Introduction
Liver cancer is one of the most lethal cancer types
worldwide and its incidence is steadily rising (1). The overall 5-year survival rate for
liver cancer is 17% (2) and drops
to only 3% in patients with advanced malignancy. At diagnosis,
<20% of patients with hepatic carcinoma have early-stage tumors
that are potentially curable with surgery. For the majority of
patients with non-resectable tumors, the treatments are largely
palliative. Sorafenib is a multi-targeted tyrosine kinase
inhibitor, and the only first-line drug for the clinical management
of primary liver cancer (3).
However, concerns have been raised about sorafenib therapy,
including acquired drug resistance (3). Therefore, it is of great significance
to elucidate the underlying mechanism and effective approaches to
enhance sensitivity to sorafenib in patients with liver cancer.
Cancer stem cells (CSCs) are stem cell-like cells
that are self-renewing and differentiating in tumors. The existence
of tumor stem cells has been identified in liver cancer and various
solid tumors (4). It was recently
hypothesized that CSCs were correlated with occurrence, metastasis
and drug resistance in many cancer types (5). The existence of liver CSCs is
directly associated with resistance to cisplatin and 5-fluorouracil
through regulation of RAC-α serine/threonine-protein kinase (Akt),
transforming growth factor (TGF)-β and other signaling pathways
(6). Liver CSCs may develop
resistance to sorafenib through diverse mechanisms (7). These previous studies indicated that
if sorafenib-resistant CSCs are eliminated, the incidence of drug
resistance may be reduced.
Biguanide drugs are commonly used in the treatment
of type 2 diabetes mellitus (T2DM). A recent study reported that
metformin may reduce the incidence and mortality rates of multiple
tumors in patients with T2DM (8).
Metformin also suppressed CSC formation in breast cancer (9), glioblastoma (10), colon cancer (11), pancreatic cancer (12), prostate cancer (13) and osteosarcoma (14). Metformin was reported to improve
overall survival by reducing the proportion of CSCs in oral
squamous cell carcinoma (15).
Metformin may improve CSC sensitivity to chemotherapeutic drugs,
reduce the proportion of CSCs in liver cancer and increase liver
cancer cell sensitivity to sorafenib (16). According to these results, it was
hypothesized that metformin may be a useful therapeutic agent in
enhancing sensitivity to sorafenib in T2DM, in addition to in
patients without T2DM, by targeting CSCs.
Sphere-forming cell subpopulations isolated from
human hepatoma cell lines possess properties that define CSCs
(17). In the present study,
cancer stem-like HepG2 spheres were generated using stem cell
conditioned culture medium, and decreased sensitivity to sorafenib
was observed in these stem-like spheres. Low doses of metformin
reduced the formation by reversing the epithelial-mesenchymal
transformation (EMT) process of HepG2 stem-like spheres.
Materials and methods
Cell culture
The HepG2 cell line was purchased from the Cell
Resource Center of the Shanghai Institutes for Biological Science,
Chinese Academy of Sciences (Shanghai, China). Cells were cultured
in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere at 37°C with 5%
CO2.
Enrichment of stem cell-like HepG2
spheres
HepG2 cells were digested with Accutase cell
detachment solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and washed with PBS (Gibco; Thermo Fisher Scientific, Inc.) twice.
Subsequently, cells were seeded at a density of 1×104
cells/100 mm Petri dish, and cultured in stem cell conditioned
culture medium [RPMI 1640 medium containing 2% B27 supplement
(Gibco; Thermo Fisher Scientific, Inc.), 20 ng/ml recombinant human
epidermal growth factor (PeproTech, Inc., Rocky Hill, NJ, USA) and
20 ng/ml recombinant human insulin-like growth factor (PeproTech,
Inc.)]. Cells were maintained in a humidified incubator at 37°C
with 5% CO2. Culture medium was half-replaced every
other day. The diameters of the spheres reached 90–100 µm after 8
days. Following culturing for 8 days, the spheres were collected,
dissociated into a single cell suspension and resuspended in fresh
medium for serial subcultivation every 6 days. The morphology of
the spheres was recorded with an inverted microscope (×200
magnification; Olympus IX73; Olympus Corporation, Tokyo, Japan)
every 3 days. The total number and diameter of spheres >50 µm in
three typical fields in each well or dish were counted, and the
mean values were calculated accordingly. For cell passages, single
cell suspensions derived from the original spheroids were obtained
using Accutase cell detachment solution and placed into a new
culture dish or plate, and the spheres grew to 100 µm in diameter
within 9 days.
Flow cytometry
In order to detect the expression of the stem cell
surface markers cluster of differentiation (CD) 133 and CD90,
spheres at day 7 were digested into single cells with Accutase cell
detachment solution. A total of ~1.0×106 cells were
washed with cold PBS twice and resuspended in 400 µl PBS. A volume
of 10 µl rabbit anti-human CD133/1 (AC133)-phycoerythrin (PE; cat.
no. 130098826; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) or
2 µl rabbit anti-human CD90 (REA897)-fluorescein isothiocyanate
(FITC; cat. no. 130114901; Miltenyi Biotec GmbH) was added and
incubated in the dark for 30 min at 4°C. Following two washes with
PBS, the markers were determined using a FACSCalibur system running
BD CellQuest™ Pro software version 3.3 (BD Biosciences,
San Jose, CA, USA) and the data were analyzed using FlowJo version
7.6 (FlowJo LLC, Ashland, OR, USA). Isotype controls of PE and FITC
were used to eliminate false positive expression.
Cell viability assay
HepG2 spheres and parental HepG2 cells were seeded
into a 96-well plate at a density of 600 cells/well for 3 days.
Cells were treated with sorafenib at concentrations of 5, 10, 15,
20 or 25 µM, alone or combined with 1, 3 or 5 mM metformin and
incubated at 37°C for 48 h. Cell proliferation analysis was
conducted using a Cell Counting Kit-8 (CCK8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) assay, according to the
manufacturer's protocol. In brief, CCK8 solution was added into the
culture and incubated for 2 h. The absorbance at 450 nm [optical
density (OD) value] was measured using an auto-microplate reader
(BERTHOLD TECHNOLOGIES GmbH & Co. KG, Bad Wildbad, Germany).
The inhibition percentage was calculated using the following
equation: Inhibition
percentage=(1-ODexperiment/ODcontrol) ×100.
The IC50 value was determined by plotting the inhibition
percentage values. The resistance index (RI) was calculated using
the following equation: RI=half-maximal inhibitory concentration
(IC50) spheres/IC50HepG2.
Western blotting
HepG2 cells and spheres were treated with sorafenib,
metformin or a combination of sorafenib and metformin for 48 h.
Cells were collected and lysed in Cell Lysis Buffer (cat. no.
P0013) containing 1 mM phenylmethylsulfonyl fluoride (cat. no.
ST506-2; both Beyotime Institute of Biotechnology, Haimen, China)
for 30 min on ice. The lysate was centrifuged at 12,000 × g for 15
min at 4°C, and the supernatant was collected. The protein
concentration was determined using a Bicinchoninic Acid Protein
Assay kit (cat. no. P0012; Beyotime Institute of Biotechnology). A
total of 30 µg of protein lysate was boiled in SDS-PAGE Sample
Loading Buffer (cat. no. P0015; Beyotime Institute of
Biotechnology), resolved by electrophoresis on 10% SDS-PAGE gels
and transferred to polyvinylidene fluoride membranes (cat. no.
FFP33; Beyotime Institute of Biotechnology). The membranes were
blocked with 5% skimmed milk in TBS/Triton-X-100 at room
temperature for 90 min. The membranes were probed overnight at 4°C
with primary antibodies against human zinc finger protein SNAI1
(Snail; rabbit monoclonal antibody; cat. no. 3879T; Cell Signaling
Technology, Inc., Danvers, MA, USA), E-cadherin (mouse monoclonal
antibody; cat. no. AF0138; Beyotime Institute of Biotechnology),
vimentin (mouse monoclonal antibody; cat. no. AF0318; Beyotime
Institute of Biotechnology) and twist-related protein 1 (Twist1;
rabbit polyclonal antibody; cat. no. 21642; Signalway Antibody LLC,
College Park, MD, USA), with GAPDH (rabbit monoclonal antibody;
cat. no. 5174; Cell Signaling Technology, Inc.) as the control. All
primary antibodies were diluted 1:1,000 with Antibody Dilution
Buffer (cat. no. P0023A; Beyotime Institute of Biotechnology).
Horseradish peroxidase (HRP)-labeled goat anti-mouse immunoglobulin
(Ig)G (H+L; cat. no. A0216; Beyotime Institute of Biotechnology)
and HRP-labeled goat anti-rabbit IgG (H+L; cat. no. A0208; Beyotime
Institute of Biotechnology) diluted 1:5,000 with Antibody Dilution
Buffer, were used to detect specific proteins. Finally, the bands
were detected using Enhanced Chemiluminescent Substrates
(http://www.bio-kits.cn; Biokits Technologies
Inc., Beijing, China).
Statistics
Data were analyzed using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). Experiments were performed at
least in triplicate, and data are presented as the mean ± standard
deviation. Data were analyzed by Bonferroni analysis (least
significant difference-t test). α risk was set at 0.05 and β risk
was set at 0.95. P<0.05 was considered to indicate a
statistically significant difference.
Results
Stem-like HepG2 spheres are enriched
by stem-cell enrichment medium
The human liver cancer cell line HepG2 was cultured
with stem cell enrichment medium as described above. The cells
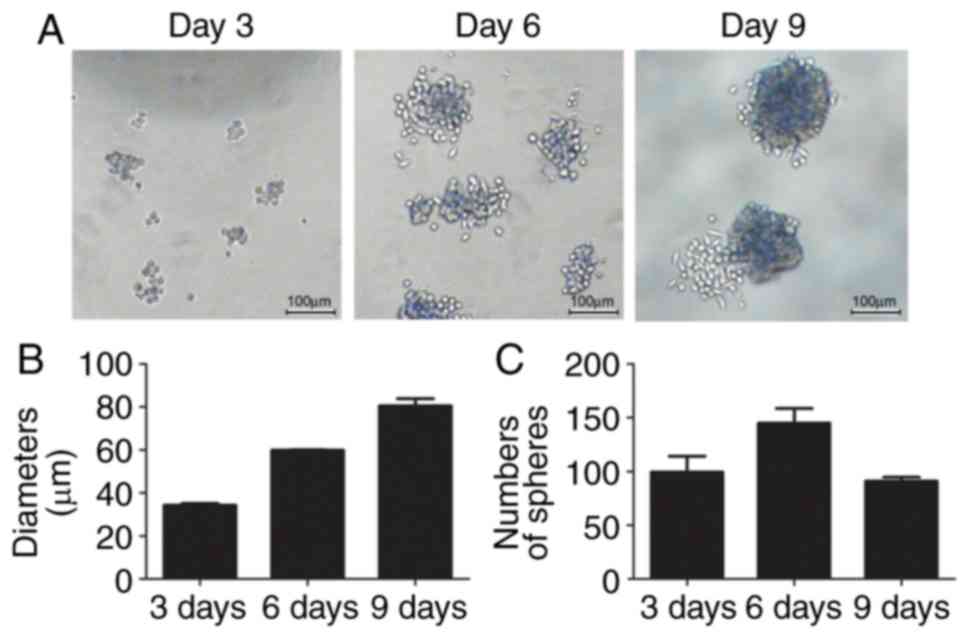
detached and formed small spheres from the 3rd day (Fig. 1A), with a diameter of ~34.32±1.07
µm (Fig. 1B). There were ~100±21.2
small spheres in three fields on day 3. The diameters of the
spheres reached ~81.97±1.87 µm on day 9. The numbers of spheroids
first increased to 145±19.1 on day 6, although they decreased to
91±5.1 on day 9 as the small spheres joined to become a large
sphere (Fig. 1B and C).
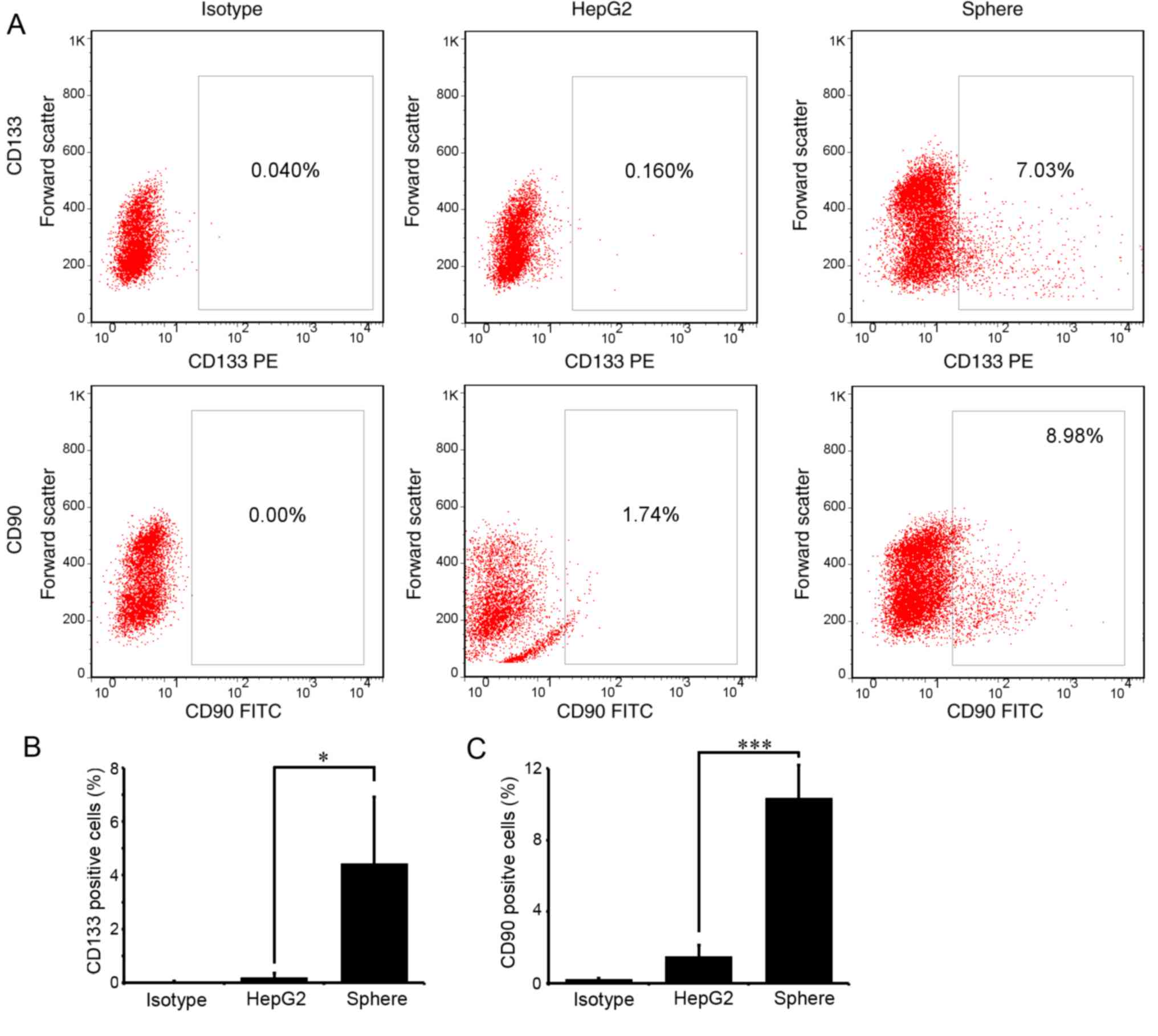
CD133 and CD90 are the most recognized liver CSC
markers. To examine the stemness of the enriched spheres, the
proportion of CD133- or CD90-positive cells in the spheres was
detected with flow cytometry. As expected, few cells expressed
CD133 or CD90 in parental HepG2 cells. In spheres passaged three
times (P3), ~4.43±2.48 and 10.33±1.23% cells expressed CD133 and
CD90, respectively (Fig. 2A and
B). The differences were statistically significant
(P<0.05).
Stem cell-like HepG2 spheres are less
susceptible to sorafenib
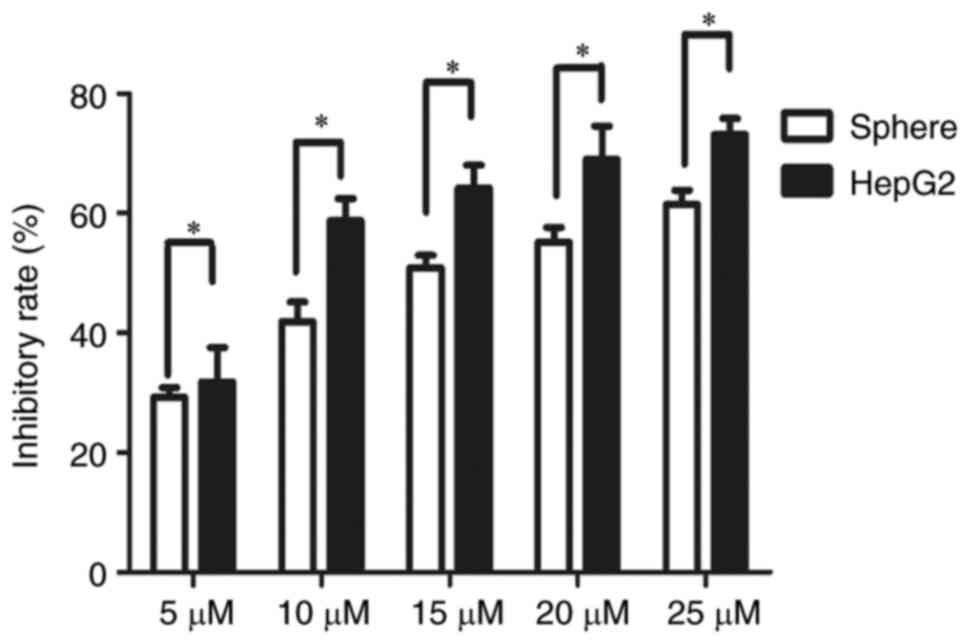
Single cell suspensions of HepG2 sphere cells and
HepG2 cells were seeded in 96-well plates at 600 cells/well. After
3 days, cells were treated with sorafenib at 5, 10, 20 and 25 µM
for 24 and 48 h, respectively. The CCK8 cell proliferation activity
assay demonstrated that the inhibition rate of sorafenib on HepG2
spheres was significantly lower compared with that on parental
HepG2 cells at 48 h post-treatment (Fig. 3). The IC50 value of
HepG2 spheres (14.84 µM) was significantly higher than that of
parental HepG2 cells (9.4 µM) (P<0.05; data not shown).
Metformin increases the sensitivity to
sorafenib of HepG2 spheres
As there was no suggestion for the dose of metformin
for non-T2DM patients, the present study sought to analyze whether
lower doses of metformin have effects on enhancing drug
sensitivity. Thus, HepG2 sphere cells were treated with 0, 1, 3 and
5 mM metformin combined with 5 µM sorafenib. As expected, 1 mM
metformin and 5 µM sorafenib significantly inhibited the cell
viability of HepG2 spheres (Fig. 4A
and B). As the concentration of metformin increased, the
diameter of the suspension spheres was significantly reduced
(Fig. 4A and C). The number of
spheres increased when 1 mM metformin was added, although it
decreased when the concentration of metformin increased (Fig. 4D).
Metformin inhibits EMT and reverses
the formation of HepG2 spheres
EMT is associated with the formation of CSCs. From
previous results, it was identified that alterations in the
diameters and numbers of spheres following treatment with metformin
indicated that metformin may reverse the formation of stem-like
spheres. As demonstrated in Fig.
5A, the diameters and numbers of spheres were markedly reduced
by 1 and 5 mM metformin. As expected, expression of the epithelial
marker E-cadherin was significantly reduced in stem-like spheres
compared with parental HepG2 cells, while the expression of the
mesenchymal markers vimentin, Snail and Twist1 were significantly
elevated in spheres. Metformin reversed the expression of these EMT
markers in a dose-dependent manner (Fig. 5B-F).
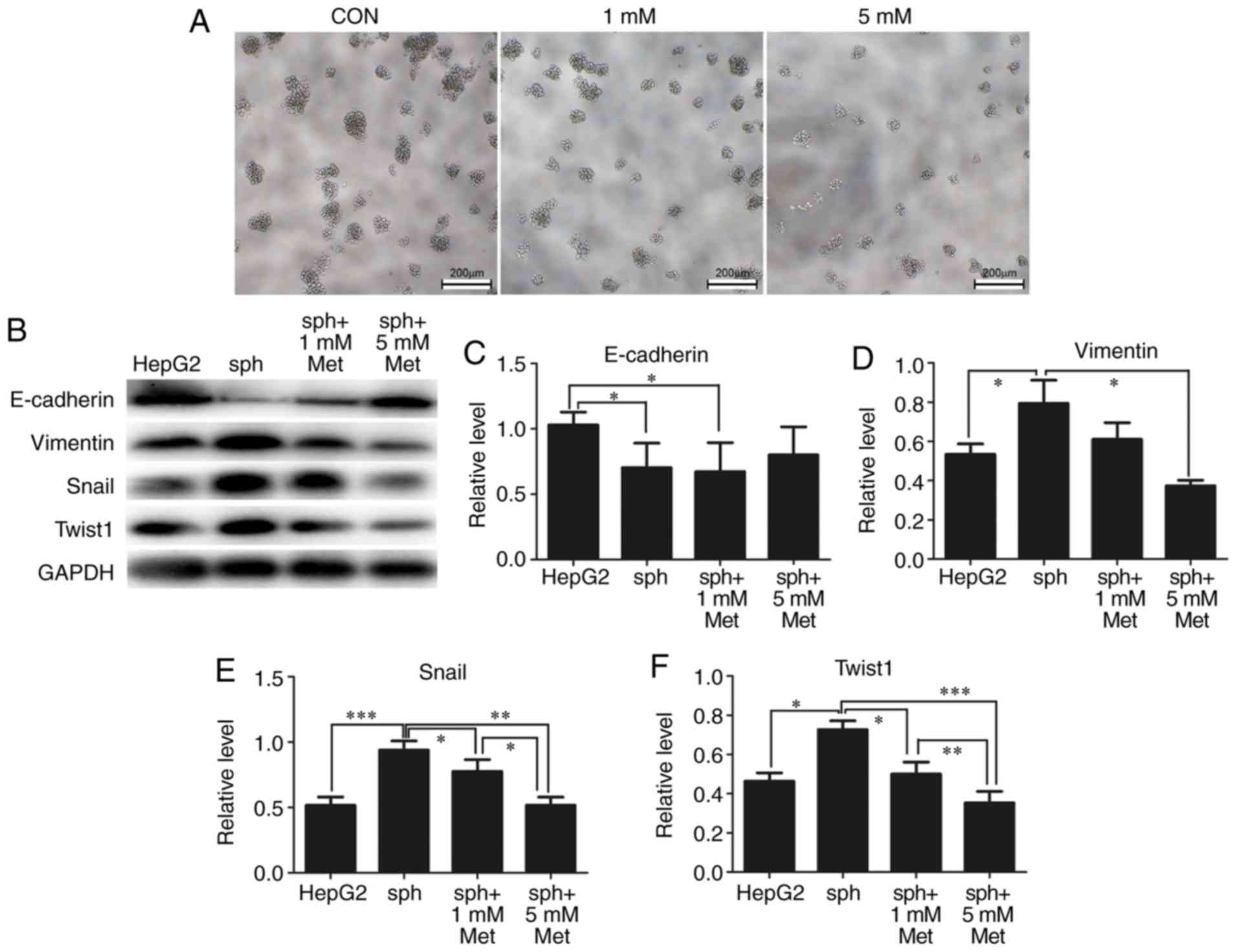 | Figure 5.Metformin reverses HepG2 sphere
formation by reducing epithelial-mesenchymal transformation. (A)
Typical bright-field images of spheroids treated with 0 (CON), 1 or
5 mM metformin for 24 h. Scale bar, 200 µm. (B) Expression of
E-cadherin, vimentin, Snail and Twist1 in parental HepG2 cells and
spheroids treated with metformin was detected by western blotting.
GAPDH was used as a loading control. Semi-quantification of protein
expression was performed for (C) E-cadherin, (D) vimentin, (E)
Snail and (F) Twist1. All experiments were repeated at least three
times independently and the data are expressed as the mean ±
standard deviation. *P<0.05, **P<0.01, ***P<0.001. CON,
control; sph, spheroid; met, metformin; Snail, zinc finger protein
SNAI1; Twist1, twist-related protein 1. |
Discussion
In this study, stem cell-like HepG2 spheres were
enriched using stem cell enrichment medium. It was demonstrated
that those spheres expressed increased levels of the stem cell
markers CD133 and CD90 compared with parental HepG2 cells, and were
more resistant to sorafenib. Metformin reduced the diameters and
numbers of spheres, and reversed their resistance to sorafenib. In
addition, it was observed that these effects were associated with
the metformin-induced reduction in EMT and stemness.
Researchers have identified that the mechanism of
tumor resistance to sorafenib is multifaceted. The anti-tumor
activity of sorafenib was largely attributed to the inhibition of
growth factor signaling pathways, including vascular endothelial
growth factor receptor and platelet-derived growth factor receptor,
and the downstream RAF proto-oncogene serine/threonine-protein
kinase (RAF)/dual specificity mitogen-activated protein kinase
kinase 1 (MEK)/extracellular signal-regulated kinase (ERK) pathway.
The activation of an escape pathway from RAF/MEK/ERK may result in
chemoresistance (7). Certain
studies have demonstrated that upregulation of hypoxia inducible
factor-2α induced by sorafenib contributes to the resistance of
hypoxic liver cancer cells by activating the TGF-α/epidermal growth
factor receptor pathway (18).
Emerging evidence has indicated that the activation of the EMT
process, the emergence of CSCs and the activation of compensatory
pathways leading to sorafenib have been implicated (18–20).
In response to treatment with sorafenib, MHCC97H cells develop a
mesenchymal phenotype and resistance is conferred (19). The formation of CSCs is also
thought to serve an important role in chemotherapy resistance to
multiple drugs, including sorafenib (7). A recent study reported that CSCs were
enriched in tumor tissues following treatment with sorafenib
(21). An increased proportion of
CD133+ CSCs may cause resistance to sorafenib. In the
present study, stem-cell like HepG2 spheres were enriched using
stem cell conditioned enrichment medium. Compared with parental
HepG2 cells, there were significantly more CD133+ cells
in enriched spheres, and the sensitivity of spheres to sorafenib
was significantly decreased. These results supported the hypothesis
that stem cells are partially responsible for sorafenib
resistance.
A recent study suggested that EMT may be a
predictive marker for the development of resistance to sorafenib
(21). Following long-term
exposure to sorafenib, liver cancer cells exhibit EMT and
resistance to sorafenib (22).
EMT-associated serum response factor was reported to induce HLE
cells to acquire a mesenchymal phenotype, which leads to resistance
against a sorafenib-mediated apoptotic effect (20). Multi-drug resistance (MDR) is a
downstream molecular event of EMT and is also responsible for
sorafenib resistance. Silencing Snail with small interfering RNA
inhibits EMT and partially reverses MDR, thereby markedly
decreasing invasion and metastasis in sorafenib-resistant liver
cancer cells (22). In the present
study, it was observed that in the stem-cell like HepG2 spheres,
expression of the markers of EMT indicated that they acquired the
mesenchymal phenotype during enrichment, which may explain the
increased resistance to sorafenib compared with parental HepG2
cells.
Metformin is the most popular drug used to treat
T2DM, and functions through inhibition of the mitochondrial
electron transport chain complex I and activation of 5′-adenosine
monophosphate-activated protein kinase
(AMPK)-serine/threonine-protein kinase mTOR (mTOR) signaling. The
anti-tumor effect of metformin has received extensive attention
(8). Metformin also exhibits great
potential in the prevention and treatment of liver cancer, as the
incidence of liver cancer reduces with increasing doses of
metformin (23). Metformin may
reduce the incidence of liver cancer in patients with T2DM
(16). Metformin promotes
apoptosis in a variety of malignancies (24), and increases the sensitivity of
sorafenib to MHCC97H cells (24).
The present study demonstrated that the combination of metformin
and sorafenib at low concentrations significantly inhibited the
cell viability of stem-like spheres.
The anti-tumor mechanism of metformin remains
unclear. Previous studies suggested that metabolic reprograming was
important in maintaining the stemness of CSCs; metformin may
interfere with oxidative phosphorylation by inhibiting the
mitochondrial electron transport chain complex, and has been
reported to delay tumor progression by reducing the formation of
CSCs (7,9,10,12,13,15,16).
Certain studies have reported that drug resistance is associated
with EMT in CSCs. Metformin inhibits tumor stem cell EMT to enhance
sensitivity to chemotherapeutic drugs (11–14,25).
Metformin at a low concentration may inhibit EMT in ovarian CSCs
(26). It has been suggested that
metformin may enhance the sensitivity of hepatoma cells to
sorafenib by inhibiting the production of CSCs, and inhibiting EMT
to reduce the formation of drug-resistant stem cells (16,19).
It was identified that metformin reduced the diameters and numbers
of HepG2 spheres. Metformin reversed the alterations in E-cadherin
and vimentin expression in a suspension of HepG2 sphere-forming
cells, and reversed the expression of the stem cell-associated
transcription factors Twist1 and Snail in a dose-dependent manner.
The above results suggested that metformin may reverse resistance
to sorafenib by attenuating EMT and stem cell enrichment.
Metformin may enhance the sensitivity of cells to
sorafenib though pathways which also regulate EMT (27), including TGF-β signaling, mothers
against decapentaplegic homolog (SMAD)-dependent signaling, the
ERK-Jun pathway and the Akt-mTOR pathway. For example, the
combination of sorafenib and metformin enhances the inhibitory
effect of metformin on the mTOR complex 1 and ERK signaling
pathways (28). Metformin
activates the AMPK pathway, which suppresses EMT by modulating
Akt-E3 ubiquitin-protein ligase Mdm2-forkhead box protein O3
signaling axis (29) and the
TGF-β-SMAD2/3 pathway (30). Thus,
it was hypothesized that metformin may affect a number of
crosslinked pathways, and further study was required clarify
this.
However, not all cancer cells respond well to
metformin or other biguanide drugs. Hepatoma cells that have a
higher mitochondrial respiration rate are more sensitive to these
treatments; whereas tumor cells that exhibit increased glycolysis
are more resistant. Altered energy metabolism in hepatoma cells,
from glycolysis to mitochondrial respiration, improves cellular
sensitivity to biguanides (31).
Since metformin is a mitochondrial complex enzyme inhibitor, this
may explain why it only affects tumor cells with enhanced oxidative
phosphorylation.
In conclusion, in the present study, human liver
cancer cell line HepG2 stem cell-like cells were enriched with
tumor stem cell conditioned medium suspension culture, and it was
verified that the sensitivity of stem cell-like spheres to
sorafenib was significantly reduced. Metformin enhanced sensitivity
to sorafenib, possibly by inhibiting EMT in the suspension spheres
and reducing the formation of stem cell-like cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 8156049 and
81360347) and the Guangxi Nanning Qingxiu District Science and
Technology Development Project (grant no. 2015S14).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF, XG and XLL conceived and designed the study. YF,
XG, XPH, MYW, XL and SSW acquired, analyzed or interpreted the
data. YF and XG drafted the manuscript. XLL revised and approved
the final version of the manuscript to be published.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oikawa T: Cancer stem cells and their
cellular origins in primary liver and biliary tract cancers.
Hepatology. 64:645–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nishida N, Kitano M, Sakurai T and Kudo M:
Molecular mechanism and prediction of sorafenib chemoresistance in
human hepatocellular carcinoma. Dig Dis. 33:771–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Safe S, Nair V and Karki K:
Metformin-induced anticancer activities: Recent insights. Biol
Chem. 399:321–335. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi P, Liu W, Tala, Wang H, Li F, Zhang H,
Wu Y, Kong Y, Zhou Z, Wang C, et al: Metformin suppresses
triple-negative breast cancer stem cells by targeting KLF5 for
degradation. Cell Discov. 3:170102017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gritti M, Würth R, Angelini M, Barbieri F,
Peretti M, Pizzi E, Pattarozzi A, Carra E, Sirito R, Daga A, et al:
Metformin repositioning as antitumoral agent: selective
antiproliferative effects in human glioblastoma stem cells, via
inhibition of CLIC1-mediated ion current. Oncotarget.
5:11252–11268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nangia-Makker P, Yu Y, Vasudevan A,
Farhana L, Rajendra SG, Levi E and Majumdar AP: Metformin: A
potential therapeutic agent for recurrent colon cancer. PLoS One.
9:e843692014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chai X, Chu H, Yang X, Meng Y, Shi P and
Gou S: Metformin increases sensitivity of pancreatic cancer cells
to gemcitabine by reducing CD133+ cell populations and suppressing
ERK/P70S6K signaling. Sci Rep. 5:144042015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mayer MJ, Klotz LH and Venkateswaran V:
Metformin and prostate cancer stem cells: A novel therapeutic
target. Prostate Cancer Prostatic Dis. 18:303–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shang D, Wu J, Guo L, Xu Y, Liu L and Lu
J: Metformin increases sensitivity of osteosarcoma stem cells to
cisplatin by inhibiting expression of PKM2. Int J Oncol.
50:1848–1856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddappa G, Kulsum S, Ravindra DR, Kumar
VV, Raju N, Raghavan N, Sudheendra HV, Sharma A, Sunny SP, Jacob T,
et al: Curcumin and metformin-mediated chemoprevention of oral
cancer is associated with inhibition of cancer stem cells. Mol
Carcinog. 56:2446–2460. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saito T, Chiba T, Yuki K, Zen Y, Oshima M,
Koide S, Motoyama T, Ogasawara S, Suzuki E, Ooka Y, et al:
Metformin, a diabetes drug, eliminates tumor-initiating
hepatocellular carcinoma cells. PLoS One. 8:e700102013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu F, Cao X, Liu Z, Guo H, Ren K, Quan M,
Zhou Y, Xiang H and Cao J: Casticin suppresses self-renewal and
invasion of lung cancer stem-like cells from A549 cells through
down-regulation of pAkt. Acta Biochim Biophys Sin (Shanghai).
46:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao D, Zhai B, He C, Tan G, Jiang X, Pan
S, Dong X, Wei Z, Ma L, Qiao H, et al: Upregulation of HIF-2α
induced by sorafenib contributes to the resistance by activating
the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell
Signal. 26:1030–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
You A, Cao M, Guo Z, Zuo B, Gao J, Zhou H,
Li H, Cui Y, Fang F, Zhang W, et al: Metformin sensitizes sorafenib
to inhibit postoperative recurrence and metastasis of
hepatocellular carcinoma in orthotopic mouse models. J Hematol
Oncol. 9:202016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bae JS, Noh SJ, Kim KM, Jang KY, Chung MJ,
Kim DG and Moon WS: Serum response factor induces epithelial to
mesenchymal transition with resistance to sorafenib in
hepatocellular carcinoma. Int J Oncol. 44:129–136. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mir N, Jayachandran A, Dhungel B, Shrestha
R and Steel JC: Epithelial-to-mesenchymal transition: A mediator of
sorafenib resistance in advanced hepatocellular carcinoma. Curr
Cancer Drug Targets. 17:698–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong J, Zhai B, Sun W, Hu F, Cheng H and
Xu J: Activation of phosphatidylinositol 3-kinase/AKT/snail
signaling pathway contributes to epithelial-mesenchymal
transition-induced multi-drug resistance to sorafenib in
hepatocellular carcinoma cells. PLoS One. 12:e01850882017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen HP, Shieh JJ, Chang CC, Chen TT, Lin
JT, Wu MS, Lin JH and Wu CY: Metformin decreases hepatocellular
carcinoma risk in a dose-dependent manner: Population-based and in
vitro studies. Gut. 62:606–615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo Z, Cao M, You A, Gao J, Zhou H, Li H,
Cui Y, Fang F, Zhang W, Song T, et al: Metformin inhibits the
prometastatic effect of sorafenib in hepatocellular carcinoma by
upregulating the expression of TIP30. Cancer Sci. 107:507–513.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang R, Zhang P, Wang H, Hou D, Li W,
Xiao G and Li C: Inhibitory effects of metformin at low
concentration on epithelial-mesenchymal transition of
CD44(+)CD117(+) ovarian cancer stem cells. Stem Cell Res Ther.
6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez DM and Medici D: Signaling
mechanisms of the epithelial-mesenchymal transition. Sci Signal.
7(re8)2014.PubMed/NCBI
|
|
28
|
Ling S, Song L, Fan N, Feng T, Liu L, Yang
X, Wang M, Li Y, Tian Y, Zhao F, et al: Combination of metformin
and sorafenib suppresses proliferation and induces autophagy of
hepatocellular carcinoma via targeting the mTOR pathway. Int J
Oncol. 50:297–309. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou CC, Lee KH, Lai IL, Wang D, Mo X,
Kulp SK, Shapiro CL and Chen CS: AMPK reverses the mesenchymal
phenotype of cancer cells by targeting the Akt-MDM2-Foxo3a
signaling axis. Cancer Res. 74:4783–4795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin H, Li N, He H, Ying Y, Sunkara S, Luo
L, Lv N, Huang D and Luo Z: AMPK inhibits the stimulatory effects
of TGF-β on Smad2/3 activity, cell migration, and
epithelial-to-mesenchymal transition. Mol Pharmacol. 88:1062–1071.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu CC, Wu LC, Hsia CY, Yin PH, Chi CW,
Yeh TS and Lee HC: Energy metabolism determines the sensitivity of
human hepatocellular carcinoma cells to mitochondrial inhibitors
and biguanide drugs. Oncol Rep. 34:1620–1628. 2015. View Article : Google Scholar : PubMed/NCBI
|