Introduction
Breast cancer remains a great threat to female
health worldwide. According to a cancer statistic (1,2),
breast cancer is the most frequently diagnosed cancer and the
leading cause of cancer-associated mortality among females in the
United States, accounting for ~232,340 of the total cases of cancer
and ~39,620 cases of cancer-associated mortality. The high
incidence has made breast cancer the most common non-skin cancer in
females (1,2). Current knowledge has attributed
several factors, including histone modification (3,4),
hormone disorder (5) and
transcription factors (6), to the
development and progression of breast cancer. Breast cancer
development is thought to occur via the accumulation of genetic
alterations or mutations, and is a multistep disease that involves
the co-ordinal interaction of numerous genes, leading to molecular
and morphologic changes within normal gland epithelium (7). Although treatments have improved over
the last decades, the prognosis of breast cancer remains poor.
Therefore, novel therapeutic strategies for the treatment of breast
cancer are still required.
With the rapid development of genome and
transcriptome sequencing technologies, as well as establishment of
genomics consortiums, including Encyclopedia of DNA Elements and
Functional Annotation of the Mammalian Genome, the classic view of
the transcriptome landscape and its mRNA-centric paradigm for
transcript annotation has undergone fundamental changes (8,9). It
is well recognized that the vast majority of the genome serves as a
template for the transcription of noncoding RNAs. Among these
noncoding RNAs, long noncoding RNAs (lncRNAs) are a novel class of
RNA that are >200 nucleotides in length and function by
regulating various biological processes, including cell
proliferation, apoptosis, differentiation, migration and invasion
(10,11). Increasing evidence has also
indicated the potential role of lncRNAs in numerous diseases, such
as cancer (12–15). lncRNAs may have pro-oncogenic or
suppressive roles in breast cancer development. A recent study used
a Global run-on sequencing, and RNA-sequencing-integrated genomic
and molecular approaches to identify and characterize
growth-regulating lncRNAs in breast cancers (12), revealing novel insight into breast
cancer diagnosis and treatments. For example, nuclear factor
κ-light-chain-enhancer of activated B cells interacting lncRNA has
been demonstrated to suppress breast cancer metastasis by blocking
IκB phosphorylation (13).
Furthermore, lncRNA HOXA11-antisense (AS) can promote cell
proliferation and metastasis in human breast cancer (14), and six lncRNAs have been identified
to be significantly altered in invasive ductal breast carcinoma
with the use of sure independence screening procedures based on
distance correlation (15).
lncRNA RUSC1-AS-N is a novel lncRNA, whose function
remains largely unknown. RUSC1-AS-N was initially identified from
microarray data (16), and has
recently been identified to be upregulated in hepatocellular
carcinoma (HCC) tissue. It was associated with poor prognosis in
patients with HCC from GSE54238 and GSE40144 datasets (16), indicating the oncogenic role of
RUSC1-AS-N in human tumorigenesis. However, the role of RUSC1-AS-N
in breast cancer remains to be elucidated.
In the present study, systemic investigation into
the role of RUSC1-AS-N in breast cancer was performed. Firstly, the
expression levels of RUSC1-AS-N in breast cancer tissues and cell
lines were examined. Subsequently, RUSC1-AS-N expression was
knocked down in breast cancer cell lines to evaluate the role of
RUSC1-AS-N in cell proliferation, migration and invasion. To the
best of our knowledge, this is the first study of its kind to
provide experimental evidence for the functional role of RUSC1-AS-N
in human solid tumors. The data from the present study indicated
that lncRNA RUSC1-AS-N as a therapeutics target may be valuable and
promising for the treatment of breast cancer.
Materials and methods
Human samples
A total of 100 breast cancer tissues and their
adjacent non-cancerous tissues were collected from female patients
(age range, 45–70 years; mean age, 59 years) diagnosed with
triple-negative breast infiltrating ductal carcinoma at The Third
Department of Breast Cancer, Tianjin Medical University Cancer
Institute and Hospital (Tianjin, China) between January
2014-January 2016. Patients did not receive radiotherapy or
chemotherapy treatment prior to surgery. All tissues were frozen in
liquid nitrogen as soon as they were collected via surgery. All
patients provided informed consent to participate in the present
study, which was approved by the Ethics Committee of Tianjin
Medical University Cancer Institute and Hospital.
Cell culture and transfection
Human breast cancer cell lines T47D, MCF7,
MDA-MB-231, MDA-MB-468 and SK-BR-3 were purchased from the Cell
Bank of Type Culture Collection of Shanghai Biological Institute,
Chinese Academy of Science (Shanghai, China). The breast epithelial
cell line MCF10A was purchased from the American Type Culture
Collection (Manassas, VA, USA) and used as control cells. Cells
were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.). Cells were maintained at 37°C in a humidified atmosphere
with 5% CO2.
Cell transfection was conducted using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturers' protocols. The
transfection was performed 48 h prior to the subsequent analysis.
The siRNAs were provided by GenePharm Co. (Shanghai, China; cat.
no. 17892). Wnt agonist 1 was purchased from Selleck Chemicals
(Shanghai, China) and used at a final concentration of 10 µM at
37°C for 24 h. The culture medium was replaced every two days
unless otherwise stated.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from human tissues and
cultured cells using TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA was quantified using a NanoDrop™
2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher
Scientific, Inc., Wilmington, DE, USA) and then immediately
reversely transcribed into cDNA using PrimeScript™ RT
Master Mix (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's protocols. Subsequently, qPCR was
performed with SYBR® Premix EX Taq™ II
(Takara Biotechnology Co., Ltd.) on an ABI PRISM® 7900HT
Sequence Detection system (Thermo Fisher Scientific, Inc.). The
thermocycling protocol was as follows: Initial denaturation at 95°C
for 5 min, followed by 45 cycles of 10 sec at 95°C (denaturation),
10 sec at 60°C (primer annealing) and 10 sec at 72°C (elongation),
and a final extension step for 10 min at 72°C. The primer sequences
used for qPCR were as follows: RUSC1-AS-N, forward,
5′-TCTTTCCCAGAAGTAGCAC-3′ and reverse, 5′-ATTTTATCAACGGAGACGC-3′;
Wnt1, forward, 5′-CGATGGTGGGGTATTGTGAAC-3′ and reverse,
5′-CCGGATTTTGGCGTATCAGAC-3′; β-catenin, forward,
5′-AAAGCGGCTGTTAGTCACTGG-3′ and reverse,
5′-CGAGTCATTGCATACTGTCCAT-3′; and GAPDH, forward,
5′-GTGGACATCCGCAAAGAC-3′ and reverse, 5′-AAAGGGTGTAACGCAACTA-3′.
GAPDH was used as the internal reference, and relative gene
expression levels were calculated using the 2−ΔΔCq
method (17). Each experiment was
repeated at least three times.
Colony formation assay
To observe the effects of RUSC1-AS-N on cell
proliferation, colony formation assays were performed. Briefly,
MDA-MB-231 and MDA-MB-468 cells were seeded in 6-well plates at a
density of 900 cells/well and were later transfected with scramble
negative control siRNA (siNC) or specific siRNA against RUSC1-AS-N
(siRUSC1-AS-N) when the cell confluence reached 10%. Transfected
cells were cultured for 14 days to form natural colonies.
Subsequently, cells were washed with PBS for three times, treated
with (1%) crystal violet for 30 min at room temperature and washed
twice with deionized water. The colonies were viewed under a light
microscope (Nikon Corporation, Tokyo, Japan) with at least five
fields randomly and the number of colonies was counted.
Cell viability analysis
Cell viability was determined using an MTT assay.
MDA-MB-231 and MDA-MB-468 cells were trypsinized and seeded in
triplicate in 96-well plates at a density of 4,000 cells/well.
Cells were transfected with siNC and siRUSC1-AS-N at the presence
or absence of RUSC1-AS-N knockdown. Cell viability was monitored
over the course of 5 days. At each indicated time point (day 1, 2,
3, 4 and 5), 10 µl/well of 5 mg/ml MTT solution was added to the
cell cultures. DMSO was used to dissolve the formazan. Following 2
h incubation at room temperature, the absorbance at 490 nm was
recorded with a Tecan microplate reader. Cell viability was defined
as the cell number ratio of experimental groups to control
cells.
Wound-healing assay
MDA-MB-231 and MDA-MB-468 cells were transfected
with siRUSC1-AS-N or siNC, seeded at a density of 5×105
cells/well in 6-well culture plates and allowed to reach 90%
confluence overnight. Subsequently, the culture medium was replaced
with serum-free DMEM, and a scratch wound was created using a 10-µl
pipette tip and the cells were washed three times with PBS.
Following incubation at 37°C for 12 h, the migrating cells were
observed and images were captured using a light microscope
(magnification, ×100; Nikon Corporation).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Nantong, China) with a
protease inhibitor cocktail (Thermo Fisher Scientific, Inc.). The
total protein extracts were quantified using a Bicinchoninic Acid
assay kit (Thermo Fisher Scientific, Inc.), according to the
manufacturers' protocols. A total of 40 µg protein was separated by
12% SDS-PAGE and transferred to a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). After blocking in 5% milk for
1 h at room temperature, proteins were detected using specific
primary antibodies (incubated at 4°C overnight) against Wnt1
(1:1,000; cat. no. ab15251; Abcam, Cambridge, UK), β-catenin
(1:1,000; cat. no. ab16051; Abcam), E-cadherin (1:1,000; cat. no.
sc-71009; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
N-cadherin (1:1,000; cat. no. sc-53488; Santa Cruz Biotechnology,
Inc.), cyclin B1 (1:1,000; cat. no. sc-70898; Santa Cruz
Biotechnology, Inc.) and GAPDH (1:1,000; cat. no. sc-32233; Santa
Cruz Biotechnology, Inc.). Goat anti-rabbit IgG-HRP secondary
antibody and goat anti-mouse IgG-HRP secondary antibody were
purchased from Santa Cruz Biotechnology (cat. nos. sc-2004 and
sc-2005; 1:2,500) and incubated with the membrane at room
temperature for 1 h. Proteins were visualized using an enhanced
chemiluminescence (Immobilon Western HRP; Millipore) and the bands
were quantified by densitometry using ImageJ software (V1.8.0;
National Institutes of Health, Bethesda, MD, USA). All experiments
were performed in triplicate.
Transwell assay
For cell migration assays, MDA-MB-231 and MDA-MB-468
cells were transfected with siRUSC1-AS-N or siNC for 48 h.
Subsequently, cells were trypsinized and collected by low-speed
centrifugation (1,000 × g, 4°C for 5 min). A total of
1×104 cells in 200 µl serum-free DMEM were seeded into
the upper chamber of Transwell cell culture chambers (pore size, 8
µm; Corning Life Sciences, New York, NY, USA). The lower chamber
was filled with 600 µl DMEM containing 10% FBS. The plate was
incubated at 37°C and the cells were allowed to migrate for 24 h.
Next, the membrane was fixed with pre-cooled methanol for 5 min at
room temperature and stained with 1% crystal violet for 5 min at
room temperature. Cell migration was assessed by counting the cells
that had migrated through the membrane to the underside of the
membrane. A total of 5 random fields of view were selected and
images were captured using a light microscope (magnification, ×100;
Nikon Corporation).
For cell invasion assays, the same protocol was
followed; however, membrane was pre-coated with Matrigel (20%;
Corning Incorporated, Corning, NY, USA) and cells were incubated
for 6 h at 37°C.
Statistical analysis
GraphPad Prism version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to perform statistical analyses. The
data are presented as the mean ± standard deviation. A two-tailed
Student's t-test was used for comparisons between two groups.
Differences between tumor and adjacent normal control samples were
analyzed using paired Student's t-test. For comparisons among
multiple groups, one-way analysis of variance was applied followed
by Least Significance Difference post hoc test. P<0.05 was
considered to indicate a statistically significant difference. Each
experiment was repeated three times.
Results
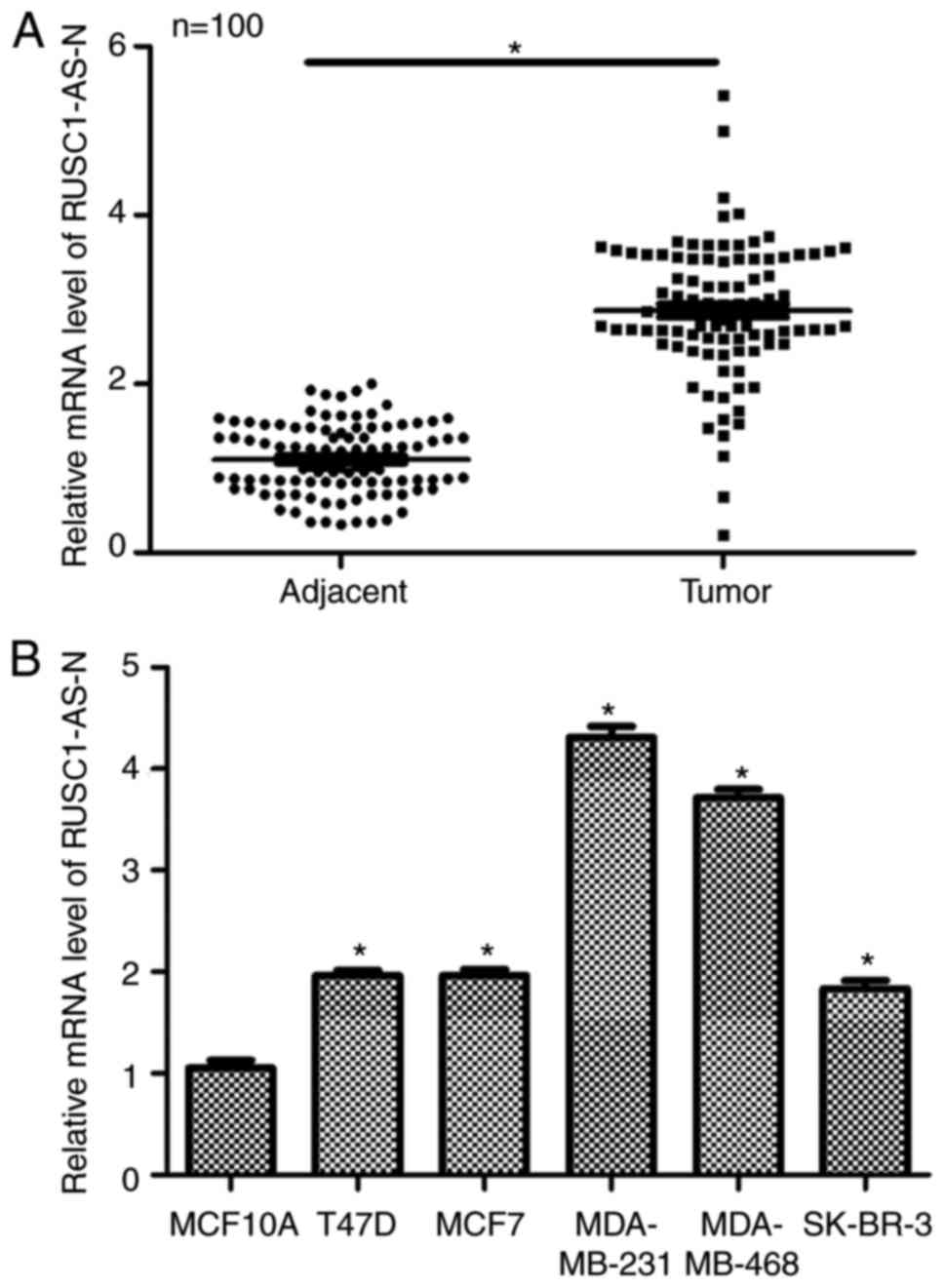
lncRNA RUSC1-AS-N is upregulated in
human breast cancer
Firstly, the relative expression levels of
RUSC1-AS-N in human breast cancer were examined in vivo and
in vitro. A total of 100 patients with breast cancer were
included in the study. As presented in Fig. 1A, patients had significantly higher
transcript levels of RUSC1-AS-N in the tumor tissues compared with
in the adjacent non-cancerous tissues. Next, five different breast
cancer cell lines were used to compare expression levels of
RUSC1-AS-N; normal MCF10A cells were included as a control. It was
demonstrated that the transcription levels of RUSC1-AS-N were
significantly upregulated in all breast cancer cell lines compared
with the control (Fig. 1B), with
MDA-MB-231 and MDA-MB-468 cells exhibiting the highest expression
of RUSC1-AS-N. Therefore, MDA-MB-231 and MDA-MB-468 were selected
for subsequent knockdown experiments. Interestingly, MDA-MB-231 and
MDA-MB-468 cells are known to have high metastatic potential
(7,14), indicating a potential association
of RUSC1-AS-N with cell metastasis. The data suggested that the
expression of RUSC1-AS-N was upregulated in human breast cancer
both in vivo and in vitro.
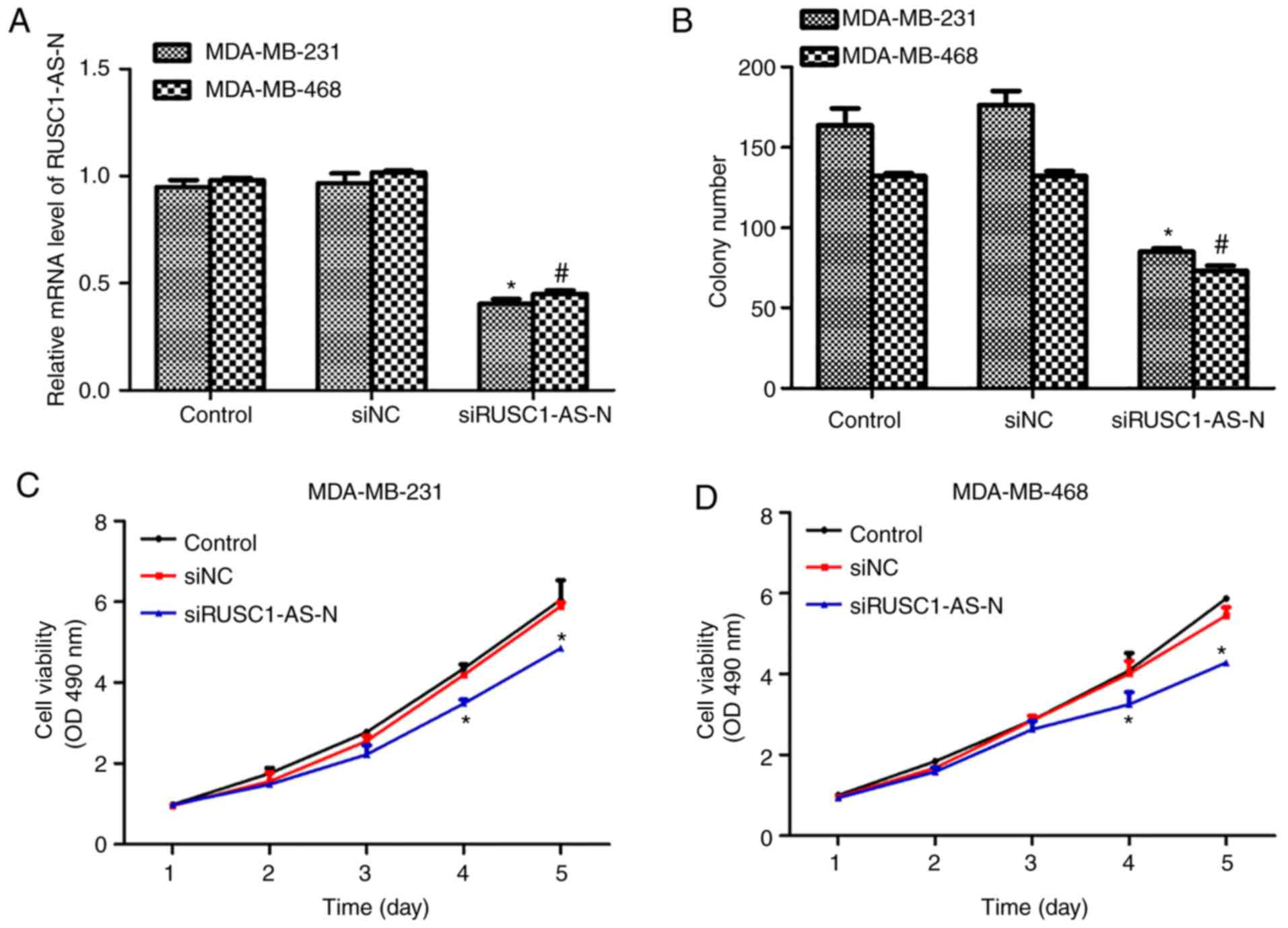
Knockdown of RUSC1-AS-N inhibits cell
proliferation and viability in human breast cancer
Next, the role of RUSC1- AS-N in human breast cancer
cell proliferation was explored. A specific siRNA against
RUSC1-AS-N, siRUSC1-AS-N, was transfected into MDA-MB-231 and
MDA-MB-468 cells. It was revealed that the transcription levels of
RUSC1-AS-N were decreased by ~50% in both cell lines following
transfection with siRUSC1-AS-N (Fig.
2A). Colony formation assays were also performed, and it was
demonstrated that knockdown of RUSC1-AS-N significantly decreased
the number of colonies by 24 and 20% in MDA-MB-231 and MDA-MB-468
cells, respectively, compared with the control (Fig. 2B). Subsequently, cell viability was
detected in both cell lines transfected with or without
siRUSC1-AS-N. For the first 3 days following transfection, there
was no notable difference amongst the three experimental groups;
however, cell viability of MDA-MB-231 cells was suppressed by 16
and 22% on days 4 and 5, respectively (Fig. 2C). Similar results were observed in
MDA-MB-468 cells (Fig. 2D),
whereby significant suppression of cell proliferation was also
recorded on day 4 and 5. These results revealed that knockdown of
RUSC1-AS-N inhibited cell proliferation of human breast cancer
cells in vitro.
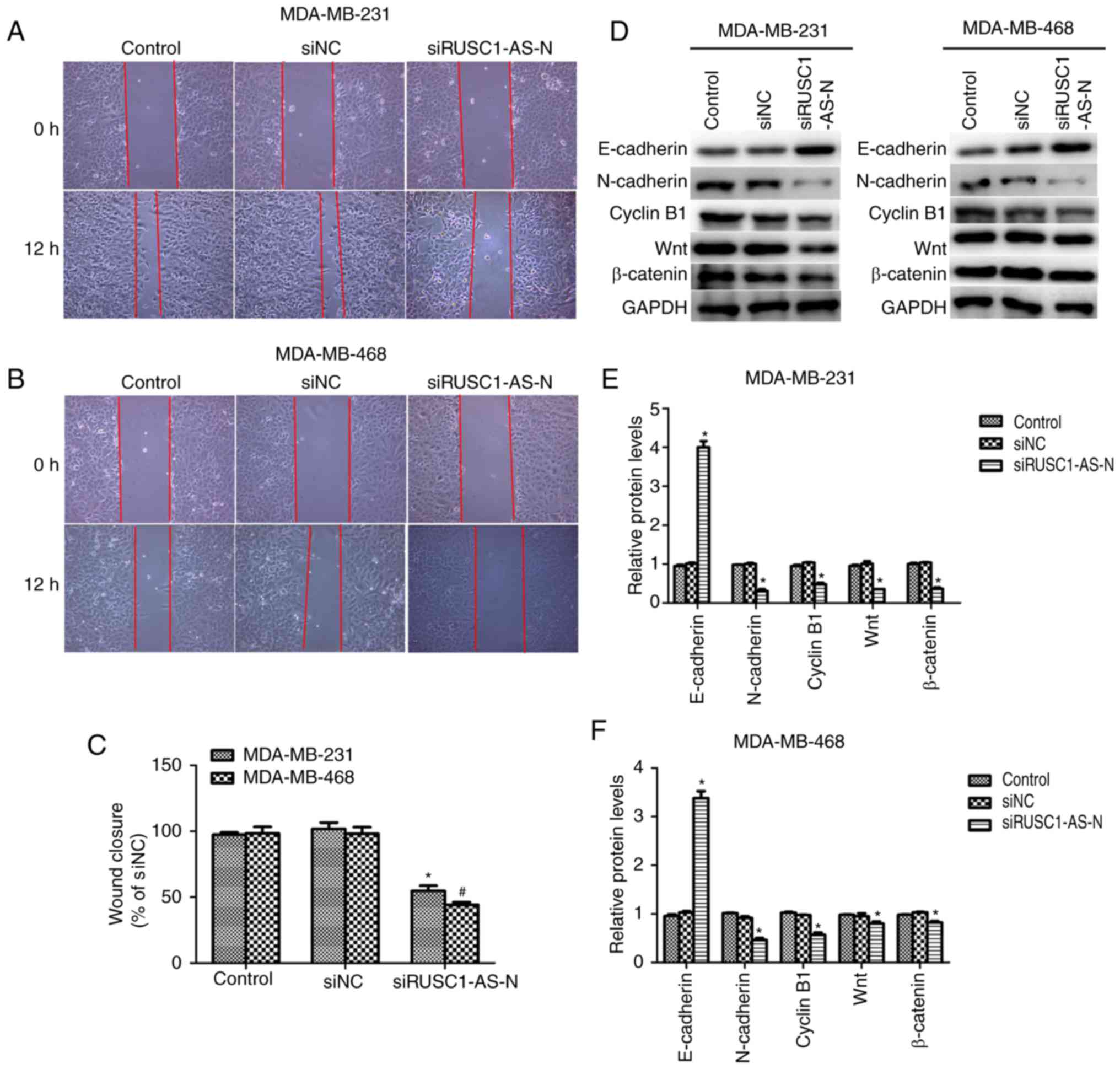
Knockdown of RUSC1-AS-N suppresses
cell migration in human breast cancer
Cell proliferation and migration are the two main
manifestations of cancer; therefore, the role of RUSC1-AS-N in cell
migration was investigated. Wound healing assays were performed and
as presented in Fig. 3A and B,
transfection with specific siRNA against RUSC1-AS-N decreased the
wound closure rate in both cell lines. Quantification of wound gap
area revealed that cell migration was significantly suppressed by
~50% in MDA-MB-231 and MDA-MB-468 cells (Fig. 3C). The protein expression levels of
epithelial-to-mesenchymal transition (EMT) markers were analyzed
using western blotting. It was demonstrated that when RUSC1-AS-N
was knocked down, the protein levels of E-cadherin were
significantly increased, while that of N-cadherin, cyclin B1, Wnt1
and β-catenin were significantly decreased in MDA-MB-231 and
MDA-MB-468 cells (Fig. 3D-F)
compared with the control. The results suggested that knockdown of
RUSC1-AS-N inhibited cell migration via the Wnt/β-catenin signaling
pathway in human breast cancer.
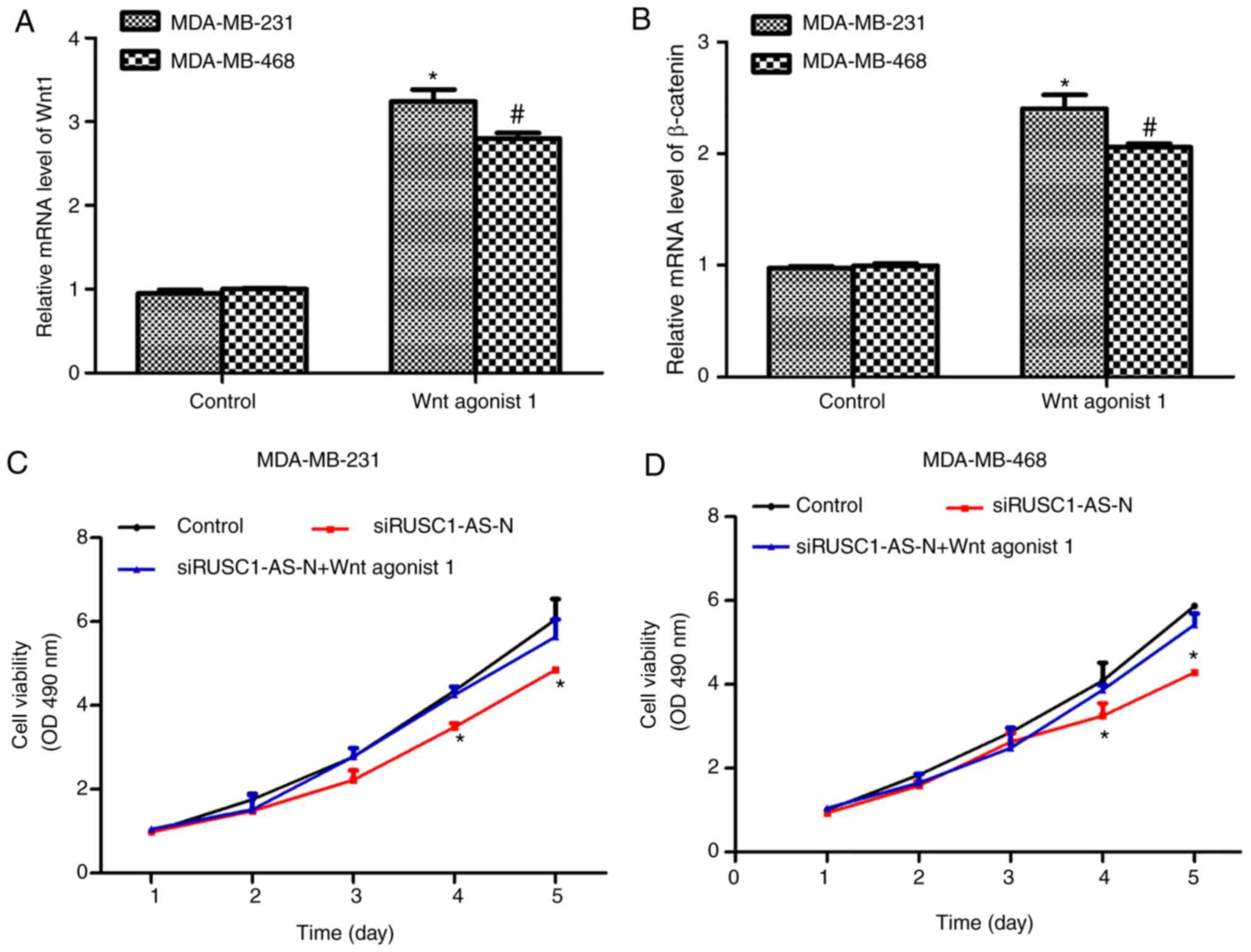
Activation of the Wnt/β-catenin
signaling pathway reverses the inhibitory effects of siRUSC1-AS-N
on cell proliferation in human breast cancer
Next, the Wnt/β-catenin signaling pathway was
activated using a specific activator, Wnt agonist 1, at a final
concentration of 10 µM. As presented in Fig. 4A and B, treatment of MDA-MB-231 and
MDA-MB-468 cells with Wnt agonist 1 significantly increased the
mRNA levels of Wnt1 and β-catenin by ~3-fold. In addition, cell
viability was assessed in cells treated with Wnt agonist 1 in the
presence or absence of siRUSC1-AS-N. As presented in Fig. 4C, knockdown of RUSC1-AS-N in
MDA-MB-231 cells significantly suppressed cell viability on days 4
and 5 following transfection compared with the control; however,
treatment with Wnt agonist 1 reversed the inhibitory effects of
siRUSC1-AS-N. Similarly, depletion of RUSC1-AS-N in MDA-MB-468
cells also decreased cell viability, whereas activation of the
Wnt/β-catenin signaling pathway suppressed the inhibitory effects
of siRUSC1-AS-N (Fig. 4D). These
data suggested that RUSC1-AS-N promoted cell viability by
regulating the Wnt/β-catenin signaling pathway in human breast
cancer cells.
Activation of Wnt/β-catenin signaling
reverses the inhibitory effects of siRUSC1-AS-N on cell metastasis
in human breast cancer
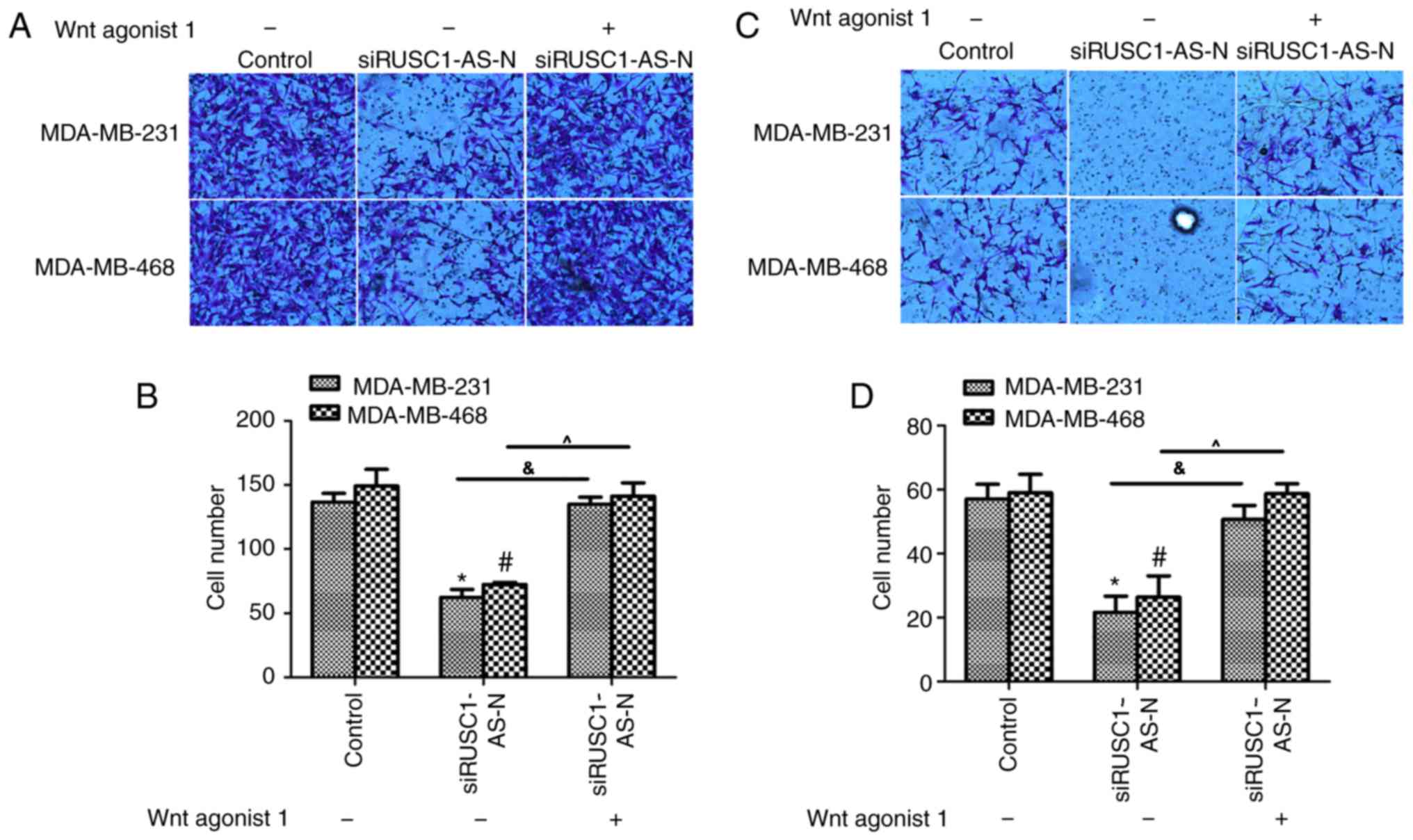
The effects of Wnt agonist 1 on human breast cancer
cell lines were also examined using Transwell assays. The migration
assays revealed that transfection with siRUSC1-AS-N significantly
inhibited cell migration via the Transwell membrane for MDA-MB-231
and MDA-MB-468 cells, while co-treatment with Wnt agonist 1
reversed the inhibitory effect of siRUSC1-AS-N (Fig. 5A and B). The invasion assay
demonstrated that cell metastasis was significantly inhibited when
cells were transfected with siRUSC1-AS-N compared with the control;
cell metastasis returned to normal levels when cells were
co-treated with Wnt agonist 1 (Fig. 5C
and D). These results indicated that RUSC1-AS-N promoted cell
metastasis via regulation of the Wnt/β-catenin signaling pathway in
human breast cancer cells.
Discussion
Breast cancer is one of the most common type of
cancer in females. According to an investigation in 2016, there are
3,053,450 females living with breast cancer, which significantly
affects the quality of life of these patients (1,3,18).
Although great efforts have been made to improve breast cancer
diagnosis and treatment, most tumors are often clinically diagnosed
at an advanced stage (19).
Additionally, karyotyping suggests that breast cancer becomes
increasingly aggressive via the stepwise accumulation of genetic
alteration (6,20); therefore, novel therapeutic
strategies for breast cancer are required.
Recent advances in gene sequencing have greatly
broadened current knowledge of gene regulation. lncRNAs are a novel
class of noncoding RNAs that have been implicated in multiple
cellular processes, including proliferation (21), apoptosis (22), migration and invasion (14,23).
lncRNA RUSC1-AS-N is a novel lncRNA that has unknown function. A
previous study revealed that RUSC1-AS-N is upregulated in HCC
tissues and its expression levels indicate poor prognosis in
patients with HCC (16). To the
best of our knowledge, the present study is the first to
demonstrate that RUSC1-AS-N is also upregulated in breast cancer
tissues compared with in non-cancerous tissues.
RUSC1-AS-N was also highly expressed in breast
cancer cell lines MDA-MB-231 and MDA-MB-468; therefore, siRNA was
used to deplete RUSC1-AS-N expression, and cell proliferation,
migration and invasion were examined. Interestingly, it was
identified that depletion of RUSC1-AS-N significant decreased cell
viability over the course of 5 days. Furthermore, depletion of
RUSC1-AS-N also decreased cell migration ability as breast cancer
cells transfected with siRUSC1-AS-N exhibited significantly lower
wound closure rates. EMT is a marker of cell invasiveness and has
been widely recognized as a critical process that implicates
distant metastases (24).
Consistent with the wound healing assay results, knockdown of
RUSC1-AS-N increased the expression of the epithelial marker
E-cadherin, but decreased that of the mesenchymal marker
N-cadherin, confirming that RUSC1-AS-N may promote the EMT
processes during breast tumorigenesis. Knockdown of RUSC1-AS-N also
decreased the protein levels of cyclin B1, a marker of cell cycle
progression, reinforcing the idea that RUSC1 also promoted cell
cycle progression, thereby promoting cell proliferation in breast
cancer. The results of the present study collectively suggested
that RUSC1-AS-N promoted cell proliferation and migration in breast
cancer.
Interestingly, it was observed that the protein
levels of Wnt and β-catenin, two pivotal proteins that execute the
Wnt/β-catenin pathway cascade, were also decreased following
knockdown of RUSC1-AS-N. With the use of Wnt agonist 1, which
selectively activates the Wnt/β-catenin pathway, it was
demonstrated that siRUSC1-AS-N-induced inhibition of cell
proliferation, migration and invasion was reversed. Activation of
the Wnt/β-catenin pathway by Wnt agonist 1 led to cell viabilities
and invasion capacities that were comparable with control untreated
cells. Previous studies have reported that the Wnt/β-catenin
pathway is crucial for the development and progression of breast
cancer (25,26). In addition, high levels of Wnt
expression and aberrant activation of β-catenin have been detected
in breast cancer tissues (27).
However, downregulation of the Wnt/β-catenin pathway can inhibit
EMT and suppress the spontaneous invasion of breast cancer cells
(28). Taken together, the results
in the present study suggested that RUSC1-AS-N positively regulated
the Wnt/β-catenin pathway, thereby promoting the proliferation and
metastasis of breast cancer cells.
In conclusion, the present study identified lncRNA
RUSC1-AS-N as a critical mediator of breast cancer cell
proliferation and metastasis. This is the first report that
systemically investigated the functional roles of RUSC1-AS-N in
solid tumors. The results suggested that RUSC1-AS-N promoted cell
proliferation and metastasis via Wnt/β-catenin signaling in human
breast cancer. The present study provided novel evidence that the
therapeutic targeting of RUSC1-AS-N or Wnt/β-catenin may be a
promising strategy for the treatment of breast cancer in
clinic.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81672623).
Availability of data and materials
All data and materials are available.
Authors' contributions
PZ performed most of the experiments, analyzed the
data and prepared the manuscript, PL helped with certain of the
experiments and revised the manuscript and JZ provided the funding,
designed the study and revised the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tianjin Medical University Cancer Institute and Hospital (Tianjin,
China). All patients gave their full consent to participate in the
present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li LL, Xue AM, Li BX, Shen YW, Li YH, Luo
CL, Zhang MC, Jiang JQ, Xu ZD, Xie JH and Zhao ZQ: JMJD2A
contributes to breast cancer progression through transcriptional
repression of the tumor suppressor ARHI. Breast Cancer Res.
16:R562014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng W, Lu Z, Luo RZ, Zhang X, Seto E,
Liao WS and Yu Y: Multiple histone deacetylases repress tumor
suppressor gene ARHI in breast cancer. Int J Cancer. 120:1664–1668.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Travis RC and Key TJ: Oestrogen exposure
and breast cancer risk. Breast Cancer Res. 5:239–247. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li L, Gao P, Li Y, Shen Y, Xie J, Sun D,
Xue A, Zhao Z, Xu Z, Zhang M, et al: JMJD2A-dependent silencing of
Sp1 in advanced breast cancer promotes metastasis by downregulation
of DIRAS3. Breast Cancer Res Treat. 147:487–500. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H, Stephens LC and Kumar R:
Metastasis tumor antigen family proteins during breast cancer
progression and metastasis in a reliable mouse model for human
breast cancer. Clin Cancer Res. 12:1479–1486. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Hoon M, Shin JW and Carninci P:
Paradigm shifts in genomics through the FANTOM projects. Mamm
Genome. 26:391–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pennisi E: Genomics. ENCODE project writes
eulogy for junk DNA. Science. 337:1159–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Wang W, Cao L, Li Z and Wang X:
Long non-coding RNA CCAT1 acts as a competing endogenous RNA to
regulate cell growth and differentiation in acute myeloid leukemia.
Mol Cells. 39:330–336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo X and Hua Y: CCAT1: An oncogenic long
noncoding RNA in human cancers. J Cancer Res Clin Oncol.
143:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun M, Gadad SS, Kim DS and Kraus WL:
Discovery, annotation, and functional analysis of long noncoding
RNAs controlling cell-cycle gene expression and proliferation in
breast cancer cells. Mol Cell. 59:698–711. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X,
Lin L, Yao H, Su F, Li D, et al: A cytoplasmic NF-κB interacting
long noncoding RNA blocks IκB phosphorylation and suppresses breast
cancer metastasis. Cancer Cell. 27:370–381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su JC and Hu XF: Long non-coding RNA
HOXA11-AS promotes cell proliferation and metastasis in human
breast cancer. Mol Med Rep. 16:4887–4894. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Yang J, Qian L and Cao T:
Aberrantly expressed mRNAs and long non-coding RNAs in patients
with invasive ductal breast carcinoma: A pilot study. Mol Med Rep.
11:2185–2190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang R, Wu JC, Zheng LM, Li ZR, Zhou KL,
Zhang ZS, Xu DF and Chen C: Long noncoding RNA RUSC1-AS-N indicates
poor prognosis and increases cell viability in hepatocellular
carcinoma. Eur Rev Med Pharmacol Sci. 22:388–396. 2018.PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Banas T, Juszczyk G, Pitynski K,
Nieweglowska D, Ludwin A and Czerw A: Incidence and mortality rates
in breast, corpus uteri, and ovarian cancers in Poland (1980–2013):
An analysis of population-based data in relation to socioeconomic
changes. Onco Targets Ther. 9:5521–5530. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meissner HI, Klabunde CN, Han PK, Benard
VB and Breen N: Breast cancer screening beliefs, recommendations
and practices: Primary care physicians in the United States.
Cancer. 117:3101–3111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee EY and Muller WJ: Oncogenes and tumor
suppressor genes. Cold Spring Harb Perspect Biol. 2:a0032362010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun H, Wang G, Peng Y, Zeng Y, Zhu QN, Li
TL, Cai JQ, Zhou HH and Zhu YS: H19 lncRNA mediates
17β-estradiol-induced cell proliferation in MCF-7 breast cancer
cells. Oncol Rep. 33:3045–3052. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arase M, Horiguchi K, Ehata S, Morikawa M,
Tsutsumi S, Aburatani H, Miyazono K and Koinuma D: Transforming
growth factor-β-induced lncRNA-Smad7 inhibits apoptosis of mouse
breast cancer JygMC(A) cells. Cancer Sci. 105:974–982. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu P, Chu J, Wu Y, Sun L, Lv X, Zhu Y, Li
J, Guo Q, Gong C, Liu B and Su S: NBAT1 suppresses breast cancer
metastasis by regulating DKK1 via PRC2. Oncotarget. 6:32410–32425.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu Y, Ginther C, Kim J, Mosher N, Chung S,
Slamon D and Vadgama JV: Expression of Wnt3 activates Wnt/β-catenin
pathway and promotes EMT-like phenotype in trastuzumab-resistant
HER2-overexpressing breast cancer cells. Mol Cancer Res.
10:1597–1606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao Z, Lu P, Zhang H, Xu H, Gao N, Li M
and Liu C: Nestin positively regulates the Wnt/β-catenin pathway
and the proliferation, survival and invasiveness of breast cancer
stem cells. Breast Cancer Res. 16:4082014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu Z and Xie L: LHX6 inhibits breast
cancer cell proliferation and invasion via repression of the
Wnt/β-catenin signaling pathway. Mol Med Rep. 12:4634–4639. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Proffitt KD, Madan B, Ke Z, Pendharkar V,
Ding L, Lee MA, Hannoush RN and Virshup DM: Pharmacological
inhibition of the Wnt acyltransferase PORCN prevents growth of
WNT-driven mammary cancer. Cancer Res. 73:502–507. 2013. View Article : Google Scholar : PubMed/NCBI
|