Introduction
Ankylosing spondylitis (AS), a common
spondyloarthropathy, is an inflammatory rheumatic disease with a
predilection for the axial skeleton. Clinical hallmarks of AS
include sacroiliitis, uveitis, enthesitis and persistent spinal
inflammation. The diagnosis of AS is usually difficult as the
pathogenic mechanism of development and maintenance of the disease
remains poorly understood (1,2).
However, it is considered to be immune-mediated and to have a
marked genetic association with the class I human leukocyte antigen
allotype HLA-B27 (3). Previous
studies have suggested that sensations of hip stiffness are usually
due to issues with the hip ligaments (4,5),
while the most common type of ligament tissue cells involved are
fibroblasts. Fibroblasts derived from mesenchymal stem cells (MSC)
with osteogenic properties are involved in ligament ectopic
ossification, the origin of ligament ossification dynamic cells
(6–9). However, the specific mechanisms
involved in AS development have not been elucidated.
C-X-C chemokine receptor type 4 (CXCR4) is a member
of the CXC chemokine factor family and stromal cell-derived
factor-1 (SDF-1) the specific receptor which consists of an even
transmembrane structure at the cell surface. The SDF-1/CXCR4
signaling axis serves an important role in several biological
processes; it is involved in tumor cells, in particular cell
transformation, migration and homing, in embryonic development,
immune regulation, and is the primary regulator of tumorigenesis
(10). In addition, the increase
level of CXCR4 in human fetal MSCs was demonstrated to improve the
success rate and the mechanical strength of bone allografts in rat
models of osteogenesis. SDF-1 is a member of the CXC family, also
known as C-X-C motif chemokine ligand 12, and has been identified
to be expressed by a number of different cell types (11–13).
Previous studies have demonstrated the role of SDF-l in the
chemotaxis of stem cells, progenitor cells, and organ-specific
homing through its interaction with CXCR4 (14,15).
Furthermore, the interaction between SDF-1 and CXCR4 may serve a
crucial role during embryogenesis in cardiogenesis, hematopoiesis,
vascular development and cerebellar development (16). Expression of the SDF-1/CXCR4
pathway in mature osteoblasts also results in feedback inhibition
of osteoclast pool size, thereby affecting the homeostasis of bone
formation and resorption (17).
Expression of SDF1 was demonstrated to be induced in a mouse model
of periosteum injury, through the SDF1/CXCR4 pathway during the
recruitment of MSCs to the injury site within the bone cells
involved in cartilage repair (18).
Therefore, in the present study, the differences in
the expression levels of CXCR4 between AS and control hip synovial
tissues was firstly examined, and whether blocking CXCR4 was able
to alleviate osteogenesis was then explored. Subsequently, the
function of differentially expressed proteins was investigated, in
an attempt to clarify the pathogenesis of AS.
Materials and methods
Tissues samples and primary culture of
hip capsule fibroblasts
The present study was approved by the Ethics
Committee of Second Military Medical University (Shanghai, China).
All samples were obtained from patients with AS (male:female=11:2,
n=13) and patients with aseptic necrosis of the femoral head
(control; n=8). All of the patients underwent total hip
arthroplasty at Changhai Hospital (Shanghai, China) between January
2017 and December 2017. Patients with AS were diagnosed according
to the Modified New York Criteria (1984) (19). The age of patients
with AS ranged from 8 to 20 years, with an mean age of 42.67 years.
Ligaments were acquired from the femoral neck during surgery. None
of the patients had taken anti-osteoporosis drugs, glucocorticoids,
anti-tumor necrosis factor-drugs or NSAIDS at least 2 months prior
to surgery. All patients agreed to participate and provided written
informed consent. Fibroblasts were isolated from ligament tissues
using the following method: The ligament tissue was washed twice
with Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and finely minced. The minced
tissue was incubated with 5 ml DMEM containing 1 mg/ml collagenase
type II (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for
6 h, then with 5 ml 0.25% trypsin at 37°C for 30 min, filtered
through a nylon mesh of 75 µm and then washed three times. The
single cell suspension was cultured in DMEM supplemented with 10%
fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml) and streptomycin (100 µg/ml) in a humidified
5% CO2 atmosphere at 37°C overnight. Subsequently,
non-adherent cells were removed, and adherent cells were
re-cultured in DMEM plus 10% FBS. Confluent cells were trypsinized
with 0.05% trypsin (Thermo Fisher Scientific, Inc.) and
re-cultured. All ligament fibroblasts from the third passage were
used for all subsequent experiments, as these cells were more
purified compared with the first and second passages, and more
similar to cells in vivo. Fibroblasts were identified by
flow cytometry with CD90-FITC from GeneTex (GeneTex, Inc., Irvine,
CA, USA) using a BD FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Western blot analysis
Hip tissues in the AS and control groups were lysed
in ice-cold lysis buffer [1% Triton X-100, 20 mmol/l Tris-HCl
(PH=8.0), 137 mmol/l NaCl, 10% glycerol (v/v), 2 mmol/l EDTA, 1
mmol/l phenylmethysulfonyl fluoride, 10 µg/ml leupeptin, 50 µg/ml
trypsin inhibitor, 1 mmol/l sodium orthovanadate] for 15 min. The
amount of protein was determined using the BCA protein assay kit
(Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein
(100 µg) was loaded onto an 8% SDS-PAGE gel and transferred onto
polyvinylidenedifluoride (PVDF) membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Membranes were blocked for 1 h at room
temperature in 5% skimmed milk, washed with TBST (150 mM NaCl, 10
mM Tris pH 7.4, 0.1% Tween-20). Primary antibodies used included:
Rabbit polyclonal CXCR4 (cat. no. sc-374159; 1:400; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA;), polyclonal β-catenin (cat.
no. sc-316059; 1:1,000; Santa Cruz Biotechnology, Inc.), polyclonal
p-β-catenin (cat. no. sc-316098; 1:500; Santa Cruz Biotechnology,
Inc.), or polyclonal GAPDH (cat. no. ab8245; 1:500; Abcam,
Cambridge, MA, USA). The antigen-antibody complexes were detected
using peroxidase-labeled goat anti-rabbit antibodies (cat. no.
ab95071; 1:800; Abcam) in TBS-T containing 2.5% non-fat dry milk
for 1 h at room temperature. The results were visualized using the
enhanced chemiluminescence Plus detection system (Abcam, Cambridge,
MA, USA), and band areas/intensities for all proteins were measured
using Image J software (version 1.8.0; National Institutes of
Health, Bethesda, MD, USA).
Immunohistochemistry
All Immunohistochemistry (IHC) analyses was
performed on formalin-fixed and paraffin-embedded samples. Paraffin
blocks were sectioned to 4-µm thickness. Then, poly-L-lysine-coated
slides were used to promote adhesion of the paraffin-section to the
slides. The sections were dewaxed, rehydrated, and
antigen-repaired. For dewaxing, the sections were baked in an
incubator at 60°C for 2 h, followed by cooling to room temperature
and soaking in xylene for 10 min 3 times. For rehydrating, the
sections were put into ethanol (I) for 5 min, ethanol (II) for 5
min, 95% ethanol solution for 5 min, 80% ethanol solution for 5
min, 70% ethanol solution for 5 min, 50% ethanol solution for 5
min, and ddH2O for 5 min successively. The sectioned
tissue slides were incubated with the primary polyclonal rabbit
antibodies: Polyclonal rabbit CXCR4 (cat. no. sc-374159; 1:400;
Santa Cruz Biotechnology, Inc.) for 18 h at 4°C in a humidity
chamber. Biotinylated secondary antibody anti-rabbit IgG (cat. no.
ab80948; 1:2,000, Abcam) was incubated with the sections for 30 min
at 37°C, then incubated with extra-avidin peroxidase for 30 min at
37°C. Images were captured under Nikon Eclipse (E600) fluorescent
microscope (Nikon Corporation, Tokyo, Japan) at magnification ×400,
and then analyzed using Image-Pro Plus software (version 4.5.1;
Media Cybernetics, Rockville, MD, USA).
Fibroblast verification
Flow cytometry was used for fibroblast verification.
The CD90 marker was used to identify fibroblasts, as described
previously (20–22). In brief, third-generation
fibroblasts were collected, and a suspension with a density of
1×105 cells/ml was prepared. Next, 12 ml trypsin/EDTA
solution was added to the 75 cm3 cell tubes with
1×106 cells/ml in each tube. A total of 20 µl
fluorescein isothiocyanate-labeled mouse anti-human CD90 monoclonal
antibody (cat. no. 11-0903-82; 1:1,000; Thermo Fisher Scientific,
Inc.) was added, followed by incubation on ice for 20 min in the
dark. Tubes without antibodies were used as the negative control.
PBS (2 ml) was added to each tube and centrifuged at 400 × g for 6
min at 25°C. Cells were washed twice with 0.5 ml PBS. Flow
cytometry was performed using BD FACSCalibur version 4.1 (BD
Biosciences). Fibroblasts were plated into chamber slides and fixed
with 3% formaldehyde, and then blocked with 5% BSA and incubated
with anti-Vimentin (cat. no. ab20346; 1:2,000; Abcam,) overnight at
4°C. Following incubation with the primary antibodies, cells were
washed three times in PBS followed by 60 min of incubation at room
temperature with anti-rabbit FITC (cat. no. Sfk12258; 1:1,000;
Shanghai Shifeng Biological Technology, Co., Ltd., Shanghai,
China,). Fluorescence was analyzed using the fluorescence
microscopy (Nikon Corporation).
Cell proliferation assay
Cell proliferation was assessed using an MTS assay.
Aliquots (10 µl) of MTS at a concentration of 0.1 mg/ml from the
MTS cell proliferation assay kit (Promega Corporation, Madison, WI,
USA) were added to the cell (1×104) cultures. After 4 h
at 37°C, the absorbance was measured at 490 nm with a
spectrophotometer.
Osteogenesis induction
The fibroblasts were digested with 0.25% trypsin and
cultured at 37°C in a humidified atmosphere containing 5%
CO2. Following attachment of the cells to the surface of
the wells, osteogenic induction medium (OM) containing DMEM/F12
with 100 nM dexamethasone, 50 mg/l ascorbic acid, 10 mM β-sodium
glycerophosphate and 10% FBS was added. The medium contained
mycillin and was changed every 2–3 days.
Cell proliferation assay following
incubation with AMD 3100 or SDF-1, and osteogenesis induction
Following the induction of osteogenesis, AMD 3100
(10 µM) or SDF-1 (10 ng/ml) was added to the fibroblasts. Cell
proliferation was then assessed using the MTS assay as
aforementioned.
Small interference RNA (siRNA)
transfection assay
For in vitro knockdown studies, siRNA
targeting rat CXCR4 (Invitrogen; Thermo Fisher Scientific, Inc.)
were used. Control cultures were transfected with scramble siRNA
(Invitrogen; Thermo Fisher Scientific, Inc.). Fibroblasts were
seeded in a 6-cm dish at a density of 5×105 cells/dish
and prepared for transfection with 1 µg CXCR4 siRNA or control
siRNA. siRNA was added to Opti-MEM with Lipofectamine®
2000 RNAi MAX (Invitrogen; Thermo Fisher Scientific, Inc.) for
transfection according to the manufacturer's protocol. A total of 8
h following incubation, the medium was changed into fresh EBM-2
medium containing 15% FBS. Cells were harvested at 48 h following
transfection of siRNA. The sequences of the CXCR4 siRNA used were
as follows: 5′-GACUGGUACUUUGGGAAAUTT-3′ and
3′-AUUUCCCAAAGUACCAGUCTT-5′. The scrambled sequence was
5′-GUAGCAGGGCAUGUAUUUATT-3′ and 3′-UAAAUACAUGCCCUGCUACTT-5′, and
was designed as a negative control. The efficiencies of CXCR4 siRNA
and control siRNA were monitored by western blot analysis, as
aforementioned. The fibroblasts were divided into two groups: i)
The AS+OM group, containing osteogenesis-induced fibroblasts; and
ii) AS+OM + si-CXCR4 group, containing osteogenesis-induced
fibroblasts treated with si-CXCR4.
Western blot analysis following
incubation with AMD 3100 or SDF-1, and osteogenesis induction
The cells were washed with ice-cold PBS and lysates
were prepared in radioimmunoprecipitation assay lysis buffer [50 mM
Tris-Cl pH 7.4, 150 mM NaCl, 0.25% sodium deoxycholate, 1% NP-40,
100 mg/ml PMSF Protease Inhibitor Cocktail (Roche Diagnostics,
Basel, Switzerland)]. Lysates were passed through a 1 ml needle
syringe to facilitate the disruption of the cell membranes and
centrifuged at 10,000 × g for 15 min at 4°C, and supernatants were
collected. Protein concentrations of lysates were determined by
PierceH BCA Protein Assay kit (Pierce; Thermo Fisher Scientific,
Inc.). Protein (100 µg) was loaded onto a 10% SDS-PAGE gel and
transferred onto polyvinylidene difluoride membranes (Merck KGaA).
Membranes were then blocked for 1 h at room temperature in 5% skim
milk, washed with TBST (150 mM NaCl, 10 mM Tris pH 7.4, 0.1%
Tween-20). Primary antibodies used included CXCR4 (cat. no.
sc-374159; 1:800; Santa Cruz Biotechnology, Inc.), β-catenin (cat.
no. sc-7963; 1:1,000; Santa Cruz Biotechnology, Inc.;),
phosphorylated (p)-β-catenin (cat. no. sc-316059; 1:700; Santa Cruz
Biotechnology, Inc.), glycogen synthase kinase 3b (cat. no.,
sc-11757; GSK3b; 1:500; Santa Cruz Biotechnology, Inc.), frizzled-7
(FZD7; cat. no. sc-31061; 1:500; Santa Cruz Biotechnology, Inc.),
alkaline phosphatase (ALP; cat. no., sc-214129; 1:1,000; Santa Cruz
Biotechnology, Inc.), osteocalcin (OCN; cat. no., sc-139134;
1:1,500; Santa Cruz Biotechnology, Inc.), v-myc avian
myelocytomatosis viral oncogene homolog (c-myc; cat. no.,
sc-261714; 1:1,000; Santa Cruz Biotechnology, Inc.) and cyclin-D1
(cat. no., sc-426371; 1:1,000; Santa Cruz Biotechnology, Inc.).
Membranes were washed three times in TBST, incubated with
horseradish peroxidase-conjugated secondary antibody (HRP; ab6721;
1:1,000; Abcam) at 1:5,000 in 5% skim milk for 1 h at room
temperature, washed and then processed with ECL Plus Western
Blotting detection kit (Amersham Biosciences; GE Healthcare,
Chicago, IL, USA). The filter was then incubated with the substrate
and exposed to X-ray films (Thermo Fisher Scientific, Inc.), and
analyzed using Image J software (version 1.8.0; National Institutes
of Health, Bethesda, MD, USA).
Alizarin red staining
As described previously (23), cultured cells were stained with
0.1% alizarin red (Thermo Fisher Scientific, Inc.) for 30 min at
37°C. Each well washed twice with PBS, then fixed in 10% formalin
for 15 min at room temperature. Then, the wells were rinsed with
distilled water, and 1 ml alizarin red S was added to each well (40
mM; pH 4.1). The 24-well plate was then placed on a shaker and
incubated at room temperature for 20 min. Then, 4 ml distilled
water was added to each well and rinsed for 5 min, and this step
was repeated 4 times.
Statistical analysis
Statistical analysis was performed using SPSS
version 18.0 software (SPSS Inc., Chicago, IL, USA). The data are
presented as mean ± standard error of the mean, and differences
between groups were analyzed by two way analysis of variance.
Post-hoc pairwise comparisons were performed using the Bonferroni
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Upregulation of CXCR4 in AS ligament
tissue
The basic clinical data of the two groups are
summarized in Tables I and
II; there were no significant
differences between the two groups in terms of sex and age. The
expression of CXCR4 in AS and control ligament tissues was then
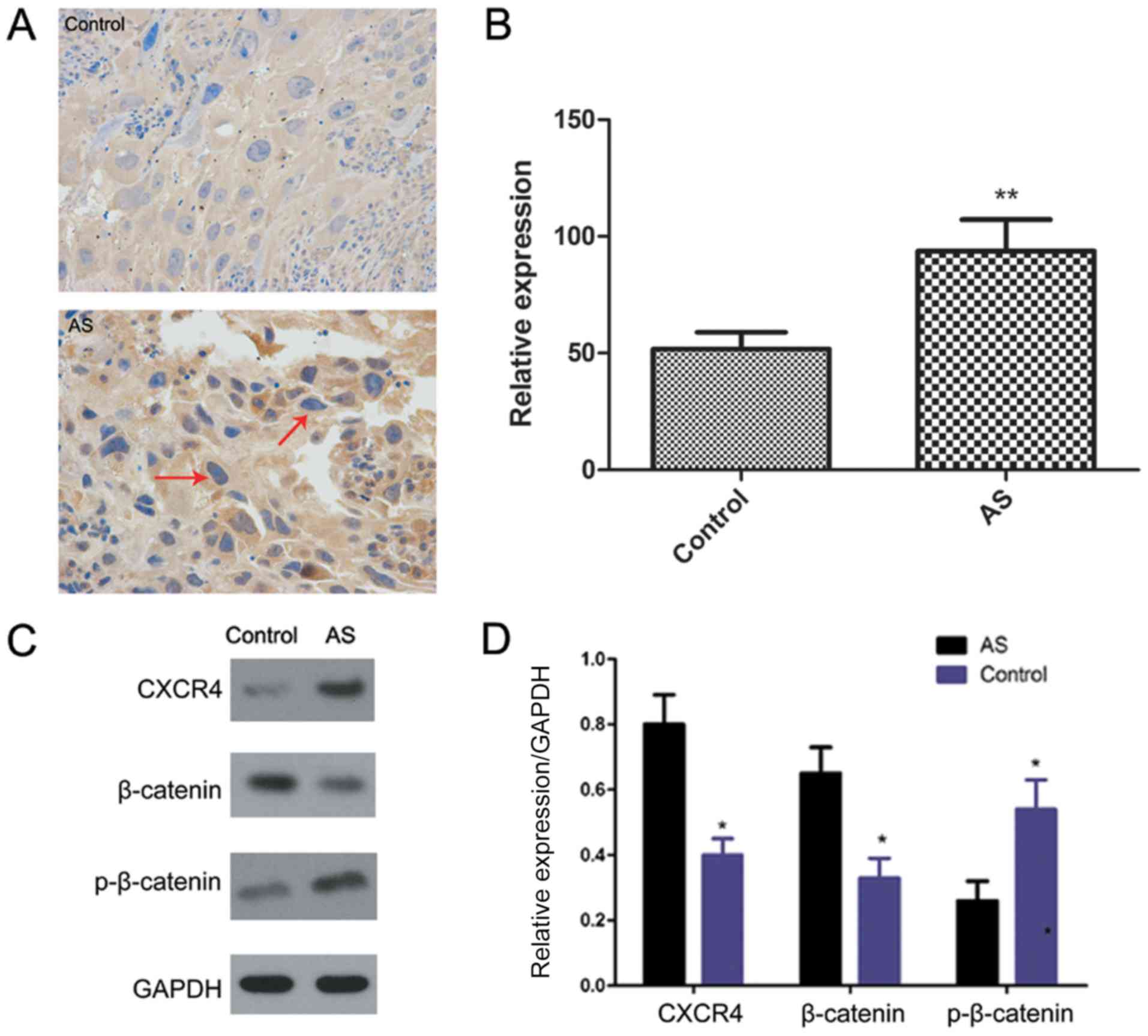
investigated. Immunohistochemistry (Fig. 1A and B) and western blot analysis
(Fig. 1C and D) indicated that the
expression of CXCR4 was upregulated in AS tissues compared to
control tissues (P<0.01).
 | Table I.Basic clinical data for patients with
AS. |
Table I.
Basic clinical data for patients with
AS.
| Patient no. | Age, y | Sex, M/F | Duration of
disease, y | HLA-B27 | NSAIDs used |
|---|
| 1 | 29 | M | 8 | + | No |
| 2 | 30 | M | 10 | + | No |
| 3 | 27 | M | 7 | + | No |
| 4 | 30 | M | 8 | + | No |
| 5 | 33 | M | 6 | + | No |
| 6 | 28 | M | 8 | + | No |
| 7 | 33 | M | 7 | + | No |
| 8 | 29 | M | 9 | + | No |
| 9 | 52 | M | 30 | + | No |
| 10 | 56 | M | 23 | + | No |
| 11 | 54 | F | 20 | + | No |
| 12 | 77 | F | 33 | + | No |
| 13 | 65 | M | 31 | + | No |
 | Table II.Basic clinical data for the control
group. |
Table II.
Basic clinical data for the control
group.
| Patient no. | Age, y | Sex, M/F | Diagnosis |
|---|
| 1 | 54 | M | NFH |
| 2 | 61 | M | NFH |
| 3 | 58 | F | NFH |
| 4 | 27 | M | NFH |
| 5 | 48 | M | NFH |
| 6 | 49 | M | NFH |
| 7 | 52 | F | NFH |
| 8 | 44 | M | NFH |
Upregulation of CXCR4 in AS
fibroblasts
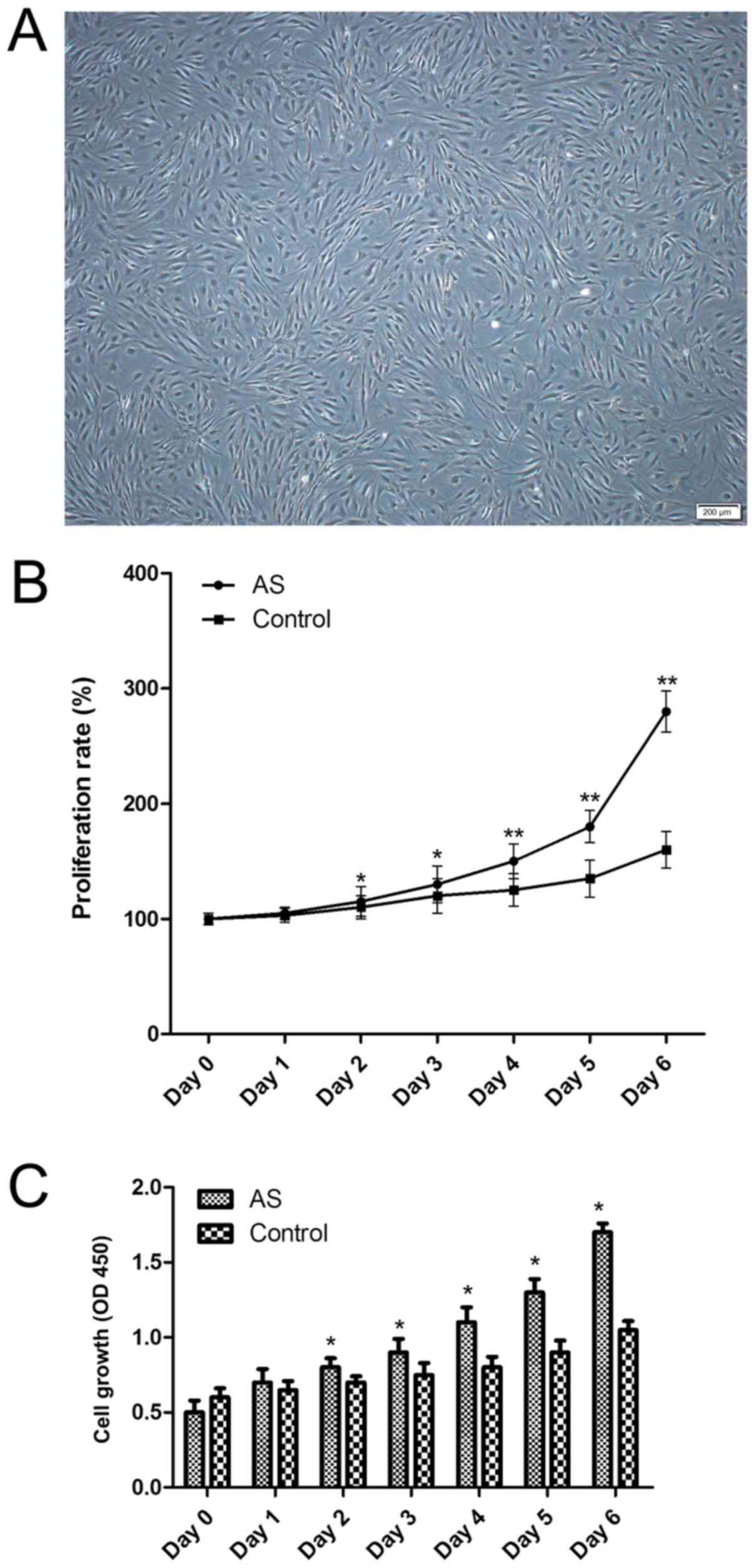
Primary fibroblasts were successfully cultured.
Following cell sorting for fibroblasts, fibroblasts morphology was
determined by vimentin immunocytochemistry (Fig. 2). To determine whether the
fibroblasts in patients with AS were different from those in the
control group, cell proliferation assays in the two fibroblasts
groups were conducted. It was identified that AS fibroblasts
exhibited an increased growth rate compared with the control group,
as determined by the growth curve.
Blocking of CXCR4 inhibits fibroblast
proliferation and osteogenic ability
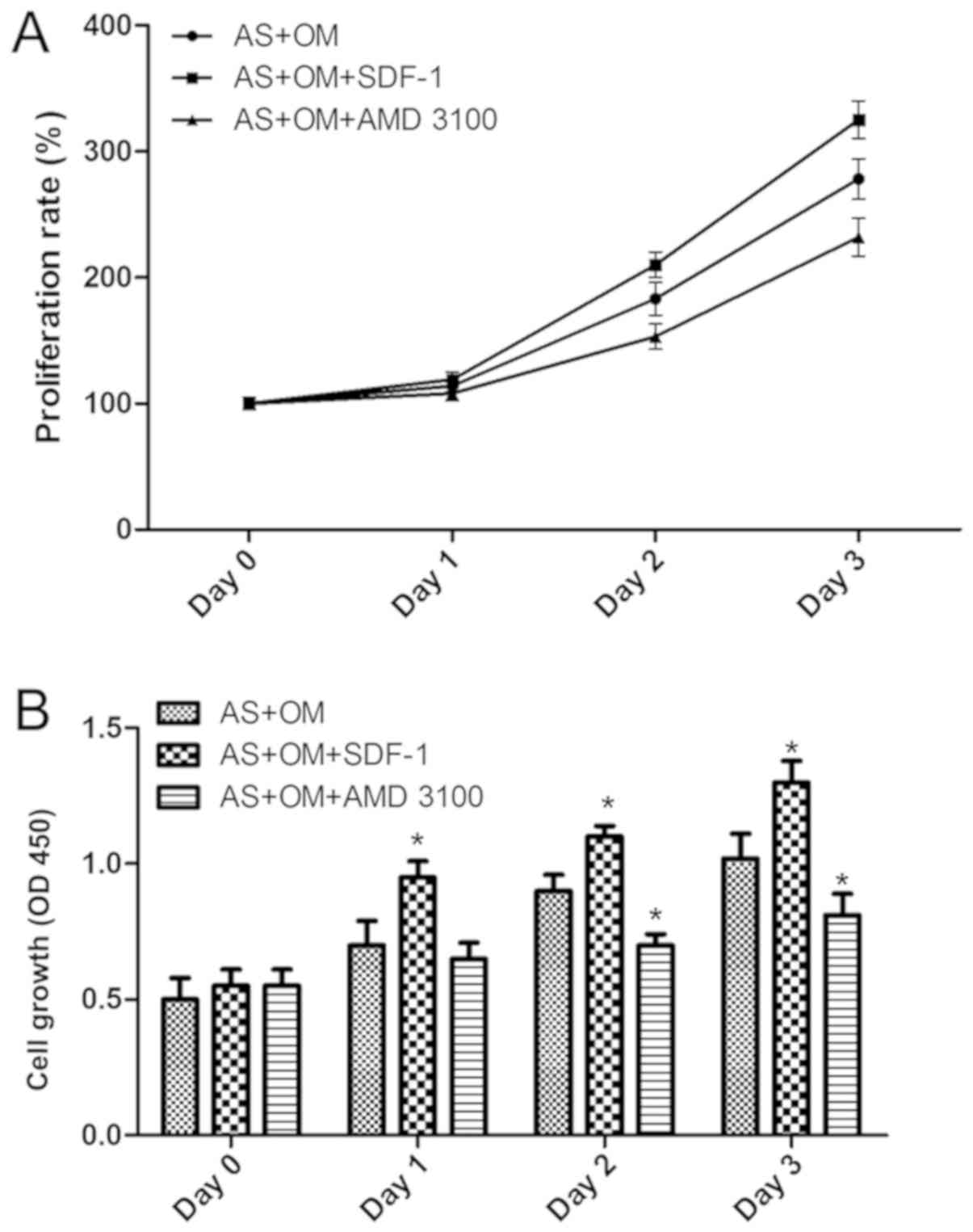
The role of CXCR4 in the proliferation and
osteogenesis of AS fibroblasts was examined by inhibiting CXCR4.
Flow cytometric analysis indicated that the inhibition of CXCR4
through the addition of AMD 3100 led to a decrease in the
proliferation of AS fibroblasts (Fig.
3). However, the proliferation of AS fibroblasts was
significantly increased following the addition of SDF-1 (Fig. 3). Therefore, these data suggest
that CXCR4 is involved in the proliferation of AS fibroblasts.
Next, it was identified that the AMD 3100
significantly suppressed the expression of CXCR4, while SDF-1
increased the level of CXCR4 in fibroblasts. Then, the
downregulation of β-catenin, GSK3b, FZD7, c-myc, cyclin D1 and the
osteogenesis markers ALP and OCN, and the upregulation of
p-β-catenin were observed in fibroblasts following the blocking of
CXCR4 using AMD 3100 (Fig. 4).
Conversely, SDF-1 in fibroblasts led to the opposite results.
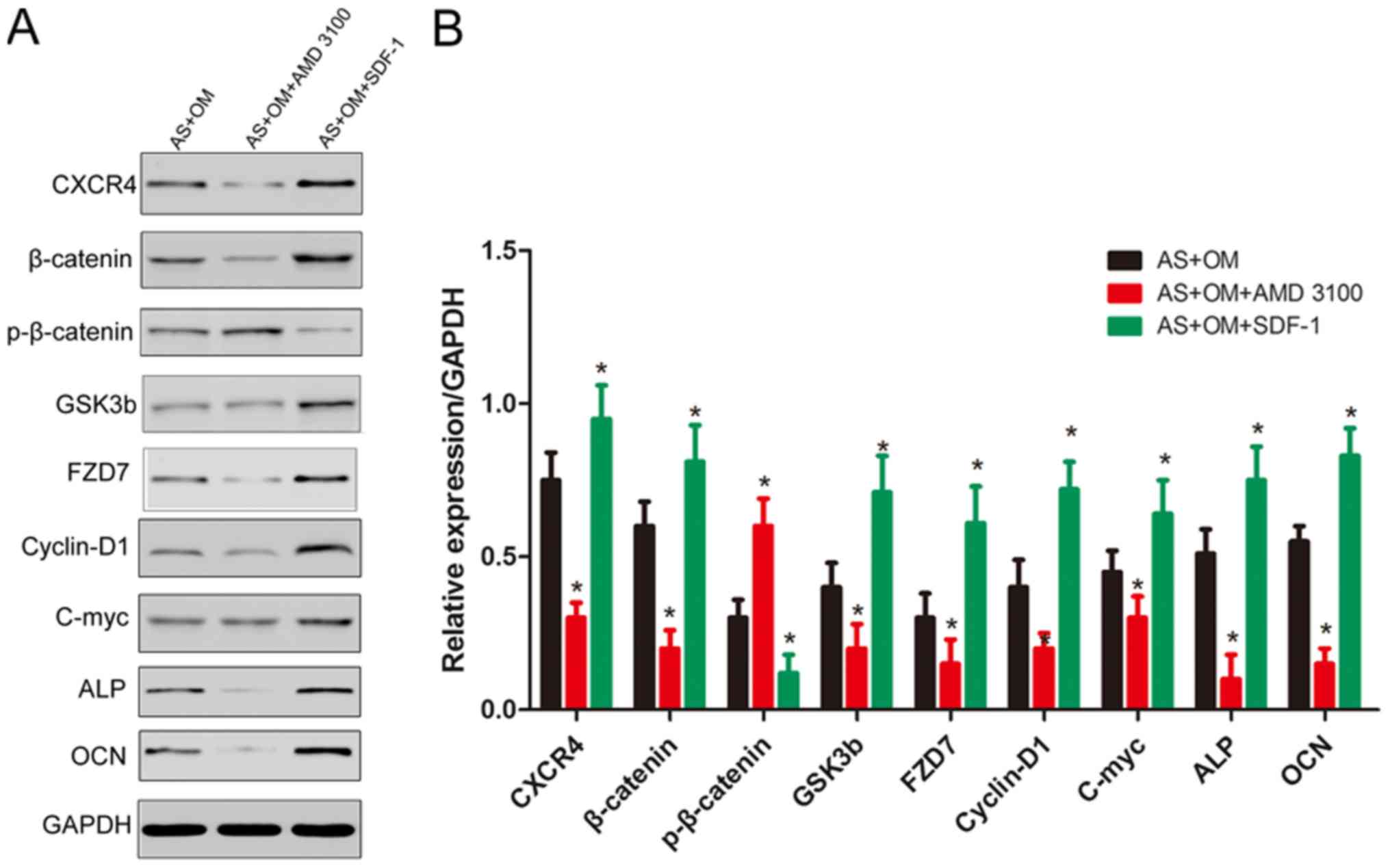 | Figure 4.Western blot analysis of the
expression levels of β-catenin, p-β-catenin, c-myc, cyclin D1,
GSK3b, FZD7, ALP and OCN following the addition of AMD 3100/SDF-1
and osteogenesis induction. (A) The data represent the mean value
from three independent experiments with 10 samples each. (B) Bar
graph demonstrates the results of quantification using Image J
software. *P<0.05 vs. AS+OM. AS, ankylosing spondylitis; OM,
osteogenic induction medium; SDF-1, stromal cell-derived factor-1;
CXCR4, C-X-C chemokine receptor type 4; p, phosphorylated; GSK3b,
glycogen synthase kinase 3b; FZD7, frizzled-7; c-myc, v-myc avian
myelocytomatosis viral oncogene homolog; ALP, alkaline phosphatase;
OCN, osteocalcin. |
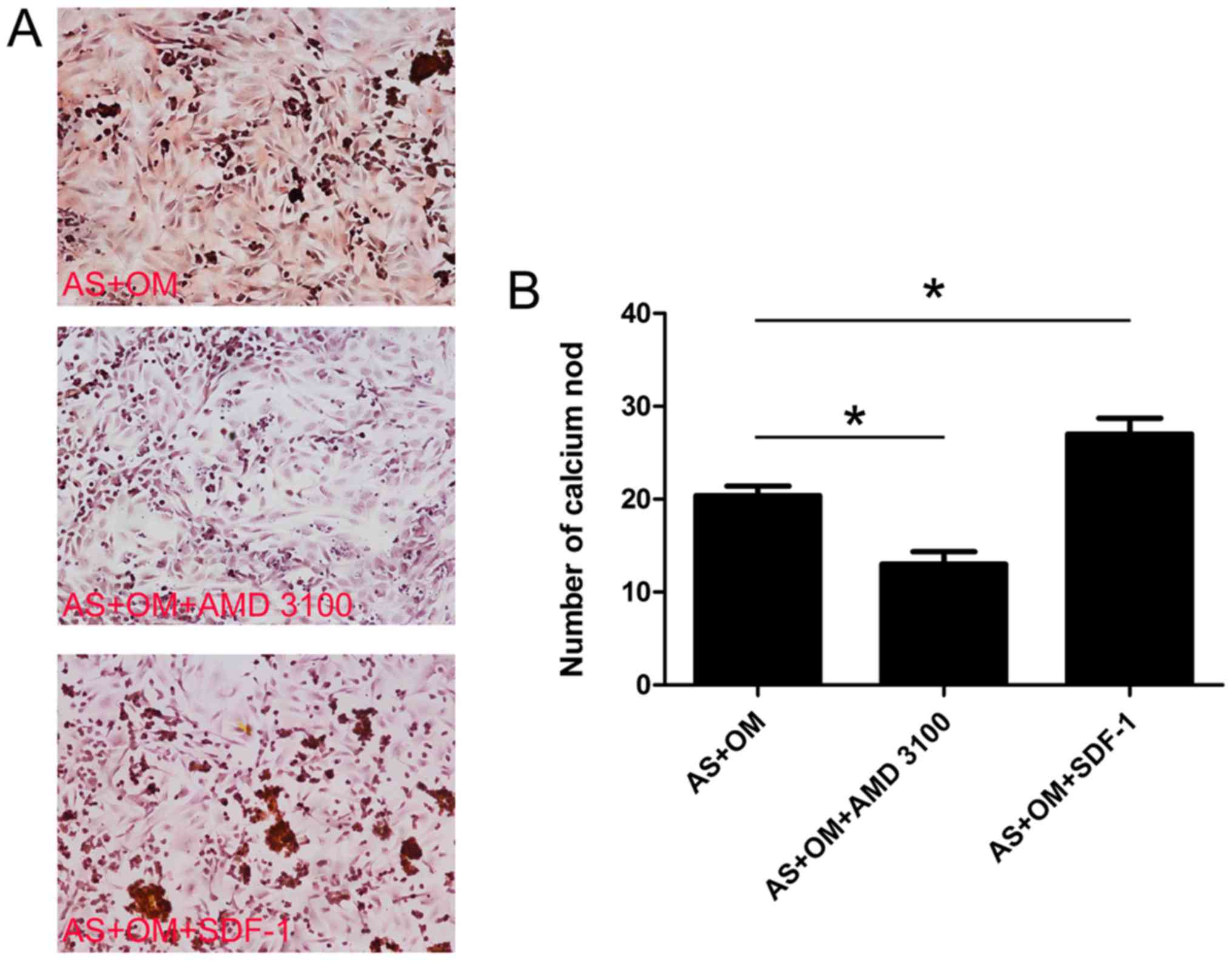
Alizarin red staining
Alizarin red staining for calcium revealed
significantly decreased levels of mineralization following the
addition of AMD 3100 (Fig. 5). By
contrast, the amount of mineralization indicated a distinctive
increasing trend following the addition of SDF-1 compared with that
observed in the AS+OM and AS+OM+AMD 3100 groups.
CXCR4 promotes osteogenic and
mineralization abilities through the Wnt pathway
Alizarin red staining was performed to investigate
the mineralization ability of CXCR4. As indicated in Fig. 6A and B, the level of mineralization
was markedly decreased following preincubation with CXCR4 siRNA
(P<0.05). It was then identified that the si-CXCR4 significantly
suppressed the expression of CXCR4, while SDF-1 increased the level
of CXCR4 in fibroblasts (Fig. 6C and
D). Then, the downregulation of β-catenin, GSK3b, FZD7, c-myc,
cyclin D1 and the osteogenesis markers ALP and OCN, and
upregulation of phosphorylated β-catenin were observed in
fibroblasts following the silencing of CXCR4. Collectively, these
data suggested that CXCR4 promoted the functions of osteogenesis
and mineralization formation in cultured fibroblasts.
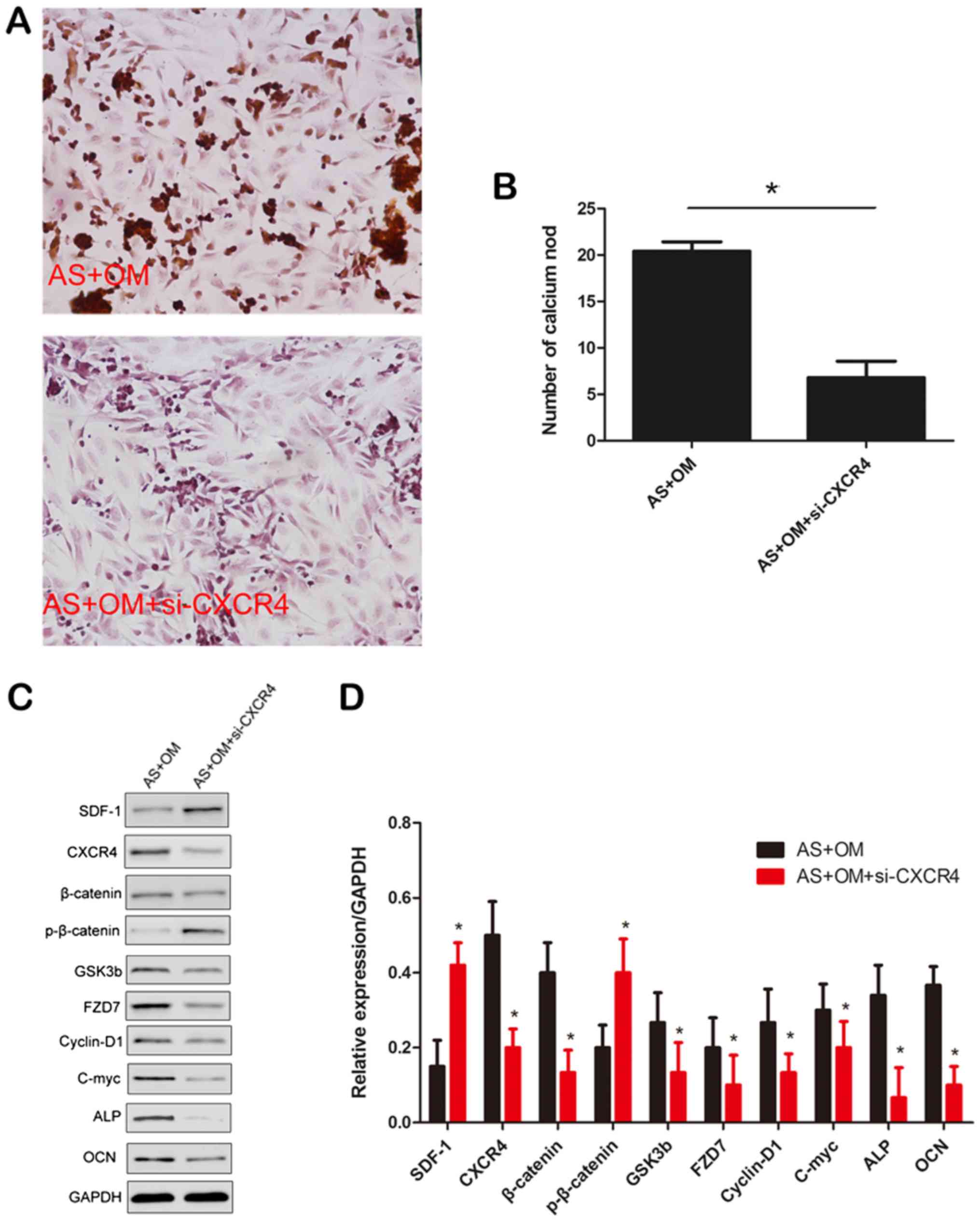 | Figure 6.Effects of CXCR4 on osteogenic and
mineralization of fibroblasts. (A) Representative images of
mineralization formation (magnification, ×400). (B) Quantification
of mineralization formation level. *P<0.05. (C) Western blot
analysis of the expression levels of β-catenin, p-β-catenin, c-Myc,
GSK3b, FZD7, cyclin D1, ALP and OCN following the silencing if
CXCR4. (D) Densitometric analysis of the western blot analysis
data. Data are presented as mean ± standard deviation of three
independent experiments. *P<0.05 vs. AS+OM. AS, ankylosing
spondylitis; OM, osteogenic induction medium; si, small interfering
RNA; SDF-1, stromal cell-derived factor-1; CXCR4, C-X-C chemokine
receptor type 4; p, phosphorylated; GSK3b, glycogen synthase kinase
3b; FZD7, frizzled-7; c-myc, v-myc avian myelocytomatosis viral
oncogene homolog; ALP, alkaline phosphatase; OCN, osteocalcin. |
Discussion
In the present study, the role of CXCR4 in AS
fibroblasts proliferation and osteogenesis was examined. To the
best of our knowledge, the present study demonstrated for the first
time that upregulation of CXCR4 led to fibroblast proliferation and
osteogenesis, while CXCR4 inhibition resulted in the opposite
effect, and for the first time demonstrated an increase in CXCR4
levels in tissues obtained from patients with AS.
AS is am autoimmune disease characterized by chronic
inflammation and abnormal ossification. At present, understanding
of its pathogenesis is limited, and therefore the design of
targeted drugs has not been available. The high morbidity rates of
AS are a common cause of the burden to families and society
(24). Musculoskeletal enthesitis
is one of the primary features of the pathogenesis of AS, which
involves ligaments in the joint capsule connected to the bone.
Long-term follow-up imaging studies have demonstrated that these
areas will eventually exhibit ossification (25). A previous study also demonstrated
that in patients with stiff hip ligament ossification, ligament
fibroblasts have been involved (9). Concomitantly, other studies have
indicated that AS ligament fibroblasts exhibit a tendency towards
ossification (26,27).
Fibroblast cells are associated with joint
remodeling, which occurs in rheumatic diseases including AS.
Cluster of differentiation 90 (CD90) is a cell adhesion molecule
and the smallest member of the immunoglobulin superfamily, with a
molecular weight of 25–35 KDa; it has been demonstrated to be
enriched in fibroblasts (28).
CD90 positivity in fibroblast, when measured, has been revealed to
be due to fibroblast contamination of the other cells, which has
also been confirmed visually (29). Previous studies have indicated that
different cell types may be identified based upon CD90 expression
(30,31). There was a loose correlation
between CD90 expression and cuboidal morphology of non-activated
cells (20). An additional
commonly used cell marker for fibroblasts is vimentin, which has a
high level of sensitivity for fibroblast labelling (32). Vimentin was originally used as an
endothelial cell marker. Although vimentin may positively identify
fibroblasts, it also positively identifies macrophages and
endothelial cells. In the present study, CD90 was used as the sole
marker for fibroblasts identification, which may present a
limitation. Additional experiments, including the use CD90 and
vimentin as markers for fibroblasts, will be performed in future
studies.
Previous studies have suggested that the SDF1-CXCR4
signaling axis is involved in a number of biological processes
in vivo (10,33). In the present study, the
experimental data indicated that the expression level of CXCR4 was
upregulated in the AS group compared with controls, indicating AS
was triggered in fibroblasts. It was also identified that the AS
group exhibited an increased level of expression of β-catenin
compared with the control group, which is similar to the results
from a previous study (34),
suggesting that upregulated CXCR4 may be activated through the
Wnt/β-catenin signaling pathway.
Increased growth rate and proliferation have been
observed in AS fibroblasts (35),
which is very similar to the results of the present study,
indicating that ossification in AS is associated not only with
fibroblast osteogenesis but also with proliferation compared with
the controls. Therefore, the inhibition of proliferation of
fibroblasts may be a potential strategy to prevent new bone
formation in AS. In the present study, inhibition of CXCR4 by AMD
3100 significantly inhibited the proliferation in AS
fibroblasts.
Wnt signaling, which is involved in the
differentiation of osteoblasts, has been indicated to direct novel
bone formation in inflammatory arthritis (36–38).
Previous studies have demonstrated that the Wnt/β-catenin signaling
pathway serves an important role in hetero-ossification (39). In the present study, the expression
of β-catenin-dependent transcription effectors was investigated,
and the results provided evidence of an association between the
expression of CXCR4 and changes to Wnt/β-catenin targets. In
addition, the expression of downstream effectors of the Wnt
signaling pathway, including cyclin D1 and c-myc, was downregulated
following the inhibition of CXCR4, suggesting that the inhibition
of CXCR4 may inhibit the proliferation of AS fibroblasts by
targeting c-myc and cyclin D1.
Other osteogenesis markers, including ALP and OCN,
were also examined. ALP and OCN are classic osteogenesis markers
that are highly expressed in ossified tissues (40,41),
which may be attenuated by CXCR4 downregulation. To additionally
assess osteogenic activity, Alizarin red staining was performed to
detect calcium. Decreased mineralization level in the AMD 3100
group was observed. By contrast, the amount of mineralization
demonstrated a marked increasing trend in the SDF-1 group,
suggesting that upregulated CXCR4 increased the level of
mineralization in fibroblasts. However, AMD 3100 also decreased
mineralization in the normal group, suggesting that additional
studies are required for the inhibition of CXCR4 during AS
treatment.
In summary, to the best of our knowledge, the
present study is the first to have cultured AS fibroblasts and
explored the role of CXCR4 in AS hip tissues. It was demonstrated
that downregulated CXCR4 levels inhibited fibroblast proliferation
and osteogenesis via the Wnt/β-catenin signaling pathways in
vitro. Therefore, we hypothesize that CXCR4 may serve an
important role in AS patient bone formation, which agrees with the
conclusions from previous studies (42). Obtaining an improved understanding
of the functions of CXCR4 in AS may provide novel insights of how
downregulating the Wnt signaling pathway may prevent or delay new
bone formation in AS. It should be noted that in the present study,
only 12 patients with AS were included, and only in vitro
experiments was performed to reveal the function of CXCR4.
Therefore, additional in vitro and in vivo
experiments are required to confirm the role of CXCR4 in AS.
Acknowledgements
Not applicable.
Funding
The present study was funding by Innovation project
fund of the Second Military Medical University (grant no. 2017QN04)
and the National Nature Science Foundation of China (grant nos.
81672126 and 81871751).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CH and DL contributed to experimental design,
manuscript writing and experimental techniques. JG performed
specimen collection and experimental techniques. JL performed
specimen collection and provided experimental assistance. ZL and WX
contributed to experimental design.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Second Military Medical University. All patients provided written
informed consent.
Patient consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
AS
|
ankylosing spondylitis
|
|
CXCR4
|
chemokine receptor 4
|
|
SDF1
|
stromal cell-derived factor-1
|
References
|
1
|
Sochart DH and Porter ML: Long-term
results of total hip replacement in young patients who had
ankylosing spondylitis. Eighteen to thirty-year results with
survivorship analysis. J Bone Joint Surg Am. 79:1181–1189. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wordsworth BP and Mowat AG: A review of
100 patients with ankylosing spondylitis with particular reference
to socio-economic effects. Br J Rheumatol. 25:175–180. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang WM and Chiu KY: Primary total hip
arthroplasty in patients with ankylosing spondylitis. J
Arthroplasty. 15:52–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brinker MR, Rosenberg AG, Kull L and Cox
DD: Primary noncemented total hip arthroplasty in patients with
ankylosing spondylitis. Clinical and radiographic results at an
average follow-up period of 6 years. J Arthroplasty. 11:802–812.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Cruyssen B, Vastesaeger N and
Collantes-Estévez E: Hip disease in ankylosing spondylitis. Curr
Opin Rheumatol. 25:448–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang HY, Liu R, Xing YJ, Xu P, Li Y and
Li CJ: Effects of hypoxia on the proliferation, mineralization and
ultrastructure of human periodontal ligament fibroblasts in vitro.
Exp Ther Med. 6:1553–1559. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang HS, Lu XH, Chen DY, Yuan W, Yang LL,
He HL and Chen Y: Upregulated expression of connexin43 in spinal
ligament fibroblasts derived from patients presenting ossification
of the posterior longitudinal ligament. Spine (Phila Pa 1976).
36:2267–2274. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sommar P, Junker JP, Strandenes E, Ness C,
Hansson T, Johnson H and Kratz G: Osteogenically-induced human
dermal fibroblasts as a tool to regenerate bone. J Plast Surg Hand
Surg. 47:8–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu F, Cui Y, Zhou X, Zhang X and Han J:
Osteogenic differentiation of human ligament fibroblasts induced by
conditioned medium of osteoclast-like cells. Biosci Trends.
5:46–51. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones GN, Moschidou D, Lay K, Abdulrazzak
H, Vanleene M, Shefelbine SJ, Polak J, de Coppi P, Fisk NM and
Guillot PV: Upregulating CXCR4 in human fetal mesenchymal stem
cells enhances engraftment and bone mechanics in a mouse model of
osteogenesis imperfecta. Stem Cells Transl Med. 1:70–78. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Fu W, Zhang S, He X, Liu Z, Gao D
and Xu T: CXCR-7 receptor promotes SDF-1α-induced migration of bone
marrow mesenchymal stem cells in the transient cerebral
ischemia/reperfusion rat hippocampus. Brain Res. 1575:78–86. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kioi M, Vogel H, Schultz G, Hoffman RM,
Harsh GR and Brown JM: Inhibition of vasculogenesis, but not
angiogenesis, prevents the recurrence of glioblastoma after
irradiation in mice. J Clin Invest. 120:694–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clift IC, Bamidele AO, Rodriguez-Ramirez
C, Kremer KN and Hedin KE: β-Arrestin1 and distinct CXCR4
structures are required for stromal derived factor-1 to
downregulate CXCR4 cell-surface levels in neuroblastoma. Mol
Pharmacol. 85:542–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Duan B, Cheng Z, Jia X, Mao L, Fu
H, Che Y, Ou L, Liu L and Kong D: SDF-1/CXCR4 axis modulates bone
marrow mesenchymal stem cell apoptosis, migration and cytokine
secretion. Protein Cell. 2:845–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cencioni C, Capogrossi MC and Napolitano
M: The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc
Res. 94:400–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Deng QJ, Xu XF and Ren J: Effects of
SDF-1/CXCR4 on the repair of traumatic brain injury in rats by
mediating bone marrow derived mesenchymal stem cells. Cell Mol
Neurobiol. 38:467–477. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shahnazari M, Chu V, Wronski TJ, Nissenson
RA and Halloran BP: CXCL12/CXCR4 signaling in the osteoblast
regulates the mesenchymal stem cell and osteoclast lineage
populations. FASEB J. 27:3505–3513. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kitaori T, Ito H, Schwarz EM, Tsutsumi R,
Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T and Nakamura T:
Stromal cell-derived factor 1/CXCR4 signaling is critical for the
recruitment of mesenchymal stem cells to the fracture site during
skeletal repair in a mouse model. Arthritis Rheum. 60:813–823.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kisselbach L, Merges M, Bossie A and Boyd
A: CD90 expression on human primary cells and elimination of
contaminating fibroblasts from cell cultures. Cytotechnology.
59:31–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sack U, Hirth A, Funke B, Wiedemeyer K,
Lange F, Tröltzsch M, Tannapfel A, Gebhardt R, Emmrich F and
Lehmann J: A novel model of fibroblast-mediated cartilage
destruction. Scand J Immunol. 61:18–28. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu WD, Yang XY, Li DH, Zheng KD, Qiu PC,
Zhang W, Li CY, Lei KF, Yan GQ, Jin SW and Wang JG: Up-regulation
of fatty acid oxidation in the ligament as a contributing factor of
ankylosing spondylitis: A comparative proteomic study. J
Proteomics. 113:57–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gregory CA, Gunn WG, Peister A and Prockop
DJ: An Alizarin red-based assay of mineralization by adherent cells
in culture: comparison with cetylpyridinium chloride extraction.
Anal Biochem. 329:77–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lories RJ, Derese I, Ceuppens JL and
Luyten FP: Bone morphogenetic proteins 2 and 6, expressed in
arthritic synovium, are regulated by proinflammatory cytokines and
differentially modulate fibroblast-like synoviocyte apoptosis.
Arthritis Rheum. 48:2807–2818. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maksymowych WP, Landewe R, Conner-Spady B,
Dougados M, Mielants H, van der Tempel H, Poole AR, Wang N and van
der Heijde D: Serum matrix metalloproteinase 3 is an independent
predictor of structural damage progression in patients with
ankylosing spondylitis. Arthritis Rheum. 56:1846–1853. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai L, Zhang J, Qian J, Li Q, Li H, Yan Y,
Wei S, Wei J and Su J: The effects of surface bioactivity and
sustained-release of genistein from a mesoporous
magnesium-calcium-silicate/PK composite stimulating cell responses
in vitro, and promoting osteogenesis and enhancing osseointegration
in vivo. Biomater Sci. 6:842–853. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marciano DP, Kuruvilla DS, Boregowda SV,
Asteian A, Hughes TS, Garcia-Ordonez R, Corzo CA, Khan TM, Novick
SJ, Park H, et al: Pharmacological repression of PPARγ promotes
osteogenesis. Nat Commun. 6:74432015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukada S, Yamamoto Y, Segawa M, Sakamoto
K, Nakajima M, Sato M, Morikawa D, Uezumi A, Miyagoe-Suzuki Y,
Takeda S, et al: CD90-positive cells, an additional cell
population, produce laminin alpha2 upon transplantation to
dy(3k)/dy(3k) mice. Exp Cell Res. 314:193–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dakhova O, Ozen M, Creighton CJ, Li R,
Ayala G, Rowley D and Ittmann M: Global gene expression analysis of
reactive stroma in prostate cancer. Clin Cancer Res. 15:3979–3989.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Andrade PZ, da Silva CL, dos Santos F,
Almeida-Porada G and Cabral JM: Initial CD34+ cell-enrichment of
cord blood determines hematopoietic stem/progenitor cell yield upon
ex vivo expansion. J Cell Biochem. 112:1822–1831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim B, Lee B, Kim MK, Gong SP, Park NH,
Chung HH, Kim HS, No JH, Park WY, Park AK, et al: Gene expression
profiles of human subcutaneous and visceral adipose-derived stem
cells. Cell Biochem Funct. 34:563–571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nim HT, Furtado MB, Costa MW, Kitano H,
Rosenthal NA and Boyd SE: CARFMAP: A curated pathway map of cardiac
fibroblasts. PLoS One. 10:e01432742015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar S and Ponnazhagan S: Mobilization of
bone marrow mesenchymal stem cells in vivo augments bone healing in
a mouse model of segmental bone defect. Bone. 50:1012–1018. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang J, Yin Z, Song G, Cui S, Jiang J and
Zhang L: Discriminating value of calprotectin in disease activity
and progression of nonradiographic axial spondyloarthritis and
ankylosing spondylitis. Dis Markers. 2017:75741472017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou YC, Yang XW, Yuan SG, Zhang P, Ye YL
and Li YK: Downregulation of dickkopf-1 enhances the proliferation
and osteogenic potential of fibroblasts isolated from ankylosing
spondylitis patients via the Wnt/β-catenin signaling pathway in
vitro. Connect Tissue Res. 57:200–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kitagaki J, Iwamoto M, Liu JG, Tamamura Y,
Pacifci M and Enomoto-Iwamoto M: Activation of beta-catenin-LEF/TCF
signal pathway in chondrocytes stimulates ectopic endochondral
ossification. Osteoarthritis Cartilage. 11:36–43. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wendling D and Claudepierre P: New bone
formation in axial spondyloarthritis. Joint Bone Spine. 80:454–458.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Li YP, Paulson C, Shao JZ, Zhang
X, Wu M and Chen W: Wnt and the Wnt signaling pathway in bone
development and disease. Front Biosci (Landmark Ed). 19:379–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Zheng Z, Cao Z, Zhuang L, Xu Y, Liu
X, Xu Y and Gong Y: Enhancing proliferation and osteogenic
differentiation of HMSCs on casein/chitosan multilayer films.
Colloids Surf B Biointerfaces. 141:397–407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Tang Y, Zhu X, Tu T, Sui L, Han
Q, Yu L, Meng S, Zheng L, Valverde P, et al: Overexpression of
MiR-335-5p promotes bone formation and regeneration in mice. J Bone
Miner Res. 32:2466–2475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dong Y, Liu H, Zhang X, Xu F, Qin L, Cheng
P, Huang H, Guo F, Yang Q and Chen A: Inhibition of SDF-1α/CXCR4
signalling in subchondral bone attenuates post-traumatic
osteoarthritis. Int J Mol Sci. 17(pii): E9432016. View Article : Google Scholar : PubMed/NCBI
|