Introduction
Cardiovascular diseases, including myocardial
infarction (MI), hypertensive heart disease, cardiomyopathy, atrial
fibrillation, peripheral artery disease and venous thrombosis, are
the leading causes of mortality worldwide (1–3). MI
causes cardiomyocyte death, scar formation, wall thinning and
collagen degradation via blockage of coronary arteries (3,4).
Therapeutic strategies that repair damaged cardiac tissue and
prevent destructive ventricular remodeling following MI are
critical for improving patient prognosis.
MI is typically treated by balloon angiography and
stents, bypass surgery, heart transplantation and artificial heart
surgeries (5–7). However, myocardial salvage is
incomplete due to restenosis in these approaches (5). Furthermore, these strategies are
costly and heart transplantations are limited due to the shortage
of donor organs (6,7). Stem cell-based therapies have been
proposed as a promising approach for the treatment of MI (6); a variety of cell types have been used
to restore damaged heart tissue, including cardiomyocyte progenitor
cells, hematopoietic stem cells, mesenchymal stem cells (MSCs),
cardiac stem cells, embryonic stem cells, skeletal myoblasts, and
fetal and umbilical cord blood cells (6,8).
MSCs may be easily obtained from the patient (9), making them an ideal cell source for
transplantation into the infarcted heart. Furthermore, MSCs have
additional advantages, including ease of isolation, multilineage
differentiation potential, and immunomodulatory and paracrine
effects (7,9,10).
Despite these favorable qualities, low
transplantation efficiency and cell viability associated with MSC
transplantation (10) have
prompted the modification of MSC-based cell therapies, including
gene delivery systems (10,11).
Gene delivery may be achieved through viral and non-viral carriers
(12). The former includes
retrovirus, lentivirus and adenovirus, which are highly efficient;
however, may additionally cause insertional mutagenesis,
immunogenicity and pathogenesis (13). In contrast, non-viral carriers are
efficient delivery systems that are safe, stable, easy to generate
and have low immunogenicity; however, are limited by a relatively
low transfection efficiency compared with viral infection (12,14,15),
which is a principal barrier for clinical applications.
Insulin-like growth factor-1 (IGF-1), which has a
structure similar to that of insulin (16), is secreted by the majority of
tissues and serves an important role in cell growth,
differentiation and transformation (17,18).
IGF-1 has been demonstrated to enhance cardiomyocyte function
(17); its overexpression was
demonstrated to prevent apoptosis of myocardial cells attenuating
ventricular dilatation and wall stress in infarcted hearts;
whereas, IGF-1 deficiency resulted in impaired cardiac remodeling
and increased apoptosis in MI (17). IGF-1 administration increased the
expression of angiogenic factors in MI animal models (19). Furthermore, IGF-1 induced the
activation of resident cardiac stem cells and increased engraftment
and survival of numerous cell types, including MSCs (20). IGF-1 may be administered by
injection of the recombinant protein; however, this method is
costly and inefficient due to the short half-life of the protein
(21). In contrast, gene transfer
using a non-viral carrier is relatively inexpensive and has low
cytotoxicity (21).
The aim of the present study was to use MSC-based
gene (IGF-1) therapy with a fluorescence-activated cell
sorting (FACS) system to improve the therapeutic outcome in a rat
MI model.
Materials and methods
Animals
Sprague-Dawley rats from Orient Bio, Inc. (Seongnam,
Korea) were handled according to the Association for Assessment and
Accreditation of Laboratory Animal Care International system.
Animal experiments conformed to the International Guide for the
Care and Use of Laboratory Animals, and experimental procedures
were examined and approved by the Animal Research Committee of
Yonsei University College of Medicine (Seoul, Korea). All rats were
allowed free access to food and water. The room was kept at a
constant temperature (22±2°C) and relative humidity (50±10%) on a
12-h light/dark cycle. The total number of rats used was 56: 10
rats used for MSC isolation (weight, 100±5 g; male; 4 weeks of age)
and 46 rats used for MI model (weight, 270±10 g; male; 8–9 weeks of
age).
Construction of the selection vector
(pEF1/His C::IGF-1::EGFP)
The pEF1/His C::IGF-1::EGFP selection vector was
constructed by inserting rat IGF-1 and enhanced green
fluorescent protein (EGFP) DNA into the pEF1/His C vector
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). In
the first step, total RNA was extracted from rat MSCs using an
RNeasy Mini kit (Qiagen GmbH, Hilden, Germany). The MSCs cDNA was
synthesized by using Promega RT reagents (Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol.
Briefly, RNA with oligo(dt) primer and Nuclease-free water was
preheated for 5 min at 70°C, and immediately chilled in 4°C for at
least 5 min. Reverse transcription reaction mix (nuclease-free
water, ImProm-II™ 5X Reaction Buffer, MgCl2, dNTP Mix,
Recombinant RNasin® Ribonuclease Inhibitor, ImProm-II™
Reverse Transcriptase) was added to RNA and primer mix. This mix
was annealed at 25°C for 5 min, extended at 42°C for 1 h,
inactivated reverse transcriptase at 70°C for 15 min. Rat
IGF-1 cDNA was amplified by polymerase chain reaction (PCR)
with DNA polymerase (Takara Bio, Inc., Otsu, Japan) using rat MSCs
cDNA as a template. The forward and reverse primers for IGF-1
contained EcoRI and XbaI restriction sites,
respectively (Table I). The
restriction site for EcoRI was 5′-GAATTC-3′ and the
restriction site for XbaI was 5′-TCTAGA-3′. The PCR
thermocycling conditions were 95°C for 5 min, 32 cycles of 95°C for
1 min, 65°C for 1 min 30 sec, 72°C for 40 sec, and then a final
extension step at 72°C for 10 min. The amplified PCR product was
purified and digested with EcoRI and XbaI restriction
enzymes. The pEF1/His C::IGF-1 vector was constructed by inserting
the digested IGF-1 fragment into the pEF1/His C vector. Expression
of IGF-1 from pEF1/His C::IGF-1 was confirmed in H9c2 cells (KCLB
no. 21446; Korean Cell Line Bank, Seoul, Korea) by reverse
transcription (RT)-PCR. The H9c2 cells were grown in DMEM (WELGENE,
Inc., Gyeongsan, Korea) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% antibiotics (Gibco; Thermo Fisher
Scientific, Inc.) in humidified atmosphere at 37°C with 5%
CO2. Subcloning was performed by inserting EGFP
DNA into the pEF1/His C::IGF-1 vector; the DNA was isolated from
the pEGFP-C1 vector (Takara Bio, Inc.) by digestion with
AseI and MluI restriction enzymes. A gap-filling DNA
precipitation step was conducted followed by DNA ligation (T4
ligase; New England BioLabs, Inc., Ipswich, MA, USA). This cloned
sequence of the pEF1/His C::IGF-1::EGFP vector was confirmed by
digesting with restriction enzymes and sequencing (Cosmo Genetech
Co., Ltd., Seoul, Korea).
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| IGF-1 |
TTTGAATTCATGGGGAAAATCAGCAGTCT |
TTTCTAGACTGCACTTCCTCTACTTGTGTTC |
| EGFP |
GTAGGTGTCATTCTATTCTGGGG |
AACCGTATTACCGCCTTTGA |
| GAPDH |
AATGCATCCTGCACCACCAACTGC |
GGAGGCCATGTAGGCCATGAGGTC |
MSC isolation and
characterization
MSCs were isolated from 4-week-old male Sprague
Dawley rats (weight, 100±5 g) as previously described (10). Rat bone marrow (BM)-derived cells
were flushed out from the femur and tibia using DMEM (WELGENE,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin solution (Gibco; Thermo Fisher
Scientific, Inc.). The medium was collected and centrifuged at 596
× g for 5 min at room temperature (RT), and cells were loaded onto
a Ficoll-Paque density gradient medium (GE Healthcare Life
Sciences, Uppsala, Sweden). Following centrifugation (596 × g, 30
min, RT), the middle layer containing mononuclear BM cells was
removed, washed and seeded in culture dishes at 1×106
cells per 100 cm2. Following incubation for 72 h in
humidified atmosphere at 37°C with 5% CO2, adherent
cells were washed and fresh MSC medium was added. MSCs were
identified using a flow cytometer (FACSVerse; BD Biosciences,
Franklin Lakes, NJ, USA) and FACSuite software (version 1.0.6; BD
Biosciences) based on the presence of cell surface markers.
Resuspended single cells were labeled with antibodies against
surface markers, including anti-cluster of differentiation
(CD)29-fluorescein isothiocyanate (FITC; cat. no. 102205; 1:50),
anti-CD54-phycoerythrin (PE; cat. no. 202405; 1:80), anti-CD90-FITC
(cat. no. 202504; 1:200), anti-CD45-PE (cat. no. 202207; 1:80), and
anti-CD49d-FITC (cat. no. 200103; 1:200) antibodies (BioLegend,
Inc., San Diego, CA, USA) in ice for 1 h. MSCs labeled with
antibodies were sorted by flow cytometry.
In vitro transfection using the
non-viral carrier dendrimer type bio-reducible polymer
[polyamidoamine-arginine grafted bio-reducible poly
(cystaminebisacrylamide-diaminohexane (PAM-ABP)] and pEF1/His
C::IGF-1::EGFP vector in MSCs
MSCs were transfected with the non-viral carrier
PAM-ABP (supplied by Professor Minhyung Lee) and selection vector
complexes at different concentrations of pEF1/His C::IGF-1::EGFP
(0, 0.5, 1, 2, 4, 8 and 16 µg). Complexes of plasmid DNA and
PAM-ABP were mixed based on weight ratio (1:5) and were used to
treat MSCs. At 4 h after transfection, MSCs were washed and the
medium was replaced with fresh complete medium. Transfected MSCs
were continuously incubated for 24 or 48 h.
RT-PCR
Cells transfected with pEF1/His C::IGF-1::EGFP were
harvested 24 or 48 h following transfection, and total RNA was
extracted using an RNeasy Mini kit (Qiagen GmbH). cDNA was
synthesized by using Promega RT reagents (Promega Corporation)
according to manufacturer's protocol. Briefly, RNA with oligo(dt)
primer and nuclease-free water was preheated for 5 min at 70°C and
immediately chilled in 4°C for at least 5 min. Reverse
transcription reaction mix (nuclease-free water, ImProm-II™ 5X
Reaction Buffer, MgCl2, dNTP Mix, Recombinant
RNasin® Ribonuclease Inhibitor, ImProm-II™ Reverse
Transcriptase) was added to RNA and primer mix. This mix was
annealed at 25°C for 5 min, extended at 42°C for 1 h with
inactivated reverse transcriptase at 70°C for 15 min. RT-PCR was
performed with an AmfiEco kit (GenDEPOT, Katy, TX, USA). Primer
sequences targeting rat IGF-1 and GAPDH are listed in
Table I. The PCR cycling
conditions of rat IGF-1 were 95°C for 5 min, 28 cycles of 95°C for
1 min, 65°C for 1 min 30 sec, 72°C for 40 sec, and then a final
extension step at 72°C for 10 min. Thermocycling conditions of
GAPDH were 95°C for 5 min, 28 cycles of 95°C for 1 min, 60°C for 1
min, 72°C for 40 sec, and then a final extension step at 72°C for
10 min. PCR products were loaded in 1.2% agarose gel containing
red-safe (iNtRON Biotechnology, Seongnam, Korea). The results were
scanned and visualized with CoreBio i-MAX™ gel imager
analysis system (CoreBiosystem, Seoul, Korea).
ELISA
Conditioned medium from transfected MSCs was
collected 24 or 48 h following incubation and analyzed using a rat
IGF-1 Quantikine ELISA kit (cat. no. MG100; R&D Systems,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Serum-free low-glucose Dulbecco's modified Eagle's medium (WELGENE,
Inc.) was used as a negative control. All samples were assayed in
triplicate, and the optical density was measured at 450 nm.
Fluorescence-activated cell sorting
(FACS) analysis
FACS analysis was performed to isolate transfected
MSCs using a FACSAria III flow cytometer (BD Biosciences). At 24 h
following transfection, transfected MSCs were harvested, washed and
resuspended in FACS buffer (2 mM EDTA, 25 mM HEPES, and 1% FBS in
1X PBS). MSCs transfected with pEF1/His C::IGF-1::EGFP were
identified based on green fluorescence using a FITC filter (488
nm). Data were analyzed with FACSDiva software (version 3.1.3; BD
Biosciences).
MI model, cell transplantation and
histology
Experimental MI was induced in male Sprague Dawley
rats at 8–9 weeks of age (weight, 270±10 g) as previously described
(10). Rats were anesthetized by
intraperitoneal injection of Zoletil (30 mg/kg; Virbac Corporation,
Carros, France) and Rompun (10 mg/kg; Bayer, Leverkusen, Germany)
and ventilated with positive pressure (180 ml/min; Harvard
Apparatus, Holliston, MA, USA). The heart was exposed through a
2-cm incision in the left lateral costal rib. The left anterior
descending (LAD) artery was ligated with 6-0 prolene (Ethicon,
Inc., Cincinnati, OH, USA) below the left atrium for 1 h.
Reperfusion and intramyocardial injection of 100 µl PBS (n=10) was
performed, and control (MSCs without transfection; n=10), unsorted
(MSC transfection-unsorted; n=13), or sorted (MSC
transfection-sorted; n=13) MSCs (1×106 cells in 100 µl
PBS) were delivered to three or four different sites in the border
zone. At 2 weeks following transplantation, the animals were
re-anesthetized and sacrificed for histological examination.
To analyze MSC engraftment (or localization) within
the infarcted myocardium, the heart was perfused, fixed in 10%
formalin solution overnight at 4°C, embedded in Optimal Cutting
Temperature compound (Sakura, Zoeterwoude, The Netherlands), frozen
on dry ice and cut into transverse sections (10 µm) with a cryostat
that were mounted with mounting medium containing DAPI (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). DAPI and EGFP fluorescence
was detected by confocal fluorescence microscopy (magnifications,
×100 and ×400).
Immunofluorescence analysis
To confirm IGF-1 expression in MSCs transfected with
pEF1/His C::IGF-1::EGFP in ischemic hearts, frozen tissue sections
were permeabilized with PBS containing Triton X-100 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), quenched with 100% methanol and
30% H2O2, washed with PBS, and blocked with
3% bovine serum albumin (GenDEPOT, Katy, TX, USA) at RT for 1 h.
Cryosections were incubated with primary antibody against IGF-1
(cat. no. sc-1422, 1:50; Santa Cruz Biotechnology, Inc.) in
blocking solution at RT for 2 h followed by Cy5.5 donkey anti-goat
secondary antibody (cat. no. sc-45102, 1:200; Santa Cruz
Biotechnology, Inc.) at RT for 1 h. The sections were mounted in
mounting medium containing DAPI and examined under a fluorescence
microscope (Olympus Corporation, Tokyo, Japan) for detection of
DAPI and Cy5.5 fluorescence (magnification, ×100).
2,3,5-Triphenyltetrazolium chloride
(TTC) staining
Myocardial infarct size was measured by staining
with TTC (Sigma-Aldrich; Merck KGaA). Hearts isolated from rats
were incubated in 1% TTC for 15 min at 37°C, cut into transverse
sections, and imaged with a digital camera (Samsung Electrics Co.,
Ltd., Suwon, Korea). Infarct size was measured by calculating the
ratio of the cumulative infarcted area to that of the entire left
ventricle using ImageJ software (version 1.51j8; National
Institutes of Health, Bethesda, MD, USA).
Massons trichrome staining
Fibrosis in the heart following MI was evaluated by
Masson's trichrome staining (Sigma-Aldrich; Merck KGaA) according
to the manufacturer's protocol. In brief, sections were stained
with Bouin's solution at 56°C for 15 min, Weigert's iron
hematoxylin solution for 15 min and Blebrich scarlet-acid fuchsin
for 5 min. The sections were placed in working
phosphotungstic/phosphomolybdic acid solution for 5 min, Aniline
blue solution for 5 min, and 1% acetic acid for 2 min. Stained
sections were mounted with Permount (Thermo Fisher Scientific,
Inc.) and scanned to digitalize at ×200 magnification using an
Aperio AT2 (Leica Biosystems, Wetzlar, Germany).
Terminal deoxynucleotide
transferase-mediated dUTP nick-end labeling (TUNEL)
Apoptotic cells in paraffin-embedded heart tissue
sections were detected with the TUNEL assay using a commercial kit
(Merck Millipore, Billerica, MA, USA) according to the
manufacturer's protocol. Briefly, the paraffin-embedded heart
tissue sections were pretreated with proteinase K (20 µg/ml) for 15
min, quenched in 3% H2O2 for 5 min at RT, and
immediately equilibrated. The tissue sections were reacted with the
TdT enzyme at 37°C for 1 h, and incubated with digoxigenin
conjugated nucleotide substrate at 37°C for 30 min. Then, the heart
sections were stained with 3,3-diaminobenzene (DAB) for 5 min,
counterstained with hematoxylin for a short time, mounted with
Permount (Thermo Fisher Scientific, Inc.). A total of 5 animals per
group were analyzed. For each animal sample, apoptotic cells were
counted in at least five different regions of the stained slide
(magnification, ×100 and ×400).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation of 5 independent experiments. Differences
between groups were evaluated by one-way analysis of variance
followed by Kruskal-Wallis test and Dunn's multiple comparison
post-hoc tests. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed using SPSS v.18.0 software (SPSS, Inc., Chicago, IL, USA)
and PRISM v.5.01 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Construction of the selection vector
expressing IGF-1 and EGFP
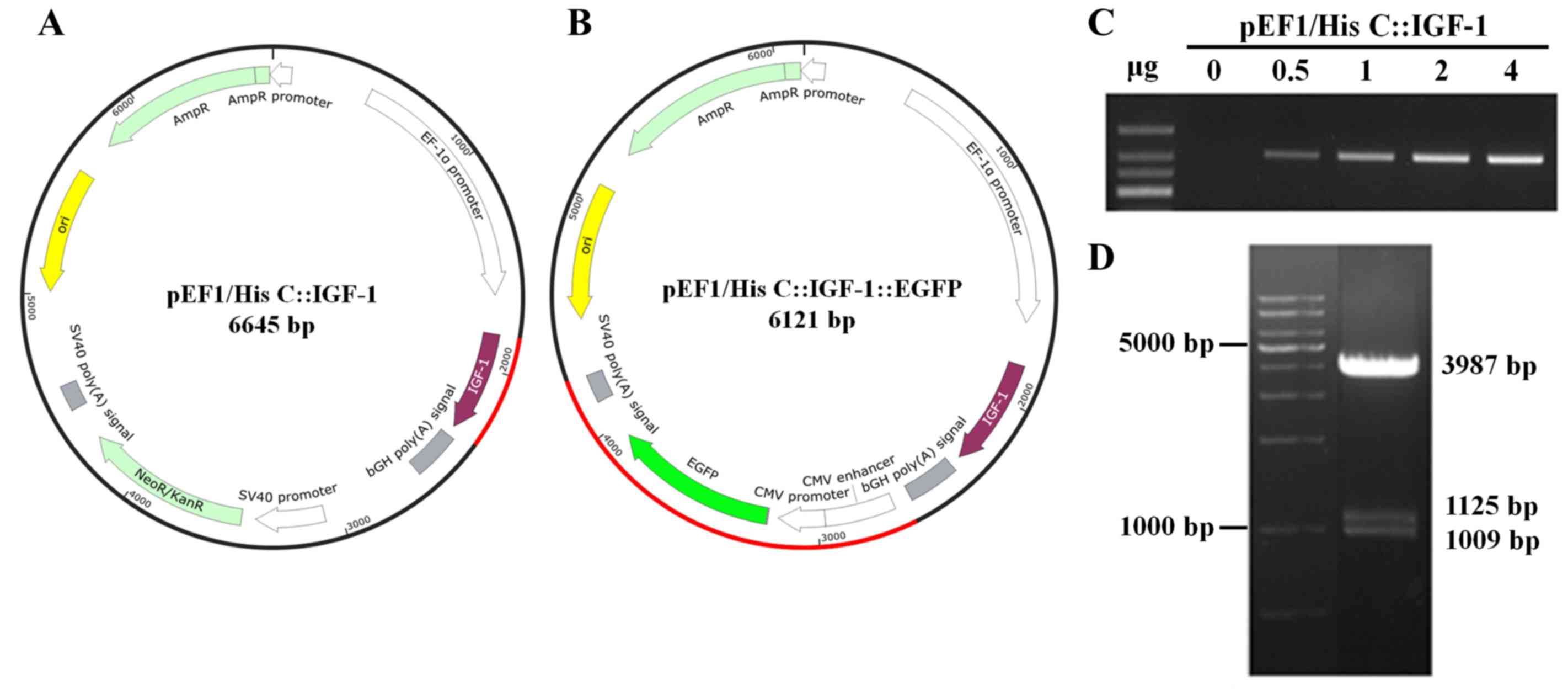
The pEF1/His C::IGF-1::EGFP selection vector was
constructed by inserting rat IGF-1 cDNA and EGFP DNA
into the pEF1/His C vector (Fig. 1A
and B). The sequence of the cloned vector (pEF1/His C::IGF-1
vector; 6,645 bp) was confirmed by gene sequencing (data not
shown). Expression of IGF-1 from pEF1/His C::IGF-1 was
confirmed in H9c2 cells by RT-PCR (0–4 µg; Fig. 1C). The EGFP gene was
subsequently inserted into the pEF1/His C::IGF-1 vector (Fig. 1D). The final size of the cloned
vector pEF1/His C::IGF-1::EGFP was 6,121 bp.
Characterization of BM derived MSCs
(BM-MSCs)
Rat primary BM-MSCs exhibited a spindle shape (data
not shown). To characterize the MSC phenotype, BM-MSCs were labeled
for positive and negative cell surface markers. Notably, BM-MSCs
were positive for MSC markers, including CD29 (99.8%), CD54 (96.3%)
and CD90 (99.7%), and were negative for CD45 (0.34%) and CD49d
(0.42%; Fig. 2A). The efficiency
of MSC transfection with the non-viral carrier PAM-ABP was assessed
by FACS based on the percentage of MSCs expressing EGFP. The
efficiency of transfection of pEF1/His C::IGF-1::EGFP and PAM-ABP
in MSCs was ~20%. Subsequent to sorting, the efficiency was
increased to 95.1% by EGFP selection, corresponding to a 4.8-times
increase in the purity of transfected MSCs (Fig. 2B and C).
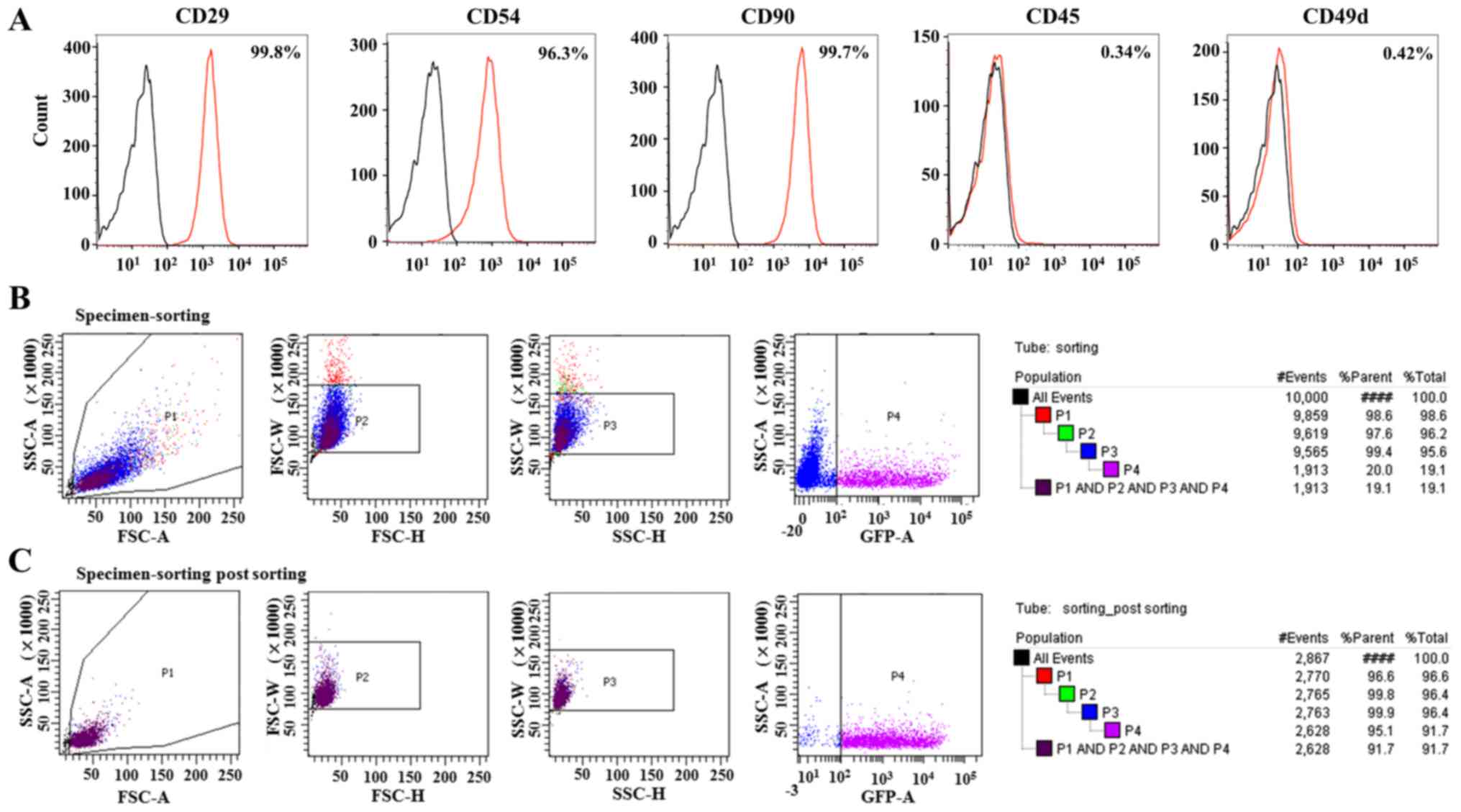 | Figure 2.Characterization of MSCs derived from
rat bone marrow and transfection efficiency of MSCs with pEF1/His
C::IGF-1::EGFP. (A) Expression levels of surface markers in the
isolated MSCs were measured by flow cytometry for CD29-FITC,
CD54-PE, CD90-FITC, CD45-PE and CD49d-FITC. The red histograms
represent staining with respective surface marker antibodies and
the black histograms represent staining with isotype controls. (B)
Prior to FACS, the transfection efficiency of MSCs with pEF1/His
C::IGF-1::EGFP and the nonviral carrier PAM-ABP was ~20% (P4). (C)
Following FACS, transfected MSCs were collected and the purity was
increased to 95.1%. Therefore, approximately all MSCs expressed a
green fluorescent signal and IGF-1. CD, cluster of differentiation;
EGFP, enhanced green fluorescence protein; IGF, insulin-like growth
factor; MSC, mesenchymal stem cells; FACS, fluorescence-activated
cell sorting cell sorting; FITC, fluorescein isothiocyanate; PE,
phycoerythrin. |
Transfection efficiency of pEF1/His
C::IGF-1::EGFP in rat BM-MSCs
To determine the optimal concentration of pEF1/His
C::IGF-1::EGFP for transfection in rat BM-MSCs, different amounts
were transfected (0–16 µg). The intensity of EGFP fluorescence was
concentration-dependent and was maximal at 8 µg following 24 and 48
h (Fig. 3A). IGF-1 mRNA
expression level was additionally increased in a
concentration-dependent manner as detected by RT-PCR, reaching a
maximum value at 8 µg after 48 h (Fig.
3B and C). IGF-1 protein expression levels in
conditioned culture medium from transfected MSCs were measured by
ELISA. Conditioned medium from pEF1/His C::IGF-1::EGFP-transfected
MSCs exhibited higher expression levels of IGF-1 compared with the
negative control (0 µg; Fig. 3D).
IGF-1 secretion was enhanced at concentrations ≤8 µg and
began to decline at 24 and 48 h following transfection (Fig. 3D). Furthermore, the amount of
IGF-1 secreted from transfected MSCs at 48 h
post-transfection was increased compared with 24 h following
transfection.
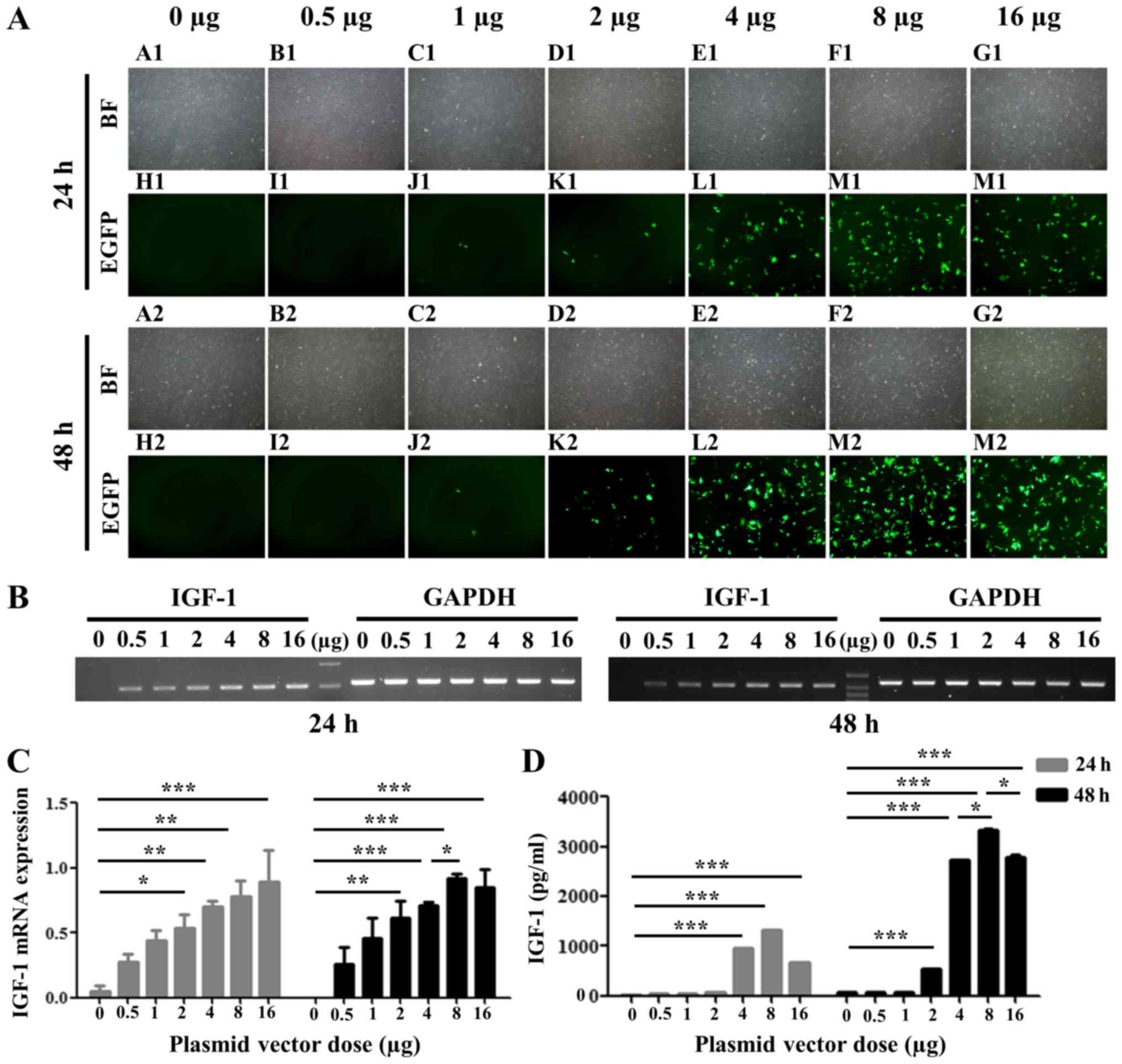 | Figure 3.EGFP and IGF-1 expression in MSCs
transfected with pEF1/His C::IGF-1::EGFP. (A) EGFP was
expressed following transfection with pEF1/His C::IGF-1::EGFP.
Magnification, ×40. A1-G1 (24 h) and A2-G2 (48 h), BF; H1-N1 (24 h)
and H2-N2 (48 h), immunofluorescence. (B) IGF-1 expression, as
detected by reverse transcription-polymerase chain reaction and (C)
quantification of its expression levels. (D) IGF-1 secretion as
measured by ELISA. *P<0.05, **P<0.01, ***P<0.001. EGFP,
enhanced green fluorescence protein; IGF, insulin-like growth
factor; MSCs, mesenchymal stem cells; BF, bright field. |
Engraftment of pEF1/His
C::IGF-1::EGFP-MSCs in ischemic hearts
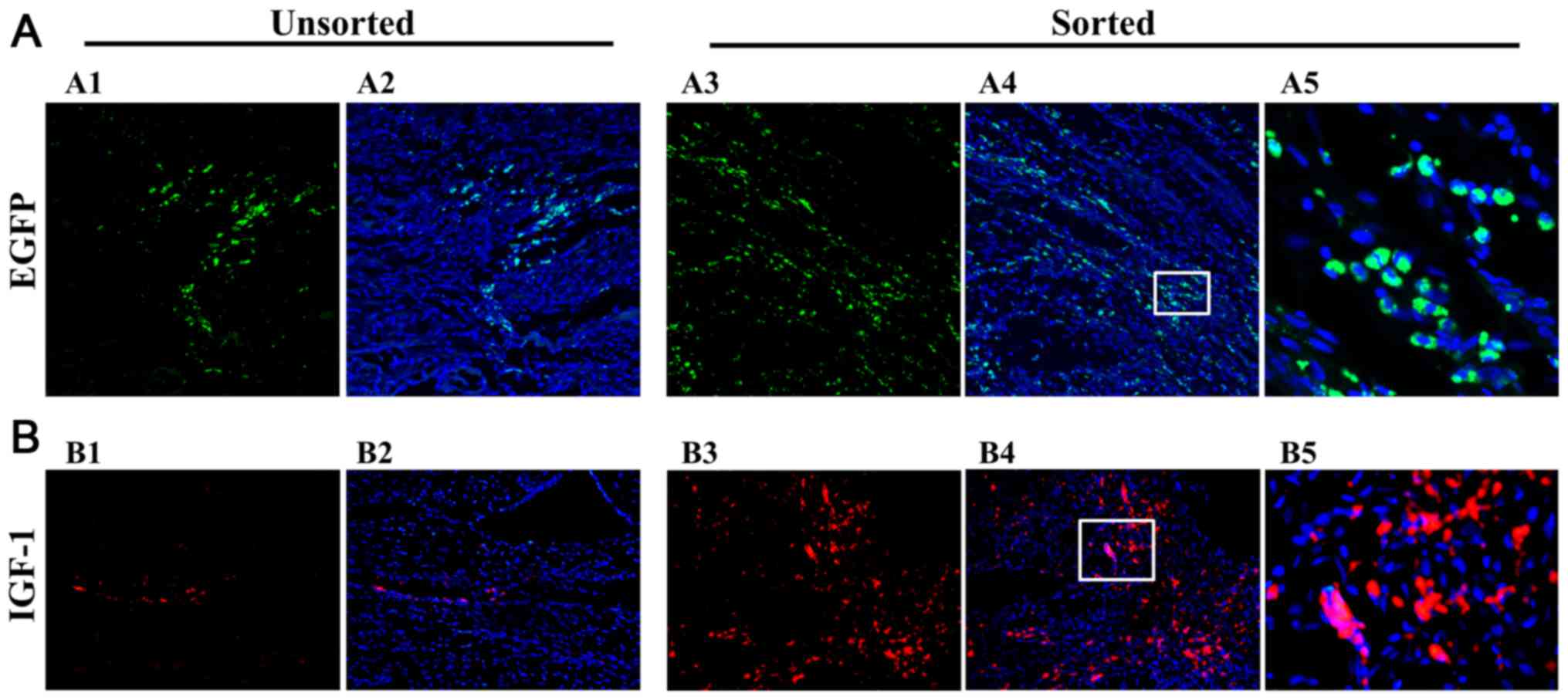
The green fluorescence associated with pEF1/His
C::IGF-1::EGFP-transfected MSCs was detected in rats injected with
unsorted and sorted cells. Transfected MSCs were more abundant in
the sorted group compared with the unsorted group (Fig. 4A). The pEF1/His
C::IGF-1::EGFP-transfected MSCs were localized in the myocardium of
the ischemic left ventricle.
Detection of IGF-1 pEF1/His
C::IGF-1::EGFP-MSCs in ischemic hearts
Red fluorescence corresponding to IGF-1
expression was detected in the myocardial layer of unsorted and
sorted groups (Fig. 4B).
IGF-1 expression levels were increased in the sorted group
compared with the unsorted group. Therefore, the sorting system
enhances the efficiency of gene delivery.
Myocardial repair is improved in
ischemic myocardium by pEF1/His C::IGF-1::EGFP transplantation
The therapeutic efficacy of the system was evaluated
through histological analysis of the infarcted tissue by TTC and
Masson's trichrome staining. Infarct size was decreased in the
sorted group compared with the PBS and MSC groups (P<0.05;
Fig. 5A and B). Although the
infarct size in the sorted was half of that of the unsorted group,
the difference was not statistically significant (Fig. 5B). Masson's trichrome staining
demonstrated that fibrotic area of the myocardial layer was
significantly decreased in the sorted group compared with the
unsorted group (P<0.05; Fig. 5C and
D).
Inhibitory effects of IGF-1 on myocyte
apoptosis
Analysis of the apoptotic cell ratio revealed that
apoptosis was decreased in the sorted group compared with the PBS,
MSC and unsorted groups (P<0.05, P<0.01; Fig. 5E and F).
Discussion
BM-MSC transplantation improves cardiac function,
capillary density and ventricular remodeling (10,22).
However, low viability and poor transplantation efficiency remain
principal challenges. Even direct transplantation of stem cells
into the infarcted heart is ineffective: Only 1–10% of cells
actually remain in the heart (23,24).
Furthermore, owing to the low gene transfection efficiency of
non-viral carriers, the proportion of stem cells harboring the
target gene is very low (11). The
aim of the present study was to maximize the effects of a
therapeutic gene using FACS to obtain a highly pure population of
cells transfected with this gene. FACS analysis has numerous
advantages over other available methods. It is the preferred method
for obtaining a pure cell population, particularly of cells
expressing a very low level of a particular marker or when
separation is based on differential marker density. In addition,
FACS is the only available technique for isolating cells based on
internal labeling or intracellular protein expression, including a
genetically modified fluorescent protein (25). It additionally allows the
purification of individual cells based on size, granularity and
fluorescence (25). However, the
cell sorting method using FACS has a disadvantage of cell loss. For
that reason, certain researchers prefer the selection of positive
transfected stem cells with chemical reagents, including puromycin
(26,27).
The pEF1/His C::IGF-1::EGFP selection vector was
constructed using the target gene IGF-1 and the selection
marker EGFP through insertion of rat IGF-1 cDNA into
the pEF1/His C vector (pEF1/His C::IGF-1) followed by insertion of
EGFP and the cytomegalovirus promoter in pEGFP-C1 into
pEF1/His C::IGF-1 (pEF1/His C::IGF-1::EGFP). The pEF1/His C vector
was used as it contains the human elongation factor-1α promoter,
which has high activity in stem cells (28). EGFP, which demonstrates
higher fluorescence compared with GFP, was used to visualize
transfected MSCs for cell sorting and for MSC tracking in infarcted
hearts (29). The two genes were
inserted under the control of its own promoter in a single vector
to avoid the generation of a fusion protein. The regulation of rat
IGF-1 and EGFP genes by the two promoters was
confirmed by transfecting pEF1/His C::IGF-1::EGFP and the non-viral
vector PAM-ABP into BM-MSCs. PAM-ABP (weight ratio, 1:5) functions
as an efficient gene carrier with low cytotoxicity (30,31).
The same weight ratio for the plasmid and non-viral carrier PAM-ABP
was applied for MSC transfection.
IGF-1-expressing MSCs were isolated by FACS with a
purity of 95.1%. When these cells were injected into MI rats, TTC
and trichrome staining revealed that infarct size and fibrosis were
decreased compared with animals injected with unsorted cells,
suggesting that the high expression levels of IGF-1 achieved by
sorting demonstrated therapeutic effects. Previous studies
suggested that IGF-1 is secreted by the majority of tissues and
serves an important role in cell growth, differentiation and
transformation (17,18). In particular, IGF-1 in the heart
has beneficial effects on cardiomyocyte function, including cell
proliferation and survival (17).
Exogenous administration of IGF-1 had cardioprotective effects that
were exerted through the Akt signaling pathway (32), and it was demonstrated that IGF-1
improved cardiac function and reduced structural damage during
ischemia/reperfusion (33). In the
present study, the TUNEL assay demonstrated that IGF-1 had an
anti-apoptotic effect on cardiomyocytes, although the apoptosis
pathway was not investigated; the effect was most marked in rats
injected with sorted cells. The present results are consistent with
a previous study, in which MSC survival and engraftment were
enhanced, and cardiac dysfunction and myocardial apoptosis were
suppressed by transplantation of IGF-1-treated MSCs (20).
Echocardiographic analysis in an MI animal model
revealed that injection of IGF-1-adipose tissue-derived SCs
improved left ventricular ejection fraction and cardiac
contractility index (34), in
addition to cardiac function (32,34,35).
Although echocardiography was not performed in the present study,
histological data provided evidence that maximizing the efficiency
of gene expression through a sorting system may improve cardiac
function in the infarcted heart.
In summary, it was demonstrated that MSC-based IGF-1
therapy may reduce fibrosis of the myocardium along with infarct
size and myocyte apoptosis in a rat MI model. Although the safety
and therapeutic efficacy of pEF1/His::IGF-1::EGFP selection vector
requires confirmation in larger animal models prior to is used in
clinical applications; this strategy may potentially be modified
for other types of cell-based therapy using different gene and stem
cell combinations.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Korea
Health Technology R&D Project through the Korea Health Industry
Development Institute (KHIDI), funded by the Ministry of Health
& Welfare, Republic of Korea (grant nos. HI17C0882 and
HI16C2211); the Mid-Career Researcher Program through a National
Research Foundation grant funded by the Ministry of Education,
Science, and Technology, Republic of Korea (grant no.
2015R1A2A2A01002731); and the Cardiovascular Research Center
(Seoul, Korea).
Availability of data and materials
The datasets used and/or analyzed generated during
the current study are available from the corresponding author on
reasonable request.
Authors contributions
DC and JK designed the study. SJ, JK, CY and HK
conducted the experiments, data analysis and statistical analysis.
ML supplied the non-viral carrier PAM-ABP and interpreted the data.
SJ and HK wrote the manuscript. All authors discussed the results
and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments conformed to the
International Guide for the Care and Use of Laboratory Animals, and
were approved by the Animal Research Committee of Yonsei University
College of Medicine (Seoul, Korea; grant no. 2015-0253).
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EGFP
|
enhanced green fluorescent protein
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
IGF-1
|
insulin-like growth factor-1
|
|
MI
|
myocardial infarction
|
|
MSC
|
mesenchymal stem cell
|
|
PCR
|
polymerase chain reaction
|
|
RT
|
reverse transcription
|
References
|
1
|
Laslett LJ, Alagona P Jr, Clark BA III,
Drozda JP Jr, Saldivar F, Wilson SR, Poe C and Hart M: The
worldwide environment of cardiovascular disease: Prevalence,
diagnosis, therapy, and policy issues: A report from the American
College of Cardiology. J Am Coll Cardiol. 60 25 Suppl:S1–S49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nabel EG: Cardiovascular disease. N Engl J
Med. 349:60–72. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sáez P and Kuhl E: Computational modeling
of acute myocardial infarction. Comput Methods Biomech Biomed
Engin. 19:1107–1115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Flynn A, Chen X, O'Connell E and O'Brien
T: A comparison of the efficacy of transplantation of bone
marrow-derived mesenchymal stem cells and unrestricted somatic stem
cells on outcome after acute myocardial infarction. Stem Cell Res
Ther. 3:362012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Michaels AD and Chatterjee K: Cardiology
patient pages. Angioplasty versus bypass surgery for coronary
artery disease. Circulation. 106:e187–e190. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsiao LC, Carr C, Chang KC, Lin SZ and
Clarke K: Stem cell-based therapy for ischemic heart disease. Cell
Transplant. 22:663–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carvalho JL, Braga VB, Melo MB, Campos AC,
Oliveira MS, Gomes DA, Ferreira AJ, Santos RA and Goes AM: Priming
mesenchymal stem cells boosts stem cell therapy to treat myocardial
infarction. J Cell Mol Med. 17:617–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krishna KA, Krishna KS, Berrocal R, Rao KS
and Rao Sambasiva KR: Myocardial infarction and stem cells. J Pharm
Bioallied Sci. 3:182–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei X, Yang X, Han ZP, Qu FF, Shao L and
Shi YF: Mesenchymal stem cells: A new trend for cell therapy. Acta
Pharmacol Sin. 34:747–754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim SH, Moon HH, Kim HA, Hwang KC, Lee M
and Choi D: Hypoxia-inducible vascular endothelial growth
factor-engineered mesenchymal stem cells prevent myocardial
ischemic injury. Mol Ther. 19:741–750. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reiser J, Zhang XY, Hemenway CS, Mondal D,
Pradhan L and La Russa VF: Potential of mesenchymal stem cells in
gene therapy approaches for inherited and acquired diseases. Expert
Opin Biol Ther. 5:1571–1584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santos JL, Pandita D, Rodrigues J, Pêgo
AP, Granja PL and Tomás H: Non-viral gene delivery to mesenchymal
stem cells: Methods, strategies and application in bone tissue
engineering and regeneration. Curr Gene Ther. 11:46–57. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nayerossadat N, Maedeh T and Ali PA: Viral
and nonviral delivery systems for gene delivery. Adv Biomed Res.
1:272012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue J, Wu J, Liu D, Zhao X and Lu WW: BMP2
gene delivery to bone mesenchymal stem cell by chitosan-g-PEI
nonviral vector. Nanoscale Res Lett. 10:2032015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang P, Wu C, Shen M, Gong F, Zhu K, Jiang
Z, Guan S, Shan H and Shuai X: An MRI-visible non-viral vector
bearing GD2 single chain antibody for targeted gene delivery to
human bone marrow mesenchymal stem cells. PLoS One. 8:e766122013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laron Z: Insulin-like growth factor 1
(IGF-1): A growth hormone. Mol Pathol. 54:311–316. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davis ME, Hsieh PC, Takahashi T, Song Q,
Zhang S, Kamm RD, Grodzinsky AJ, Anversa P and Lee RT: Local
myocardial insulin-like growth factor 1 (IGF-1) delivery with
biotinylated peptide nanofibers improves cell therapy for
myocardial infarction. Proc Natl Acad Sci USA. 103:8155–8160. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Delafontaine P, Song YH and Li Y:
Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1
binding proteins in blood vessels. Arterioscler Thromb Vasc Biol.
24:435–444. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lisa M, Haleagrahara N and Chakravarthi S:
Insulin-like growth factor-1 (IGF-1) reduces ischemic changes and
increases circulating angiogenic factors in experimentally-induced
myocardial infarction in rats. Vasc Cell. 3:132011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Zheng D, Li WF, Li HR, Zhang AD and
Li ZC: Insulin-like growth factor 1 treatment of MSCs attenuates
inflammation and cardiac dysfunction following MI. Inflammation.
37:2156–2163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schertzer JD and Lynch GS: Comparative
evaluation of IGF-I gene transfer and IGF-I protein administration
for enhancing skeletal muscle regeneration after injury. Gene Ther.
13:1657–1664. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyle AJ, Schulman SP, Hare JM and Oettgen
P: Is stem cell therapy ready for patients? Stem cell therapy for
cardiac repair. ready for the next step. Circulation. 114:339–352.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Chen X, Wang WE and Zeng C: How to
improve the survival of transplanted mesenchymal stem cell in
ischemic heart? Stem Cells Int. 2016:96827572016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Terrovitis JV, Smith RR and Marbán E:
Assessment and optimization of cell engraftment after
transplantation into the heart. Circ Res. 106:479–494. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Basu S, Campbell HM, Dittel BN and Ray A:
Purification of specific cell population by fluorescence activated
cell sorting (FACS). J Vis Exp. 41:pii: 1546. 2010.
|
|
26
|
Kolossov E, Bostani T, Roell W, Breitbach
M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel
D, et al: Engraftment of engineered ES cell-derived cardiomyocytes
but not BM cells restores contractile function to the infarcted
myocardium. J Exp Med. 203:2315–2327. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jacot JG, Kita-Matsuo H, Wei KA, Chen HS,
Omens JH, Mercola M and McCulloch AD: Cardiac myocyte force
development during differentiation and maturation. Ann N Y Acad
Sci. 1188:121–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim S, Kim GJ, Miyoshi H, Moon SH, Ahn SE,
Lee JH, Lee HJ, Cha KY and Chung HM: Efficiency of the elongation
factor-1alpha promoter in mammalian embryonic stem cells using
lentiviral gene delivery systems. Stem Cells Dev. 16:537–545. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang G, Gurtu V and Kain SR: An enhanced
green fluorescent protein allows sensitive detection of gene
transfer in mammalian cells. Biochem Biophys Res Commun.
227:707–711. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nam HY, Nam K, Lee M, Kim SW and Bull DA:
Dendrimer type bio-reducible polymer for efficient gene delivery. J
Control Release. 160:592–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Won YW, McGinn AN, Lee M, Nam K, Bull DA
and Kim SW: Post-translational regulation of a hypoxia-responsive
VEGF plasmid for the treatment of myocardial ischemia.
Biomaterials. 34:6229–6238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khan RS, Martinez MD, Sy JC, Pendergrass
KD, Che PL, Brown ME, Cabigas EB, Dasari M, Murthy N and Davis ME:
Targeting extracellular DNA to deliver IGF-1 to the injured heart.
Sci Rep. 4:42572014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pi Y, Goldenthal MJ and Marín-García J:
Mitochondrial involvement in IGF-1 induced protection of
cardiomyocytes against hypoxia/reoxygenation injury. Mol Cell
Biochem. 301:181–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bagno LL, Carvalho D, Mesquita F, Louzada
RA, Andrade B, Kasai-Brunswick TH, Lago VM, Suhet G, Cipitelli D,
Werneck-de-Castro JP and Campos-de-Carvalho AC: Sustained IGF-1
secretion by adipose-derived stem cells improves infarcted heart
function. Cell Transplant. 25:1609–1622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boucher M, Pesant S, Lei YH, Nanton N,
Most P, Eckhart AD, Koch WJ and Gao E: Simultaneous administration
of insulin-like growth factor-1 and darbepoetin alfa protects the
rat myocardium against myocardial infarction and enhances
angiogenesis. Clin Transl Sci. 1:13–20. 2008. View Article : Google Scholar : PubMed/NCBI
|