Introduction
Tourette syndrome (TS) is a disorder that is
characterized by tics. Tics are best described as normally
voluntary movements that are instead made automatically, and are
therefore not under the control of the individual. It is estimated
that 0.3–0.9% of children worldwide have TS (1). TS affects males more frequently than
females and is often associated with attention deficit
hyperactivity disorder (ADHD) and obsessive-compulsive disorder
(OCD). There is frequently an urge that precedes the tic, sometimes
in the form of a specific sensory feeling (a sensory tic) (2). Patients state that they perform the
tic in order to reduce the urge, although shortly after the tic
manifests, the urge recurs.
The precise aetiological and pathophysiological
mechanisms underlying TS with or without co-morbid conditions
remain unknown. Nevertheless, the symptoms can be aggravated by
psychosocial stress, anxiety, infection, emotional tension and/or
fatigue. There is evidence to show that TS is passed down through
families (3), although the genetic
component or combination of components has not yet been identified
(4,5). Clinical, neuropathological and
neuroimaging studies, as well as autopsy, have suggested that
abnormalities of the basal ganglia and in neurotransmitter function
are associated with TS (6,7). Of the known neurotransmitters,
catecholamines and serotonin (5-HT) may play significant roles in
the development and occurrence of tics (8–10).
Treatments for TS are variable and include pharmacological and
behavioural treatments, surgery and deep brain stimulation, among
other methods (11). Current
commonly used anti-tic drugs include α-adrenergic receptor
agonists, typically neuroleptics and atypical neuroleptics.
However, there is no method that can fully cure TS at present. Due
to the high efficacy of dopamine (DA) D2 receptor (D2-R)
antagonists, including haloperidol (Hal), abnormalities in DA
metabolism have been proposed to be associated with TS (9,12).
Increasingly, nuclear imaging studies and neurochemical assays of
post-mortem brain tissues isolated from patients with TS have
demonstrated dysfunction in the dopaminergic system (13,14).
Meanwhile, the 5-HT system has also been implicated as having an
important role in the pathophysiology of TS (15). Individuals with OCD often respond
to 5-HT re-uptake inhibitors (16), and it has been reported that
dual-acting serotonergic/dopaminergic agents, including
risperidone, help to alleviate the symptoms of TS and OCD (17). Consequently, it may be speculated
that 5-HT contributes to the symptoms of TS.
3,3′-Iminodipropionitrile (IDPN) has frequently been
used to develop animal models of tics, as it can cause persistent
behaviour syndromes that include head shaking, random circling,
hyperactivity and increased acoustic startle responses (18). These stereotyped behaviours are
similar to the symptoms of TS, and are attributed to variations in
processes involving DA, 5-HT and certain other peptides, including
norepinephrine (19). Traditional
Chinese Medicine (TCM) is a practice that has been used for
thousands of years due to its apparent effectiveness and infrequent
side effects. Ningdong granule (NDG), a compound preparation used
in TCM, is applied to alleviate tics, for which it has been
indicated to be effective (20–22).
In the present study, the focus was on the DA and 5-HT systems in
order to evaluate the anti-tic function of NDG in an animal model.
First, IDPN was used to develop a rat model of TS; subsequently,
the rats were divided into four groups, treated with saline, Hal or
NDG. After 8 weeks, micro-positron emission tomography (PET) was
used to evaluate the binding of D2Rs, DA transporters
(DATs), 5-HT2A receptors (5-HT2ARs) and 5-HT
transporters (SERTs) in brain regions of interest (ROIs).
Materials and methods
Drugs and reagents
IDPN was purchased from Sigma-Aldrich (Merck KGaA),
Hal was from Shanghai Pharmaceutical Group Co., Ltd., and isotopic
tracers (radiochemical purity >99%) were synthesized and
provided by the Jiangsu Institute of Atomic Medicine (Wuxi,
China).
Preparation of NDG
The NDG formula includes 10 different Chinese
medicinal herbs (Table I). All of
these formulations were provided and prepared by Tianjiang Medicine
Co., Ltd. After being dried, the ingredients were mixed at the
proportions listed in Table I and
then macerated for 1 h at room temperature in distilled water
(1,000 ml). Subsequently, the mixture was decocted twice for 1 h
each time at 100°C. The filtrates were mixed and condensed and then
dried in a vacuum drier at 60°C until they became granular. The
resulting granules were stored at 4°C.
 | Table I.Composition and active compounds of
Ningdong granule. |
Table I.
Composition and active compounds of
Ningdong granule.
| Components | Voucher specimens
no. | Part used | Amount used (g) |
|---|
| Gastrodia
elata Blume | 1404620 | Root | 9 |
| Uncaria
rhynchophylla (Miq.) Jacks | 1404631 | Ramulus | 15 |
| Buthus
martensii Karsch | 1404676 | Dried body | 3 |
| Scolopendra
subspinipes mutilans L. Koch | 1404674 | Dried body | Single band |
| Fossil fragments | 1405688 | Skeletal fossils | 30 |
| Radix Paeoniae
Alba | 1406089 | Root | 20 |
| Dwarf lilyturf
tuber | 1407051 | Root | 10 |
| Dried human
placenta | 1311055 | Dried placenta | 3 |
| Codonopsis
pilosula | 1403701 | Rhizome | 10 |
| Glycyrrhiza
uralensis Fisch | 1403710 | Rhizome | 3 |
Experimental animals
All experimental procedures were performed in
compliance with the relevant guidelines and regulations of the
American Physiological Society, and the protocols were approved by
the medical ethics committee of The Provincial Hospital Affiliated
to Shandong University.
A total of 24 male Wistar rats (5 weeks old, 150±10
g) were purchased from Shanghai Laboratory Animal Co., Ltd.; the
rats were divided into five cages and housed in an air-conditioned
animal room under a 12 h light/dark cycle (lights on at 06:00 a.m.
and off at 6:00 p.m.). Animals were provided access to water and
food ad libitum, were maintained at a constant temperature
of 22±2°C and a humidity of 50±10%, and were given a 1-week
adaptation period. After this period, the rats were randomly
divided into a control group (n=6) and an experimental group
(n=18). Animals in the control group were intraperitoneally (i.p.)
injected with normal saline (NS; 0.9%, 5 ml/kg); animals in the
experimental group were injected with IDPN (150 mg/kg, i.p.).
Injections for both groups were administered once a day for 7
consecutive days. The experimental rats were further randomly
assigned to three groups: An IDPN+NS group (n=6), an IDPN+Hal group
(n=6) and an IDPN+NDG group (n=6). In these groups, following the
i.p. injection of IDPN, rats were treated by intragastric
administration of NS (0.9%) at 10 ml/kg (IDPN+NS group), Hal at 1.0
mg/kg (IDPN+Hal group) or NDG at 22 g/kg (IDPN+NDG group; 6.25-fold
greater than a clinical dose) once a day for 8 weeks. The
behaviours of the rats were observed by researchers who were
familiar with stereotypical behaviours, but blinded to the
experimental group of each rat, once every 7 days after IDPN and
drug administration. Each animal was observed for 1 min out of
every 5 min over a total of 6 periods. Episodes that were in
accordance with specific behavioural categories (Table II) received the corresponding
score, and an average score was calculated on the basis of the
results from two observers to determine an objective indicator of
behavioural changes.
 | Table II.Scales for stereotypical
behaviours. |
Table II.
Scales for stereotypical
behaviours.
| Score | Stereotypical
behaviours |
|---|
| 0 | Asleep, resting in
place or normal activity in place |
| 1 | Increased sniffing
and head raising |
| 2 | Discontinuous
increased sniffing with body raising |
| 3 | Discontinuous
increased sniffing and/or licking with head and body raising
primarily in one place, with occasional rapid bursts of locomotor
activity (2–5 steps) |
| 4 | Continuous
sniffing, biting and head bobbing and repetitive body raising/wall
climbing in place |
| 5 | Continuous
sniffing, biting, licking and head bobbing, and continuous body
raising/wall climbing wherein forepaws do not touch the cage
floor |
MicroPET scans
In preparation for the scans, the rats were
anaesthetized with 1–1.5% isoflurane and then injected via the tail
vein with 150–250 µl (250±50 µCi) 18F-fallypride (a
compound that binds predominantly to D2-Rs),
18F-FECNT (binds to DATs), 18F-altanserin
(binds to 5-HT2ARs) or 18F-FPBM (binds to
SERTs). After 10 min to allow for radiotracer uptake, the rats were
positioned on a bed in pairs, with the two rats lying in prone
positions, and then placed in a microPET scanner (Focus 220
scanner; Siemens AG). Scans lasted 10 min. Images were
reconstructed using an iterative three-dimensional ordered-subset
expectation maximization algorithm with an image matrix of
128×128×159, resulting in a pixel size of 0.77 mm and a slice
thickness of 0.78 mm. The anatomical ROIs, namely the striatum,
cortex, nucleus accumbens, putamen and amygdala were delineated
with the PMOD software package (version 3.8; PMOD Technologies
LLC), and the uptake of the radiotracers [standard uptake ratio
(SUV)] was calculated using the following formulae: SUV =
Radioactive material ingestion in the area of interest
(µCi/g)/Total injection dose (µCi)/Weight (g).
Statistical analysis
The results are expressed as the mean ± SEM.
Statistical differences between groups were determined by one-way
analysis of variance. The Least Significant Difference test was
used for the comparison of parameters between different groups. All
data were analysed with the SPSS statistical software package
(version 17.0; SPSS Inc.), and P<0.05 was considered to indicate
a statistically significant difference.
Results
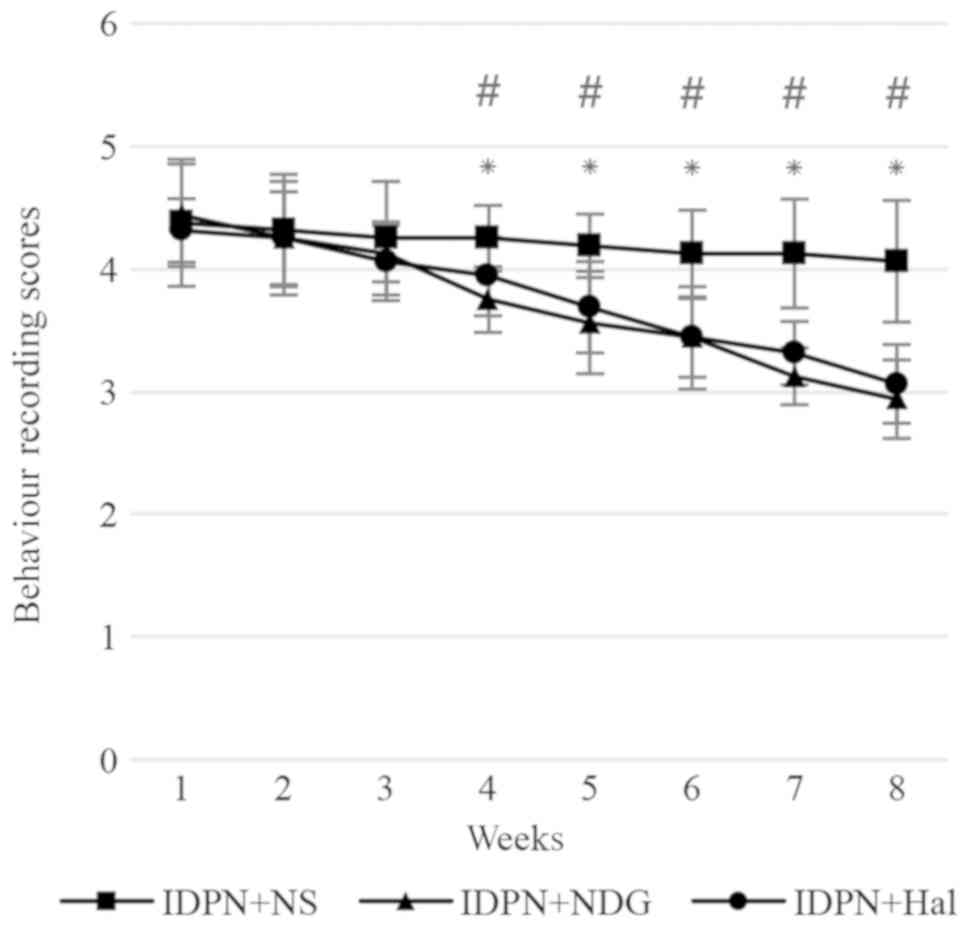
Behavioural evaluation
As indicated in Fig.
1, IDPN caused abnormal, stereotypical behaviours in rats.
After 8 weeks of treatment, these abnormal behaviours were
significantly reduced in the IDPN plus NDG and IDPN plus Hal groups
compared with the IDPN plus NS group (P<0.05). There was no
statistically significant difference in the behaviours of rats
between the NDG and Hal groups.
MicroPET imaging
D2R
In the striatum, IDPN increased the level of
D2R binding compared with that in the control group
(P<0.05). Following treatment, NDG or Hal significantly reduced
the upregulation in striatal D2R content (P<0.01). No
significant difference was observed between the NDG and Hal groups
(Fig. 2).
DAT
The DAT content in the striatum was higher in the
IDPN group than in the control group (P<0.05). Following
treatment with NDG, the DAT content in the striatum was increased
(IDPN+NDG group vs. IDPN+NS group, P<0.05). However, no
significant difference was determined in the Hal group compared
with the IDPN group (Fig. 3).
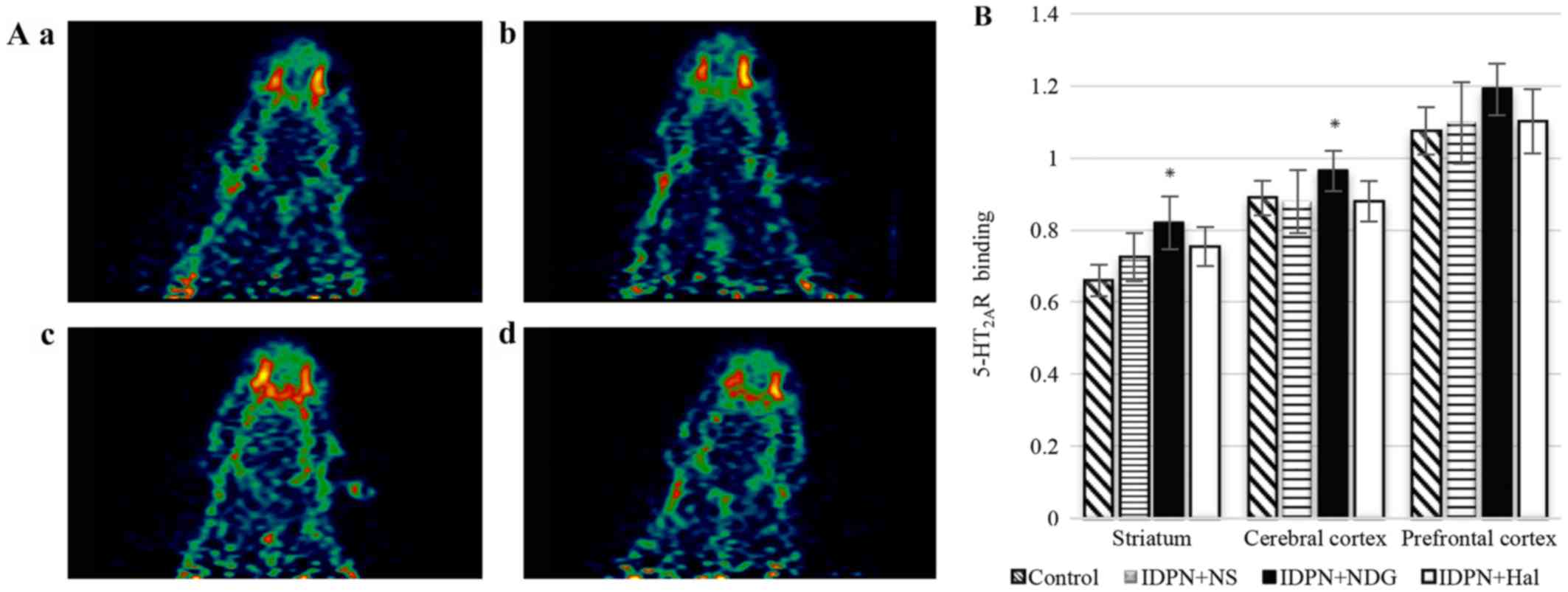
5-HT2AR
There was a slight increase in the
5-HT2AR content in the striata of TS model rats, though
this was not significant when compared with the content in control
rats. There were no differences in the 5-HT2AR content
in other brain regions among the groups. After 8 weeks of NDG
administration, the uptake ratio of 18F-altanserin was
significantly increased in the striatum and cerebral cortex
(IDPN+NDG group vs. IDPN+NS group, P<0.05). Meanwhile, Hal
failed to stimulate any changes in 5-HT2AR content
(IDPN+Hal group vs. IDPN+NS group, P>0.05; Fig. 4).
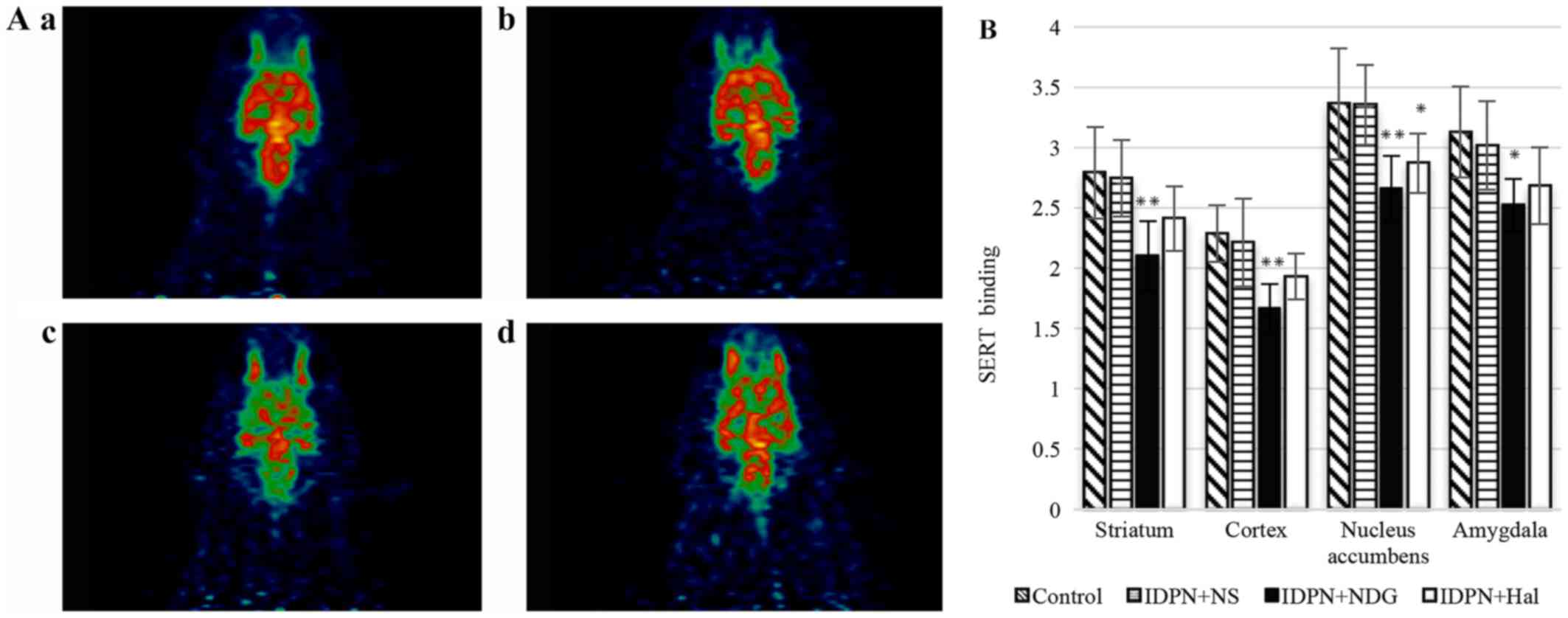
SERT
IDPN-treated rats exhibited no significant
differences relative to the control group for all brain ROIs.
Following treatment, NDG significantly reduced the SERT content in
the nucleus accumbens, putamen, amygdaloid nucleus, striatum and
cerebral cortex (IDPN+NDG group vs. IDPN+NS group, P<0.01). Hal
reduced the SERT content in the nucleus accumbens and putamen
(IDPN+Hal group vs. IDPN+NS group, P<0.05). However, in other
brain regions there were no significant differences between these
groups (Fig. 5).
Discussion
Currently there is no model treatment for TS.
Although the commonly used anti-tic drugs can alleviate the
symptoms of tics, a series of side effects cause many patients to
stop using these drugs. Hal is the drug most commonly used to
suppress tics, attributable to its high efficacy as an antagonist
of DA receptors; however, many patients stop using Hal due to its
side effects that include sedation, electrocardiographic changes
and extrapyramidal symptoms (23).
NDG includes 10 Chinese herbal medicines, as described here, which
are strictly based on the compatibility theory of TCM, and this
formulation is specifically prepared for the treatment of TS.
Previous clinical trials by our group have demonstrated the notable
effects of NDG, with nearly one-half of the tested patients
exhibiting positive outcomes; furthermore, NDG has also been
demonstrated to ameliorate the symptoms of ADHD with few side
effects [nausea (5.6%), abdominal pain (5.6%), increased appetite
(13.9%), difficulty in falling asleep (2.8%), hypersomnia (16.7%)
and anxiety/nervousness (2.8%)] (22). Meanwhile, our previous animal study
demonstrated that NDG had dual ameliorative effects on the DA
system (20). These initial
results were confirmed in the current study with the use of
high-resolution tomography in vivo that can provide more
accurate parameters. Furthermore, the binding potential of DAT was
measured in the current study. From the variation in DAT that was
observed, it may be speculated that NDG functions by upregulating
DAT expression, thus weakening the hyperinnervation of DA
fibers.
It has been suggested that there is a link between
dopaminergic dysfunction and TS pathophysiology, but the results
surrounding this assumption are heterogeneous. Increased as well as
decreased receptor binding associated with TS, or no differences in
receptor binding between patients with TS and control groups have
been reported (24–26). Grace (27) proposed two components to describe
the DA system: The phasic (spike-dependent) and tonic (homeostatic)
components. In the model of tonic-phasic DA, increased DAT activity
may lead to reduced tonic levels of DA, which could increase the
levels of phasically released DA. Singer et al (7,14)
also reported increased DAT binding and proposed that increased DA
release is a primary defect in TS. It may be speculated that DATs
have both positive and negative effects on the regulation of DA.
Normally, reuptake of the DA released into nerve terminals would be
expected to reduce the concentration of DA in the synaptic cleft;
however, the reduction of tonic DA levels may lead to phasic DA
release. In the current study, IDPN exposure caused an increase in
D2R and DAT binding compared with the levels in
saline-treated control rats. Similar to the effects of Hal, NDG
relieved the stereotypical symptoms of TS in rats and reversed the
upregulation of striatal D2R density to normal levels.
However, a significant increase in DAT binding was observed in the
NDG-treated group, which was not observed in the Hal-treated
group.
5-HT is primarily produced in the brainstem by the
raphe nuclei (28), which
innervate virtually all regions of the central nervous system
(29) and are responsible for
regulating mood, eating behaviour, sleep and cognitive function.
Serotonergic dysfunction was initially considered as the basis for
OCD, a co-morbidity that is sensitive to selective 5-HT reuptake
inhibitors. Experimental and clinical data have suggested that
abnormalities in the serotonergic system are also associated with
TS, and observations of interactions with the dopaminergic system
have led to the hypothesis that 5-HT is associated with TS. Early
studies revealed that 5-HT2AR binding is slightly
increased in the occipital cortex and parietal cortex, while SERT
binding potential (BP) was decreased (15). Lodge and Grace (30) documented that a deficit of 5-HT led
to an increase in DA release; meanwhile, increased DA content could
aggravate the symptoms of TS. Consistent with these findings, the
present data indicated the presence of increased 5-HT2AR
binding and decreased SERT binding in the rat model of TS.
Treatment with NDG enhanced the increase in 5-HT2AR
binding in the striatum and reduced the decrease in SERT BP in most
brain ROIs. The increased 5-HT2AR binding and decreased
SERT BP could be interpreted as a compensatory function to
upregulate the content of 5-HT; increased 5-HT led to a decrease in
DA release, which may alleviate the stereotypical abnormalities
associated with TS.
In the DA system, NDG can decrease the content of DA
by downregulating the binding of DA receptors and upregulating the
content of DA transporters. In the 5-HT system, NDG can increase
the content of 5-HT by upregulating the binding of 5-HT receptors
and downregulating the content of 5-HT transporters, and indirectly
decrease the content of DA. By interacting with these two
neurotransmitter systems, NDG could effectively reduce the symptoms
of TS.
Extensive experiments are being performed to study
the relationship between tics and the dysfunction of the DA system,
but few studies have assessed tics in the context of the
dysfunction of serotonin metabolism. In the current study, the
density, affinity and brain distribution of D2R, DAT,
5-HT2AR and SERT were simultaneously measured in
vivo, providing a more precise and greater insight into the
pathogenesis of TS. Even so, the experimental procedure has certain
limitations. Firstly, the diversity of individuals renders it
difficult to reproduce the pathogenesis of human TS completely.
Secondly, due to the limitation of time, the long-term efficacy of
drugs cannot be observed. In the future, other monoamine
neurotransmitters beyond DA and 5-HT, which might also participate
in the pathophysiological course of TS, should be studied. In
addition, it is important to try to develop a more effective and
safe alternative treatment for TS.
The present study has demonstrated the effects of
NDG treatment on stereotypical TS-related movement disorders in
rats, involving neurotransmitter regulation. In conclusion, NDG may
have promise as a safe and effective medication that can be used as
an alternative therapy for TS. Additionally, the current findings
suggest a model for an interaction between DA and 5-HT, where
variation in the 5-HT system could be a compensatory mechanism to
correct for dysfunction of the DA system. However, further
understanding of the specific relationship between DA and 5-HT, as
well as other neurotransmitters, is now required.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of China (grants no. 81273798 and no. 81503613),
the Natural Science Foundation of Shandong province (grants no.
ZR2012HM030 and no. BS2015YY030), the Development Project of
Science and Technology of Traditional Chinese Medicine of Shandong
Province (grant no. 2013ZDZK-085), the Development Projects of
Shandong Province Science and Technology (grant no. 2011GSF11903)
and the Postdoctoral Science Foundation of China (grant no.
2014M551924).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW conducted the experiments and wrote the
manuscript. AYL conceptualized and designed the study. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were performed in
compliance with the relevant guidelines and regulations of the
American Physiological Society, and the protocols were approved by
the medical ethics committee of The Provincial Hospital Affiliated
to Shandong University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Scharf JM, Miller LL, Gauvin CA, Alabiso
J, Mathews CA and Ben-Shlomo Y: Population prevalence of Tourette
syndrome: A systematic review and meta-analysis. Mov Disord.
30:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Houghton DC, Capriotti MR, Conelea CA and
Woods DW: Sensory phenomena in tourette syndrome: Their role in
symptom formation and treatment. Curr Dev Disord Rep. 1:245–251.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pauls DL, Fernandez TV, Mathews CA, State
MW and Scharf JM: The inheritance of tourette disorder: A review. J
Obsessive Compuls Relat Disord. 3:380–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paschou P, Yu D, Gerber G, Evans P,
Tsetsos F, Davis LK, Karagiannidis I, Chaponis J, Gamazon E,
Mueller-Vahl K, et al: Genetic association signal near NTN4 in
Tourette syndrome. Ann Neurol. 76:310–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prontera P, Napolioni V, Ottaviani V,
Rogaia D, Fusco C, Augello B, Serino D, Parisi V, Bernardini L,
Merla G, et al: DPP6 gene disruption in a family with Gilles de la
Tourette syndrome. Neurogenetics. 15:237–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leckman JF: Tourette's syndrome. Lancet.
360:1577–1586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singer HS, Hahn IH and Moran TH: Abnormal
dopamine uptake sites in postmortem striatum from patients with
Tourette's syndrome. Ann Neurol. 30:558–562. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolf SS, Jones DW, Knable MB, Gorey JG,
Lee KS, Hyde TM, Coppola R and Weinberger DR: Tourette syndrome:
Prediction of phenotypic variation in monozygotic twins by caudate
nucleus D2 receptor binding. Science. 273:1225–1227. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Minzer K, Lee O, Hong JJ and Singer HS:
Increased prefrontal D2 protein in Tourette syndrome: A postmortem
analysis of frontal cortex and striatum. J Neurol Sci. 219:55–61.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haugbol S, Pinborg LH, Regeur L, Hansen
ES, Bolwig TG, Nielsen FA, Svarer C, Skovgaard LT and Knudsen GM:
Cerebral 5-HT2A receptor binding is increased in patients with
Tourette's syndrome. Int J Neuropsychopharmacol. 10:245–252. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martino D and Pringsheim TM: Tourette
syndrome and other chronic tic disorders: An update on clinical
management. Expert Rev Neurother. 18:125–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leckman JF, Bloch MH, Smith ME, Larabi D
and Hampson M: Neurobiological substrates of Tourette's disorder. J
Child Adolesc Psychopharmacol. 20:237–247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
George MS, Robertson MM, Costa DC, Trimble
MR, Pilowsky L and Verhoeff NP: Dopamine receptor availability in
Tourette's syndrome. Psychiatry Res. 55:193–203. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singer HS, Szymanski S, Giuliano J, Yokoi
F, Dogan AS, Brasic JR, Zhou Y, Grace AA and Wong DF: Elevated
intrasynaptic dopamine release in Tourette's syndrome measured by
PET. Am J Psychiatry. 159:1329–1336. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong DF, Brasic JR, Singer HS, Schretlen
DJ, Kuwabara H, Zhou Y, Nandi A, Maris MA, Alexander M, Ye W, et
al: Mechanisms of dopaminergic and serotonergic neurotransmission
in Tourette syndrome: Clues from an in vivo neurochemistry study
with PET. Neuropsychopharmacology. 33:1239–1251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kakhi S and Soomro GM: Obsessive
compulsive disorder in children and adolescents: Duration of
maintenance drug treatment. BMJ Clin Evid. 2015:2015.
|
|
17
|
McDougle CJ, Epperson CN, Pelton GH,
Wasylink S and Price LH: A double-blind, placebo-controlled study
of risperidone addition in serotonin reuptake inhibitor-refractory
obsessive-compulsive disorder. Arch Gen Psychiatry. 57:794–801.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cadet JL: The iminodipropionitrile
(IDPN)-induced dyskinetic syndrome: behavioral and biochemical
pharmacology. Neurosci Biobehav Rev. 13:39–45. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ogawa N, Mizukawa K, Haba K and Sato H:
Neurotransmitter and receptor alterations in the rat persistent
dyskinesia model induced by iminodipropionitrile. Eur Neurol. 30
(Suppl 1):S31–S40. 1990. View Article : Google Scholar
|
|
20
|
Zhang F and Li A: Dual ameliorative
effects of Ningdong granule on dopamine in rat models of Tourette's
syndrome. Sci Rep. 5:77312015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao L, Li AY, Lv H, Liu FY and Qi FH:
Traditional Chinese medicine Ningdong granule: The beneficial
effects in Tourette's disorder. J Int Med Res. 38:169–175. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li JJ, Li ZW, Wang SZ, Qi FH, Zhao L, Lv H
and Li AY: Ningdong granule: A complementary and alternative
therapy in the treatment of attention deficit/hyperactivity
disorder. Psychopharmacology (Berl). 216:501–509. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Egolf A and Coffey BJ: Current
pharmacotherapeutic approaches for the treatment of Tourette
syndrome. Drugs Today (Barc). 50:159–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ernst M, Zametkin AJ, Jons PH, Matochik
JA, Pascualvaca D and Cohen RM: High presynaptic dopaminergic
activity in children with Tourette's disorder. J Am Acad Child
Adolesc Psychiatry. 38:86–94. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gilbert DL, Christian BT, Gelfand MJ, Shi
B, Mantil J and Sallee FR: Altered mesolimbocortical and thalamic
dopamine in Tourette syndrome. Neurology. 67:1695–1697. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hwang WJ, Yao WJ, Fu YK and Yang AS:
[99mTc]TRODAT-1/[123I]IBZM SPECT studies of the dopaminergic system
in Tourette syndrome. Psychiatry Res. 162:159–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grace AA: Phasic versus tonic dopamine
release and the modulation of dopamine system responsivity: A
hypothesis for the etiology of schizophrenia. Neuroscience.
41:1–24. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dahlstroem A and Fuxe K: Evidence for the
existence of monoamine-containing neurons in the central nervous
System. I. demonstration of monoamines in the cell bodies of brain
stem neurons. Acta Physiol Scand. 232 (Suppl):S231–S255. 1964.
|
|
29
|
Butcher LL and Woolf NJ: Cholinergic and
serotonergic systems in the brain and spinal cord: Anatomic
organization, role in intercellular communication processes, and
interactive mechanisms. Prog Brain Res. 55:1–40. 1982.PubMed/NCBI
|
|
30
|
Lodge DJ and Grace AA: The hippocampus
modulates dopamine neuron responsivity by regulating the intensity
of phasic neuron activation. Neuropsychopharmacology. 31:1356–1361.
2006. View Article : Google Scholar : PubMed/NCBI
|