGAGGCAGTC | 119 |
| GAPDH |
GAAGGTGAAGGTCGGAGT |
GAAGATGGTGATGGGATTTC | 226 |
Western blot analysis
HaCaT cells were harvested in PBS. The cells were
then centrifuged at 12,000 g for 20 min at 4°C to remove the
supernatant, lysed using radioimmunoprecipitation assay (RIPA)
lysis buffer (Pierce Biotechnology, Rockford, IL, USA), and kept on
ice for 30 min. The extracted proteins were mixed with 5X SDS
sample buffer. Lysates were separated by 10% sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked with 5% bovine serum albumin (BSA) in Tris-buffered saline
containing 0.1% Tween 20 (TBST) for 1 h at room temperature. The
membranes were then incubated with primary antibodies at 4°C
overnight. After incubation, the membranes were washed with TBST
buffer three times for 15 min and then incubated for 1 h at room
temperature with HRP-conjugated secondary antibodies diluted to
1:5,000. Membranes were then washed four times with TBST buffer and
protein signals were developed using an enhanced chemiluminescence
(ECL) detection kit (Merck Millipore, Burlington, MA, USA). Images
were obtained using the Fusion Fx gel documentation system (Vilber
Lourmat, Marne-la-Vallee, France).
Statistical analysis
Results are expressed as mean values ± standard
error of the mean (SEM). Statistical significance was determined
using one-way analysis of variance (ANOVA) with Tukey's post-hoc
test to determine differences between groups. All statistical
analyses were performed using Graph Pad Prism software 5.0 (Graph
Pad Software, Inc. La Jolla, CA, USA). *P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of TAEE on AD-like symptoms in
DNCB-treated mice
To investigate the effect of TAEE on AD-like skin
lesions, the DNCB-treated mice were orally administered TAEE for 10
days. As a positive control, the Dexa group was orally administered
dexamethasone. On day 15 after the start of the experiment, the
mice were sacrificed (Fig. 1A). To
examine the effect of TAEE on skin thickness, sections of dorsal
skin tissue were stained with hematoxylin and eosin (H&E) and
observed under a microscope. Repeated DNCB application caused
severe skin changes, including skin hypertrophy and fibrosis of the
dermis in the dorsal skin tissues of DNCB-treated mice. TAEE
treatments lead to reduced skin thickness in a dose-dependent
manner. Oral administration of 200 mg/kg TAEE and 1 mg/kg
dexamethasone markedly decreased the skin thickness (Fig. 1B and D). Furthermore, to analyze
the effect of TAEE on the number of mast cells, sections of dorsal
skin tissue were stained with toluidine blue and examined under a
microscope. Repeated DNCB application increased the number of mast
cells that infiltrated the dermis of the dorsal skin of
DNCB-treated mice. Oral administration of TAEE reduced the number
of infiltrated mast cells in a dose-dependent manner. Oral
administration of 200 mg/kg TAEE and 1 mg/kg dexamethasone
significantly decreased the number of mast cells (Fig. 1C and E).
Effects of TAEE on transepidermal
water loss (TEWL) in dorsal skin of DNCB-treated mice
AD increases skin moisture loss and impairs the skin
barrier function. Therefore, skin hydration is essential to control
AD (19). We examined the effect
of TAEE on loss of skin moisture induced by DNCB. Fig. 1F shows the moisture content of
dorsal skin after 10 days of oral drug administration. We observed
a marked decrease in the skin moisture content of down to 40.77% in
the DNCB-treated group. Compared with the DNCB-treated group, the
skin moisture content increased in a dose-dependent manner by oral
administration of TAEE. Oral administration of 200 mg/kg TAEE and 1
mg/kg dexamethasone significantly recovered the TEWL to 55.61 and
55.83%, respectively.
Effects of TAEE on elevation of serum
IgE levels in DNCB-treated mice
IgE binds to receptors on the surface of mast cells
or white blood cells, leading to an allergic reaction. In addition,
IgE antibody production is related to the T helper 2 (Th2) immune
response (20). IgE plays an
important role in AD occurrence and progression, and patients with
AD usually have high serum IgE levels. We analyzed the effect of
TAEE on serum levels of total IgE using ELISA. The serum IgE levels
were elevated in the DNCB-treated group. Compared with the
DNCB-treated group, the serum IgE levels were markedly decreased in
a dose-dependent manner by oral administration of TAEE (Fig. 1G).
Effects of TAEE on expression of
chemokines in DNCB-treated mice
Keratinocytes are activated by inflammatory
stimulation to produce a variety of chemokines. These chemokines
include regulated upon activation, normally T-expressed, and
presumably secreted (RANTES, also known as CCL5),
macrophage-derived chemokine (MDC, also known as CCL22), and
IFN-γ-induced protein of 10 kDa (IP-10, also known as CXCL10)
(21). To analyze the effects of
TAEE on inflammatory chemokines in dorsal skin tissue, we assessed
the mRNA levels of RANTES, MDC, and IP-10 using
real-time PCR. In skin lesions, the expression levels of RANTES,
MDC, and IP-10 were elevated in the DNCB-treated group.
Oral administration of TAEE decreased the mRNA levels of RANTES,
MDC, and IP-10 in a dose-dependent manner. Oral
administration of 200 mg/kg TAEE and 1 mg/kg dexamethasone
considerably lowered the mRNA levels of the said chemokines
(Fig. 2).
 | Figure 2.TAEE decreases inflammatory
chemokines in DNCB-treated mice. The mRNA levels of the chemokines
(A) RANTES, (B) MDC and (C) IP-10 were determined using reverse
transcription-polymerase chain reaction. Values are presented as
the mean ± standard error of the mean of three independent
experiments. Data were analyzed by Tukey's post-hoc test.
###P<0.001 vs. the normal group; *P<0.05,
**P<0.01 and ***P<0.001 vs. the DNCB-treated group. TAEE, 70%
EtOH extract of T. aestivum sprouts; DNCB,
2,4-dinitrochlorobenzene; Dexa, dexamethasone; RANTES, regulated
upon activation, normally T-expressed, and presumably secreted;
MDC, macrophage-derived chemokine IP-10, IFN-γ-induced protein of
10 kDa. |
Effects of TAEE on expression of
chemokines in TNF-α- and IFN-γ-treated HaCaT cells
We analyzed the effect of TAEE on cell viability in
HaCaT cells using the CCK-8 assay. The cells were pretreated with
TAEE at doses of 0, 25, 50, 100, 200, and 400 µg/ml for 24 and 48
h. As shown in Fig. 3A, the
viability of HaCaT cells was similar at all concentrations. To
investigate the effects of TAEE on the expression of inflammatory
chemokines in HaCaT cells, we analyzed the mRNA levels of
RANTES, MDC, and IP-10 using real-time PCR. As shown
Fig. 3, the expression levels of
RANTES, MDC, and IP-10 were elevated in TNF-α- and
IFN-γ-treated cells. TAEE treatment significantly decreased the
mRNA levels of RANTES, MDC, and IP-10.
 | Figure 3.TAEE suppresses inflammatory
chemokines in TNF-α- and IFN-γ-stimulated HaCaT cells. (A) HaCaT
cells were treated with various concentrations of TAEE for 24 and
48 h and the cell viability was determined using the Cell Counting
kit assay. Cells were pre-treated with TAEE (50 µg/ml) for 2 h and
in the presence of TNF-α (10 ng/ml) and IFN-γ (10 ng/ml) for the
indicated time. The mRNA levels of (B) RANTES, (C)
MDC and (D) IP-10 were examined using reverse
transcription-polymerase chain reaction. GAPDH was used as the
internal control. Values are presented as the mean ± standard error
of the mean of three independent experiments. Data were analyzed by
Tukey's post-hoc test. **P<0.01 and ***P<0.001 vs. 0 h;
###P<0.001. TAEE, 70% EtOH extract of T.
aestivum sprouts; TI, TNF-α + IFN-γ; TNF, tumor necrosis
factor; IFN, interferon; RANTES, regulated upon activation,
normally T-expressed, and presumably secreted; MDC,
macrophage-derived chemokine IP-10, IFN-γ-induced protein of 10
kDa. |
Effects of TAEE on TNF-α- and
IFN-γ-induced STAT1 phosphorylation in HaCaT cells
Previous reports have shown that TNF-α and IFN-γ
activate the STAT pathway in human epidermal keratinocytes and that
TNF-α- and IFN-γ-induced release of chemokines involves
phosphorylation of the STAT1 transcription factor (22). Therefore, we examined STAT1
phosphorylation in TNF-α- and IFN-γ-treated HaCaT cells, with or
without TAEE. The results showed that, when compared with the
control, treatment with TAEE for 1 h effectively inhibited STAT1
phosphorylation (Fig. 4A and
B).
Effects of TAEE on SOCS1 expression in
TNF-α- and IFN-γ-treated HaCaT cells
SOCS1 exhibits inhibitory activity against STAT1
(23,24). Therefore, we assessed the effects
of TAEE on SOCS1. The SOCS1 mRNA levels were examined in
TNF-α- and IFN-γ-treated HaCaT cells in the presence or absence of
TAEE. As shown in Fig. 5, TAEE
markedly enhanced SOCS1 expression compared with the
control.
Discussion
AD is characterized by relapsing, eczematous skin
lesions, skin hypersensitivity, and dry skin, caused by the
interaction of Th1 and Th2 cells (2,25).
Despite extensive research, the exact cause of AD and a definitive
cure remain elusive. Although AD is usually treated with
anti-inflammatory or immunosuppressive drugs such as steroids and
antihistamines, many of these treatments have serious side effects
(26,27). Currently, many patients are turning
to alternative strategies that use plant-based natural products
with fewer side effects. Therefore, it is essential to investigate
health products and new drugs for the safe and effective prevention
and treatment of AD. Many plant-based products have been used to
treat and meliorate AD (28,29).
T. aestivum sprouts, known as wheatgrass, are
consumed in the form of juices or dried powders, and are known as a
health food. T. aestivum sprouts contain vitamins A, B, C,
and K, calcium, potassium, iron, magnesium, sodium, amino acids,
chlorophyll, and minerals (30).
In our previous studies, we showed that a dichloromethane fraction
isolated from T. aestivum sprouts attenuated the allergic
immune response in ovalbumin (OVA)-sensitized mice, which indicated
that T. aestivum sprouts might have the potential to
regulate the immune response in allergic diseases (18). However, until now, the effect of
T. aestivum sprouts on AD, an allergic diseases, was not
known. In this study, we examined whether TAEE, as a promising
plant candidate compound, could attenuate AD in a DNCB-treated
mouse model and in inflammatory cytokine-treated human
keratinocytes.
AD patients have skin barrier dysfunctions, such as
skin hyperkeratosis and increased TEWL (31). In addition, AD increases the levels
of IgE antibodies. When IgE binds to cell surface receptors, mast
cells become activated and secrete histamine, causing inflammation
and worsening the skin condition. Thus, it is important to reduce
serum IgE levels and TEWL. For this reason, we measured skin
thickness and moisture content and serum IgE levels in DNCB-treated
dermatitis. Oral administration of TAEE significantly reduced TEWL
and serum IgE levels when compared to DNCB-treated mice. A
histological section of dorsal skin tissues showed that TAEE
markedly suppressed an increase in the thickness of the epidermis
and dermis, as well as the infiltration of mast cells.
Keratinocytes make up to 90% of cells in the
epidermis and, when activated by inflammation (e.g., by TNF-α, and
IFN-γ), produce a variety of chemokines. Th2-related chemokines
attract inflammatory cells to promote their infiltration into
inflammatory skin lesions. These infiltrating inflammatory cells
promote a switch from acute to chronic responses in AD. In chronic
AD skin lesions, Th1 cells produce TNF-α and IFN-γ. In HaCaT cells,
Th2-related chemokines are induced by TNF-α and IFN-γ stimulation.
The downregulation of inflammatory chemokine production in
keratinocytes may be an effective therapeutic strategy for
inflammatory skin diseases.
Many studies have shown that TNF-α and IFN-γ induce
the production of Th2-chemokines through STAT in human epidermal
keratinocytes (32,33).
The SOCS proteins are cytokine-inducible negative
regulators of cytokine signaling, and their levels are increased by
IFN-γ treatment. The family consists of three proteins, of which
SOCS1 and SOCS3 inhibit increased STAT1 phosphorylation in response
to IFN-γ stimulation (23,24).
In the present study, we found that TAEE suppressed
the mRNA levels of chemokines such as RANTES, MDC and IP-10 in
TNF-α- and IFN-γ-stimulated HaCaT cells and in DNCB-treated mice.
In addition, TAEE decreased STAT1 phosphorylation and increased the
mRNA levels of SOCS1. Thus, TAEE appears to decrease the
expression of chemokines by inhibiting the STAT1 pathway and
increasing the level of SOCS1. These results suggest that TAEE
exerts its protective effect in skin inflammation by regulating
pro-inflammatory chemokines via phosphorylation of STAT1.
In conclusion, the results of our study demonstrate
that TAEE is a natural anti-AD compound, which inhibits AD
like-skin lesions and the release of inflammatory chemokines in the
skin by regulating inflammation and allergy mediators. Thus, TAEE
has potential as a natural treatment for inflammatory allergic
responses of the skin.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by
grants from Wonkwang University (2018).
Availability of data and materials
All data sets generated or analyzed during this
article are included within the published article.
Authors' contributions
Y-ML, D-KK and H-HK designed the study. J-HL and
H-HK wrote the manuscript. H-HK collected clinical samples. J-HL
reviewed and analyzed the data. All authors confirmed and approved
review for this manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Experiment Ethics Committee of Chonbuk National University
(approval no. CBNU 2017-0002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leung DY and Bieber T: Atopic dermatitis.
Lancet. 361:151–160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Udompataikul M and Limpa-o-vart D:
Comparative trial of 5% dexpanthenol in water-in-oil formulation
with 1% hydrocortisone ointment in the treatment of childhood
atopic dermatitis: A pilot study. J Drugs Dermatol. 11:366–374.
2012.PubMed/NCBI
|
|
4
|
Schäkel K, Döbel T and Bosselmann I:
Future treatment options for atopic dermatitis-small molecules and
beyond. J Dermatol Sci. 73:91–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeziorkowska R, Sysa-Jędrzejowska A and
Samochocki Z: Topical steroid therapy in atopic dermatitis in
theory and practice. Postepy Dermatol Alergol. 32:162–166. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tirgar PR, Thumber BL and Desai TR:
Isolation, characterization and biological evaluation of iron
chelator from Triticum aestivum (wheat grass). Int J Pharma
Bio Sci. 2:288–296. 2011.
|
|
7
|
Das A, Raychaudhuri U and Chakraborty R:
Effect of freeze drying and oven drying on antioxidant properties
of fresh wheatgrass. Int J Food Sci Nutr. 63:718–721. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SH, Lee YM, Lee HS and Kim DK:
Anti-oxidative and anti-hyperglycemia effects of Triticum
aestivum wheat sprout water extracts on the
streptozotocin-induced diabetic mice. Korean J Pharmacogn.
40:408–411. 2009.
|
|
9
|
Ben-Arye E, Goldin E, Wengrower D, Stamper
A, Kohn R and Berry E: Wheat grass juice in the treatment of active
distal ulcerative colitis: A randomized double-blind
placebo-controlled trial. Scand J Gastroenterol. 37:444–449. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shermer M: Wheatgrass juice and folk
medicine. Sci Am. 299:422008. View Article : Google Scholar
|
|
11
|
Gore RD, Palaskar SJ and Bartake AR:
Wheatgrass: Green blood can help to fight cancer. J Clin Diagn Res.
11:ZC40–ZC42. 2017.PubMed/NCBI
|
|
12
|
Lee SH, Lim SW, Lee YM, Lee HS and Kim DK:
Polysaccharide isolated from Triticum aestivum stimulates
insulin release from pancreatic cells via the ATP-sensitive K+
channel. Int J Mol Med. 29:913–919. 2012.PubMed/NCBI
|
|
13
|
Poudel B, Nepali S, Xin M, Ki HH, Kim YH,
Kim DK and Lee YM: Flavonoids from Triticum aestivum inhibit
adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol
Med Rep. 12:3139–3145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luyen BT, Thao NP, Tai BH, Lim JY, Ki HH,
Kim DK, Lee YM and Kim YH: Chemical constituents of Triticum
aestivum and their effects on adipogenic differentiation of
3T3-L1 preadipocytes. Arch Pharm Res. 38:1011–1018. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nepali S, Ki HH, Lee JH, Lee HY, Kim DK
and Lee YM: Wheatgrass-derived polysaccharide has antiinflammatory,
anti-oxidative and anti-apoptotic effects on lps-induced hepatic
injury in mice. Phytother Res. 31:1107–1116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nepali S, Ki HH, Lee JH, Cha JY, Lee YM
and Kim DK: Triticum aestivum sprout-derived polysaccharide
exerts hepatoprotective effects against ethanol-induced liver
damage by enhancing the antioxidant system in mice. Int J Mol Med.
40:1243–1252. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ki HH, Poudel B, Lee JH, Lee YM and Kim
DK: In vitro and in vivo anti-cancer activity of dichloromethane
fraction of Triticum aestivum sprouts. Biomed Pharmacother.
96:120–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ki HH, Hwang SW, Lee JH, Kim YH, Kim DK
and Lee YM: A dichloromethane fraction of Triticum aestivum
sprouts reduces allergic immune response through inhibiting Th2
differentiation in ovalbumin-immunized mice. Mol Med Rep.
16:3535–3541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Werner Y and Lindberg M: Transepidermal
water loss in dry and clinically normal skin in patients with
atopic dermatitis. Acta Derm Venereol. 65:102–105. 1985.PubMed/NCBI
|
|
20
|
Bieber T: Atopic dermatitis. Ann Dermatol.
22:125–137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sallusto F, Mackay CR and Lanzavecchia A:
The role of chemokine receptors in primary, effector, and memory
immune responses. Annu Rev Immunol. 18:593–620. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Kim MS, Jeong GS and Yoon J:
Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines
production via blockade of NF-κB, STAT1 and p38-MAPK activation in
human epidermal keratinocytes. J Ethnopharmacol. 171:85–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song MM and Shuai K: The suppressor of
cytokine signaling (SOCS) 1 and SOCS3 but not SOCS2 proteins
inhibit interferon-mediated antiviral and antiproliferative
activities. J Biol Chem. 273:35056–35062. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Croker BA, Kiu H and Nicholson SE: SOCS
regulation of the JAK/STAT signalling pathway. Semin Cell Dev Biol.
19:414–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spergel JM and Paller AS: Atopic
dermatitis and the atopic march. J Allergy Clin Immunol. 112 6
Suppl:S118–S127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saeki H, Nakahara T, Tanaka A, Kabashima
K, Sugaya M, Murota H, Ebihara T, Kataoka Y, Aihara M, Etoh T, et
al: Clinical practice guidelines for the management of atopic
dermatitis 2016. J Dermatol. 43:1117–1145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arellano FM, Wentworth CE, Arana A,
Fernández C and Paul CF: Risk of lymphoma following exposure to
calcineurin inhibitors and topical steroids in patients with atopic
dermatitis. J Invest Dermatol. 127:808–816. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santoro D, Bohannon M, Ahrens K, Navarro
C, Gatto H and Marsella R: Evaluation on the effects of 0.1%
Peumus boldus leaf and Spiraea ulmaria plant extract
combination on bacterial colonization in canine atopic dermatitis:
A preliminary randomized, placebo controlled, double-blinded study.
Res Vet Sci. 118:164–170. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin J, Yoon SH, Ahn HS and Lee MW:
Inhibitory activity of allergic contact dermatitis and atopic
dermatitis-like skin in BALB/c mouse through oral administration of
fermented barks of Alnus sibirica. Molecules. 23:pii: E450.
2018.
|
|
30
|
Rajesh M and Ramesh BB: A study on wheat
grass and its nutritional value. Food Sci Qual Manage. 2:1–8.
2011.
|
|
31
|
Knor T, Meholjić-Fetahović A and
Mehmedagić A: Stratum corneum hydration and skin surface pH in
patients with atopic dermatitis. Acta Dermatovenerol Croat.
19:242–247. 2011.PubMed/NCBI
|
|
32
|
Jeong SJ, Lim HS, Seo CS, Kim JH, Jin SE,
Yoo SR and Shin HK: Traditional herbal formula Jakyakgamcho-tang
(Paeonia lactiflora and Glycyrrhiza uralensis)
impairs inflammatory chemokine production by inhibiting activation
of STAT1 and NF-κB in HaCaT cells. Phytomedicin. 22:326–332. 2015.
View Article : Google Scholar
|
|
33
|
Lim HS, Jin SE, Kim OS, Shin HK and Jeong
SJ: Alantolactone from saussurea lappa exerts
antiinflammatory effects by inhibiting chemokine production and
STAT1 phosphorylation in TNF-α and IFN-γ-induced in HaCaT cells.
Phytother Res. 29:1088–1096. 2015. View
Article : Google Scholar : PubMed/NCBI
|