Introduction
Peritoneal dialysis (PD) is one of the most common
treatments for managing patients with end-stage renal disease
(ESRD). On average, ~11% of patients with ESRD receive PD globally
(1); however, PD solution can
induce pathological changes in the peritoneum with regards to
function and structure, such as peritoneal fibrosis (PF), which may
result in the failure of PD (2,3).
These changes occur due to the high glucose concentrations (HG),
products of glucose degradation, lactate and low pH of PD solution
(4).
Accumulating evidence indicates that the
epithelial-mesenchymal transition (EMT) is a pivotal point in the
progression and pathogenesis of peritoneal fibrosis (PF), resulting
in the loss of epithelial characteristics and gain of mesenchymal
characteristics (5–7). Previous studies reported that HG
increased the expression of transforming growth factor-β1 (TGF-β1),
which induced the EMT in peritoneal mesothelial cells (PMCs)
(8,9). Additionally, it has been reported
that HG-based PD solution induced peritoneal EMT by generating
reactive oxygen species (ROS), whereas antioxidants effectively
inhibited the HG-induced EMT in the rat peritoneum and human PMCs
(HPMCs) (10,11).
Zinc (Zn) is an essential microelement that is
involved in various cellular functions, including the transcription
and replication of genes, signal transduction, and the regulation
of cell apoptosis and differentiation (12–15).
Previous studies demonstrated that Zn supplementation inhibited
fibrosis in various animal models, including models of myocardial
fibrosis, liver fibrosis, perivascular fibrosis, vasculitis and
cystic fibrosis (16–19). Our previous study reported that Zn
serves an important role in preventing the HG-induced EMT of rat
PMCs in vitro (20);
however, its functions and mechanisms in vivo remain
unclear. In the present study, the role of Zn in the HG-induced EMT
was investigated in a rat model of PF in vivo, and the
effects of Zn on HPMCs were determined, in addition to the
underlying molecular mechanisms.
Materials and methods
Main reagents
ZnSO4 (Xinhua Pure Chemical Industries),
4.25% dextrose PD fluid (Baxter Healthcare Ltd.), Zn chelator
N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine (TPEN;
Sigma-Aldrich; Merck KGaA) and clioquinol (CQ; Sigma-Aldrich; Merck
KGaA) were used during the study. Monoclonal antibodies against
E-cadherin (cat. no. 3195), vimentin (cat. no. 5741), NAD(P)H
quinone dehydrogenase 1 [cat. no. 3187, (NQO-1)], heme oxygenase-1
[cat. no. 5853, (HO-1)], Nrf-2 (cat. no. 12721), GAPDH (cat. no.
5174) and Lamin B (cat. no. 13435) were purchased from Cell
Signaling Technology, Inc. A Pierce ECL detection kit was obtained
from Pierce (Thermo Fisher Scientific, Inc.). All reagents used
were of analytical grade.
Animal model of PF
A total of 40 male Sprague-Dawley rats (200–250 g,
8–10 weeks) were obtained from the Center of Experimental Animals
of China Medical University (Shenyang, China). Rats were housed in
controlled conditions, at a temperature of 20–25°C with 40–70%
humidity under a 12:12-h light/dark cycle, with access to food and
water ad libitum.
Rats were randomly allocated into four groups with
10 rats in each group: The negative control (NC) group, which were
treated daily via intraperitoneal administration of 100 ml/kg of
0.9% saline for 4 weeks; the HG group, which received daily
intraperitoneal administration of 100 ml/kg of 4.25% dextrose PD
fluid for 4 weeks; the HG + Zn group, which received daily
intraperitoneal administration of 3 mg/kg ZnSO4 and 100
ml/kg of 4.25% dextrose PD fluid for 4 weeks; the HG + CQ group,
which were treated daily via intraperitoneal administration of 20
mg/kg CQ and 100 ml/kg of 4.25% dextrose PD fluid for 4 weeks.
Prior to sacrifice, 100 ml/kg of 4.25% dextrose PD fluid was
intraperitoneally administered to the rats. At 2 h later, the rats
were anaesthetized, the peritonea were opened, and all the fluid
contents were immediately collected to determine the volume (ml).
The ultrafiltration volume (UV) was calculated using the following
formula: Drainage volume-initial injection volume. The glucose
concentration in the dialysate was determined using a glucose assay
kit (cat. no. ab65333, Abcam) to assess the transfer capacity of
glucose, according to the manufacture's protocols. In addition, the
visceral and parietal peritonea were kept for subsequent
examination.
All animal experiments in the present study were
approved by the Institutional Animal Ethics Committee of China
Medical University. All animal procedures were performed according
to the Animal Care Institutional Guidelines for experimental
animals.
Pathology and
immunohistochemistry
For pathological examination, the parietal
peritoneum was fixed in 4% paraformaldehyde for 12 h at room
temperature. Paraffin-embedded sections (4 µm) were prepared and
stained with hematoxylin (room temperature for ١٥ min) and eosin
(room temperature for 3 min; H&E) and/or Masson's trichrome
(room temperature for 10 min); 5 random visual fields from each
section were imaged under a light microscope (magnification, ×10
and 20) and selected for measuring the thickness of the peritoneum.
Image-Pro Plus version 6.0 (Media Cybernetics, Inc.) was used to
analyze the images. ‘Peritoneal thickness’, which indicated the
extent of fibrosis, was defined as the thickness of the
submesothelial zone measured from the inner surface of the
abdominal muscle to the mesothelium (21). The average of five random visual
fields was used to quantify the thickness of each section.
For immunohistochemical staining, paraffin-embedded
sections were heated at 60°C for 10 min, washed with xylene and
rehydrated in a descending alcohol series. The deparaffinized
sections were blocked with 3% hydrogen peroxide (room temperature
for 10 min) and non-immune goat serum (KIT-9710; Fuzhou Maixin
Biotech Co., Ltd.; room temperature for 20 min). Then, tissues were
incubated with primary antibodies against E-cadherin (1:200; Cell
Signaling Technology, Inc.) and vimentin (1:200; Cell Signaling
Technology, Inc.) at 4°C overnight following antigen retrieval in
boiling sodium citrate buffer (10 mM, pH 6.0) for 5 min. After
washing with phosphate-buffered saline (PBS), tissue sections were
incubated with a horseradish peroxidase (HRP)-conjugated secondary
antibody (KIT-9710; Fuzhou Maixin Biotech Co., Ltd.) at 37°C for 30
min. Reaction products were visualized by incubation with
3,3′-diaminobenzidine at 37°C for 30 min and then counterstained
with hematoxylin at room temperature for 3 min. As a negative
control, tissue samples were subject to the same staining
procedures with no incubation with primary antibodies. A minimum of
five fields were randomly selected in each section and images were
acquired by a fluorescence microscope (Nikon Corporation) at a
magnification of ×20. Image-Pro Plus was used to calculate the
number of cells positive for E-cadherin and vimentin in each field.
According to the proportion of positive cells, the following
standard was used: 0, negative; 1, 1–20%; 2, 21–50%; 3, 51–80%; 4,
81–100%.
Cell culture
HMrSV5, the HPMC line, was provided by Professor
Huimian Xu of The First Affiliated Hospital of China Medical
University (Shenyang, China). Cells were isolated from human
omentum by enzymatic disaggregation and cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.) (22). All cells were incubated at 37°C in
a 5% CO2. atmosphere. Confluent cells presented as a
monolayer with characteristic cobblestone-like appearance. The
following groups were allocated: The NC group (HPMCs without any
treatment); the HG group [HPMCs treated with HG (126 mM)]; the HG +
Zn group [HPMCs co-treated with HG (126 mM) and ZnSO4
(10 µM)]; the HG + TPEN group [HPMCs co-treated with HG (١٢٦ mM)
and TPEN (1 µM)]; and the Zn group [HPMCs treated with
ZnSO4 (10 µM) alone]. Cultures were incubated for ٤٨
h.
Cell migration assay
Cell migration ability was assessed using a
Transwell assay with 24-well Transwell chambers (Corning, Inc.)
with 8-µm pores. A cell suspension containing 5×105
cells/ml was seeded into the upper chamber in serum-free medium.
The medium in the lower chamber contained 10% FBS. Cells were
untreated or pre-treated with ZnSO4 (10 µM) or TPEN (١
µM) in the presence or absence of HG (١٢٦ mM). Following incubation
at 37°C for 24 h, a cotton-tipped swab was used to carefully remove
the cells that had not migrated through the pores; the cells that
had migrated onto the lower surface were stained with 0.1% crystal
violet for 5 min at room temperature. Five random visual fields in
each section were analyzed under a light microscope (magnification,
×20) and cells were counted. Three independent experiments were
performed.
Detection of intracellular ROS
levels
An ROS assay was performed using an ROS assay kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocols. Briefly, HPMCs were treated as
aforementioned. Then, 5×106 cells were incubated with 10
µmol/l dichlorodihydrofluorescein diacetate (DCFH-DA; Beyotime
Institute of Biotechnology) probes at 37°C for 30 min. Following
washing with PBS three times, DCFH-DA was deacetylated by a
intracellular nonspecific esterase to 2,7-dichlorofluorescein
(DCF), a fluorescent compound. The fluorescence spectrum of DCF
which is excited at 488 nm and emitted at 525 nm is very similar to
FITC. So FITC can be used to detect DCF fluorescence using a flow
cytometer (BD FACSCaliber; BD Biosciences). Results were analyzed
using Cell Quest software version 5.1 (BD Biosciences).
Western blotting
For western blotting, tissues and HPMCs were lysed
in RIPA buffer (Sigma-Aldrich; Merck KGaA) with protease inhibitors
(Sigma-Aldrich; Merck KGaA). Supernatants containing total proteins
were harvested to detect HO-1, NQO1, E-cadherin, vimentin and
GAPDH. Nuclear proteins of the HPMCs were extracted using a nuclear
protein extraction kit (Sigma-Aldrich; Merck KGaA) to detect Lamin
B and nuclear factor-like 2 (Nrf2). Protein (30 µg) determined by
BCA assay (Beyotime Institute of Biotechnology) was separated via
10% SDS-PAGE and transferred to polyvinylidene difluoride
membranes, which were blocked with 5% BSA (Beijing Solarbio Science
& Technology Co., Ltd.) in Tris-Buffered saline containing 0.1%
Tween-20 for 1 h at room temperature, and then incubated with
specific primary antibodies (1:1,000; Cell Signaling Technology,
Inc.) against HO-1, NQO, GAPDH, E-cadherin, vimentin, Nrf2 and
Lamin B at 4°C overnight. Blots were subsequently incubated with a
horseradish peroxidase-conjugated secondary antibody (cat. no.
E030120-02; 1:2,000; EarthOx Life Sciences) for 1 h at room
temperature. Proteins were visualized using chemiluminescence and
clarity western ECL substrate (Bio-Rad Laboratories, Inc.).
Densitometric analysis of the western blots was performed using
ImageJ v1.48 software (National Institutes of Health).
Statistical analysis
SPSS (Version 17.0; SPSS, Inc.) was used to analyze
statistics. All data were presented as the mean ± standard
deviation, and all experiments were performed at least three times.
After statistical significance was established by one-way ANOVA,
post hoc multiple comparisons were performed using Tukey's multiple
comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Zn prevents fibrosis in a rat model of
PF
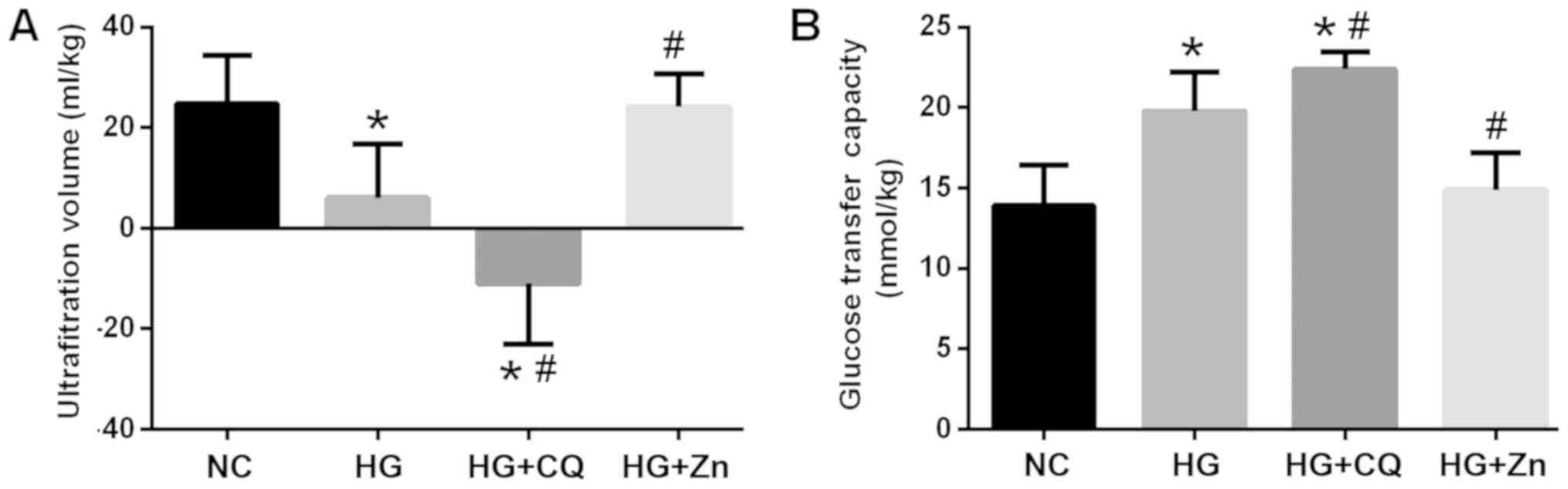
As presented in Fig.
1, the rats in the HG group exhibited significantly reduced UV
values and an increased glucose transfer capacity compared with the
NC. Zn supplementation significantly reduced the glucose transfer
capacity and increased the UV compared with HG treatment alone,
whereas CQ treatment significantly increased the glucose transfer
capacity, but decreased the UV.
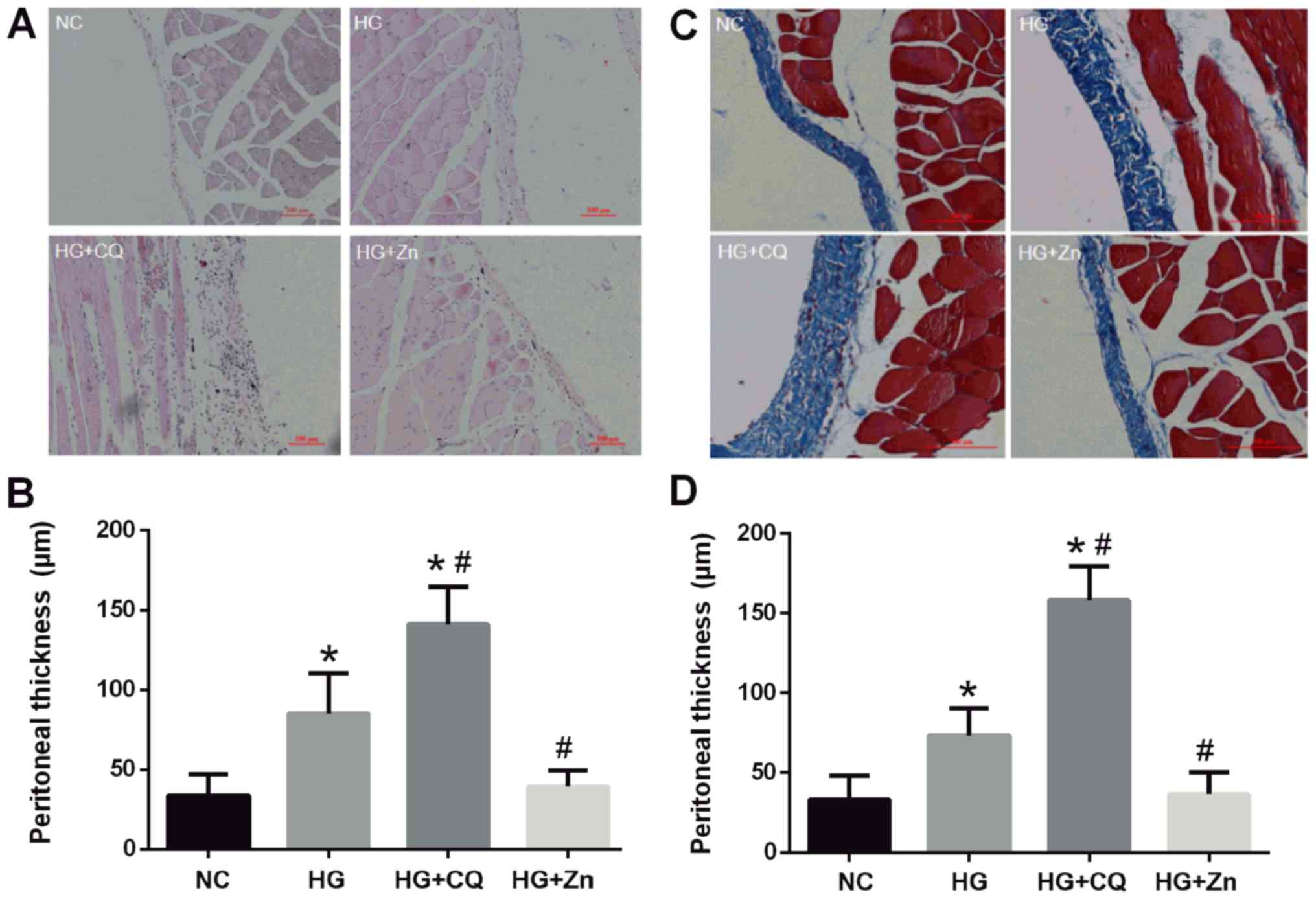
It was observed via H&E staining that
inflammatory cells infiltrated the interstitium, fibers were
exposed, mesothelial cells became round and cylindrical, and cells
shed in the HG group (Fig. 2A and
B). Furthermore, the HG + CQ group presented a further elevated
inflammatory response and significantly thicker peritoneum compared
with the HG group. Masson's trichrome staining also demonstrated
that HG treatment significantly increased the thickness of the
peritoneal collagen fibers compared with the NC group (Fig. 2C and D). This thickening may
increase the permeability of peritoneal membrane and the absorption
of glucose, which could reduce the osmotic pressure of peritoneal
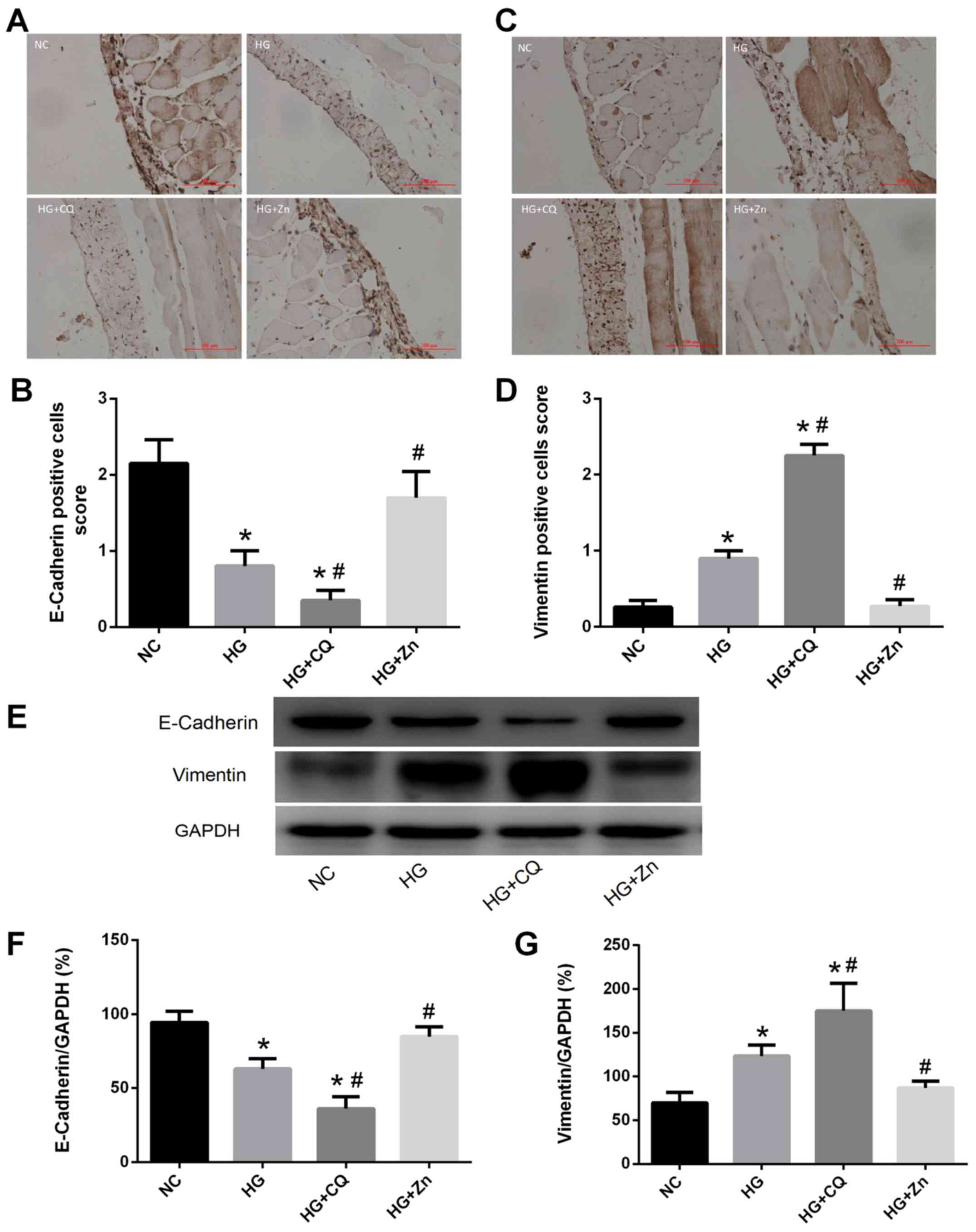
permeation and affect ultrafiltration. Immunohistochemical staining
revealed significantly increased expression of vimentin, and
decreased expression of E-cadherin in the HG group, compared with
the NC group (Fig. 3). Zn
supplementation significantly ameliorated these pathological
alterations, whereas CQ aggravated the effects of HG on the PMCs
(Figs. 2 and 3).
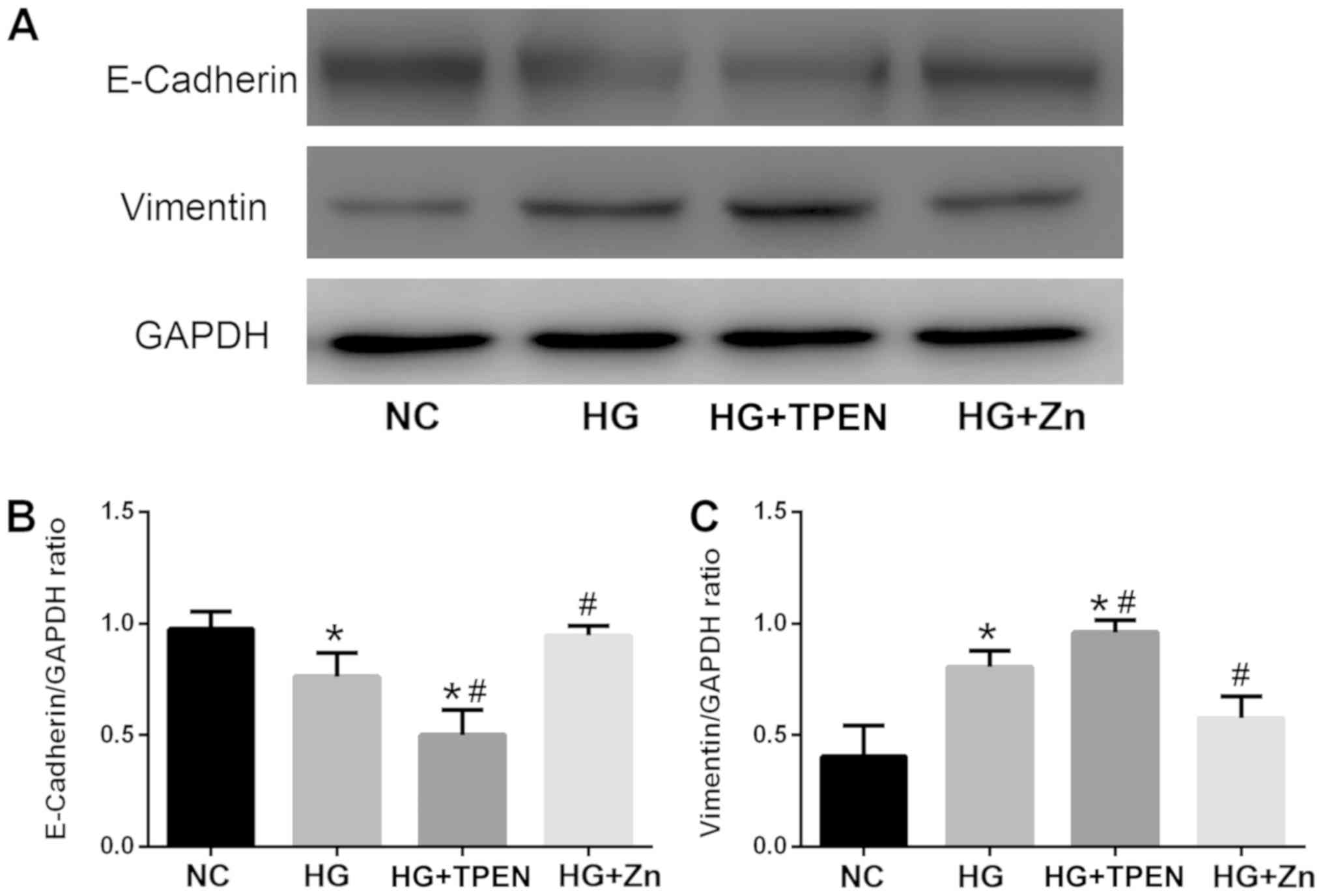
Zn inhibits the HG-induced EMT of
HPMCs
Concentrations and durations of treatment of HG, Zn
and TPEN were established according to our previous studies
(20,23,24).
Following exposure of HPMCs to HG for 48 h, E-cadherin expression
decreased, whereas the expression of vimentin was significantly
upregulated compared with the NC group (Fig. 4). Co-treatment with Zn (10 µM) for
٤٨ h in HPMCs resulted in a significant alleviation of HG-induced
EMT-associated alterations, whereas co-treatment with the Zn
chelator TPEN (1 µM) significantly aggravated HG-induced changes in
expression, consistent with the results of the in vivo
experiments.
Zn inhibits the HG-induced migration
of HPMCs
The roles of Zn in the migration of HPMCs were
investigated by using a Transwell cell migration assay; cells
exhibit increased migration following EMT (25,26).
HG stimulation for 24 h significantly increased the migration of
HPMCs compared with the NC group (Fig.
5). Co-treatment with ZnSO4 significantly reversed
the HG-stimulated increase in migration, whereas co-treatment with
the Zn chelator TPEN further promoted the migration of HPMCs
(Fig. 5).
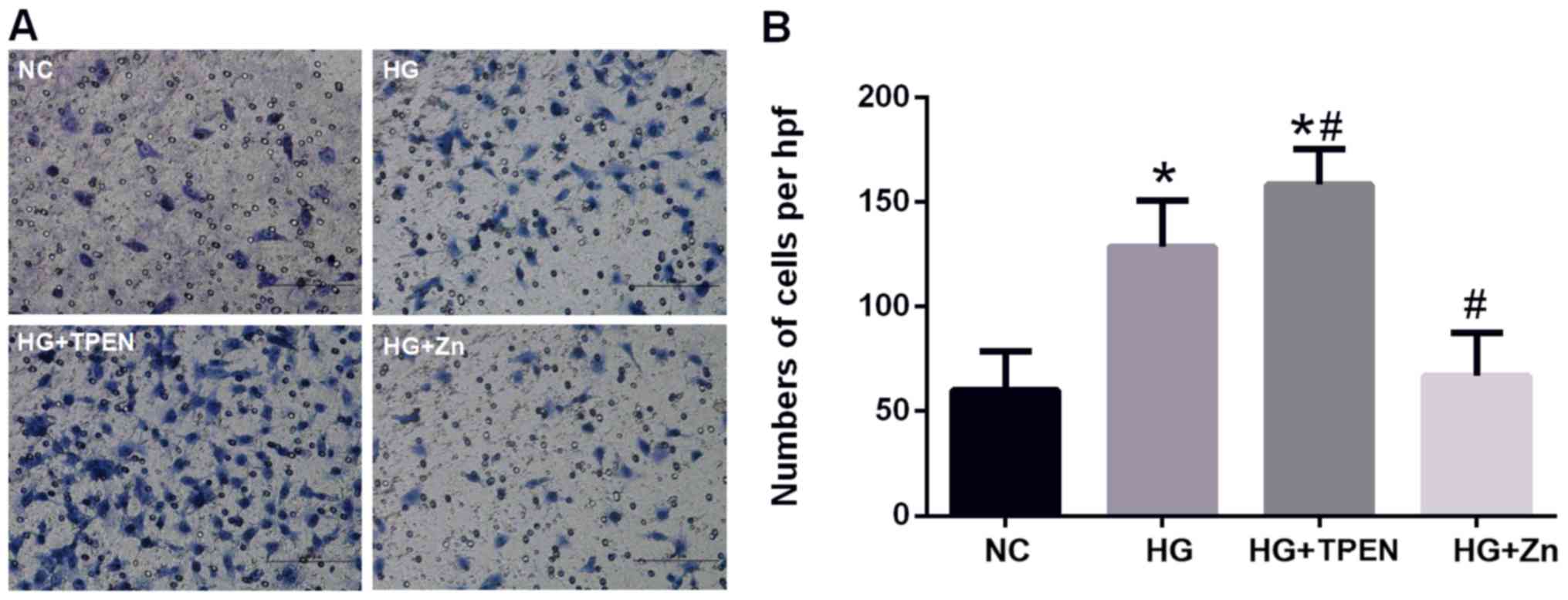 | Figure 5.Effects of Zn on the HG-induced
increase in the migration of HPMCs. (A and B) Transwell assay
revealed the migration ability of HPMCs following co-treatment with
HG, and TPEN or ZnSO4 for 24 h (magnification, ×20). Data are
presented as the mean ± standard deviation. P-values were
determined using ANOVA with Tukey's post hoc test. *P<0.05 vs.
NC; #P<0.05 vs. HG. NC, negative control; HG, high glucose; hpf,
high-power field; TPEN, N,N,N',N'-tetrakis (2-pyridylmethyl)
ethylenediamine; Zn, ZnSO4; HPMC, human peritoneal mesothelial
cell. |
Zn inhibits HG-induced ROS generation
of HPMCs
The effects of Zn on the intracellular generation of
ROS were also determined, as ROS generation may promote the onset
of EMT. HG significantly increased the levels of ROS in HPMCs
compared with the NC group (Fig.
6). Co-treatment with ZnSO4 significantly inhibited
the HG-stimulated increase in ROS levels, whereas co-treatment with
the Zn chelator TPEN further increased ROS levels in HPMCs
(Fig. 6).
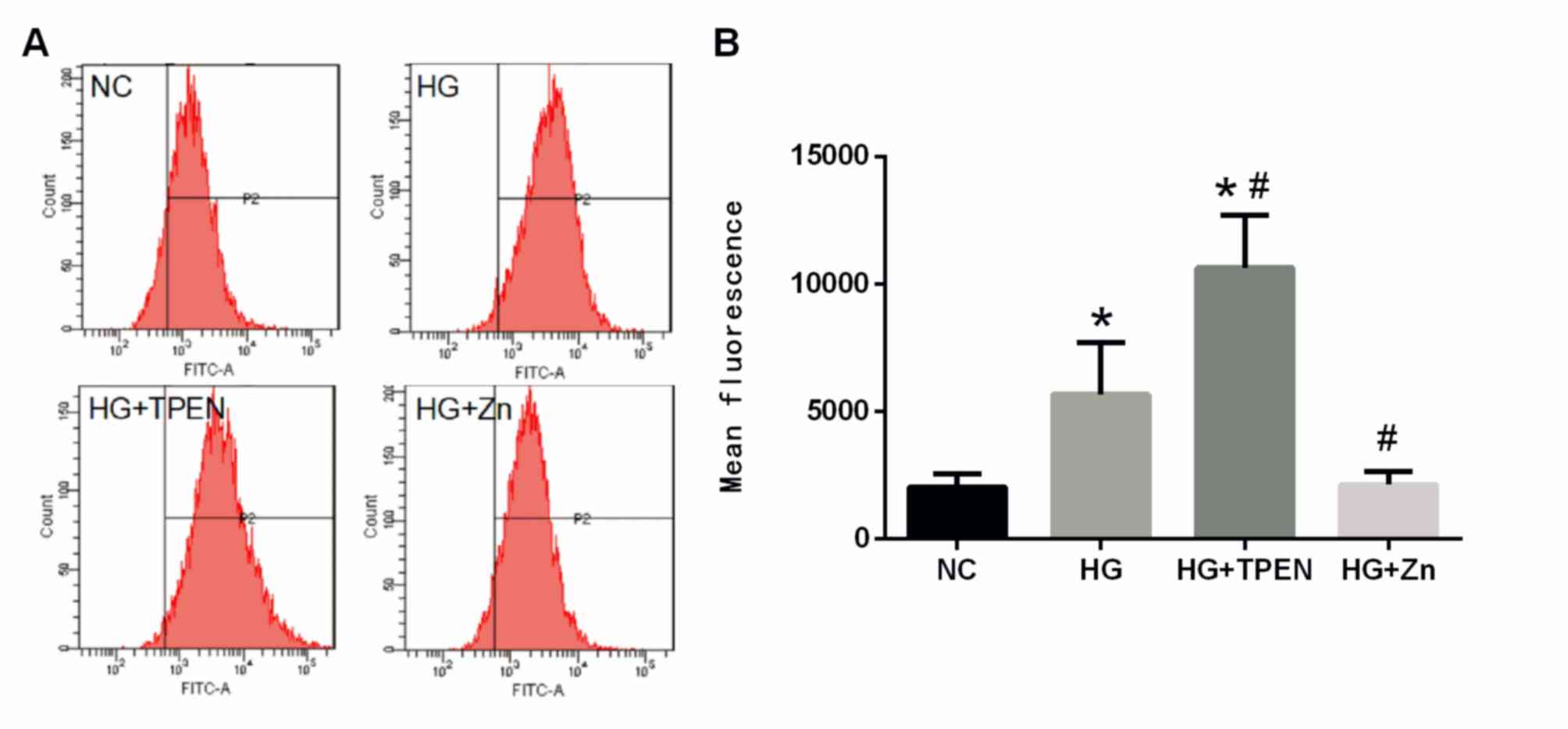 | Figure 6.Effects of Zn on intracellular ROS in
the HPMCs stimulated by HG. HPMCs were co-treated with HG, and TPEN
or ZnSO4 for 48 h. (A) Intracellular ROS were measured by flow
cytometry using an oxidation-sensitive fluorescent probe,
dichlorodihydrofluorescein diacetate, which is oxidized to
2,7′-dichlorofluorescein in the presence of ROS. (B) Data are
presented as the mean ± standard deviation. P-values were
determined using ANOVA with Tukey's post hoc test. *P<0.05 vs.
NC; #P<0.05 vs. HG. NC, negative control; HG, high glucose;
TPEN, N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine; Zn,
ZnSO4; HPMC, human peritoneal mesothelial cell; ROS, reactive
oxygen species. |
Zn activates the Nrf2 antioxidant
pathway of HPMCs induced by HG
The Nrf2 antioxidant pathway has been reported to be
involved in regulating ROS generation and the EMT (27,28).
Therefore, the effects of Zn on the activity of the Nrf2
antioxidant pathway in HPMCs was determined via western blotting.
As presented in Fig. 7, the
expression levels of nuclear Nrf2 and target proteins of the Nrf2
antioxidant pathway, including NQO1 and HO-1, were upregulated in
HG-induced HPMCs compared with the NC group. Co-treatment with TPEN
(1 µM) notably inhibited the expression of these proteins, whereas
co-treatment with ZnSO4 (10 µM) significantly promoted
the expression of nuclear Nrf٢, NQO١ and HO-١ compared with the HG
group. The group treated with Zn alone presented no significant
alterations in expression compared with the NC group.
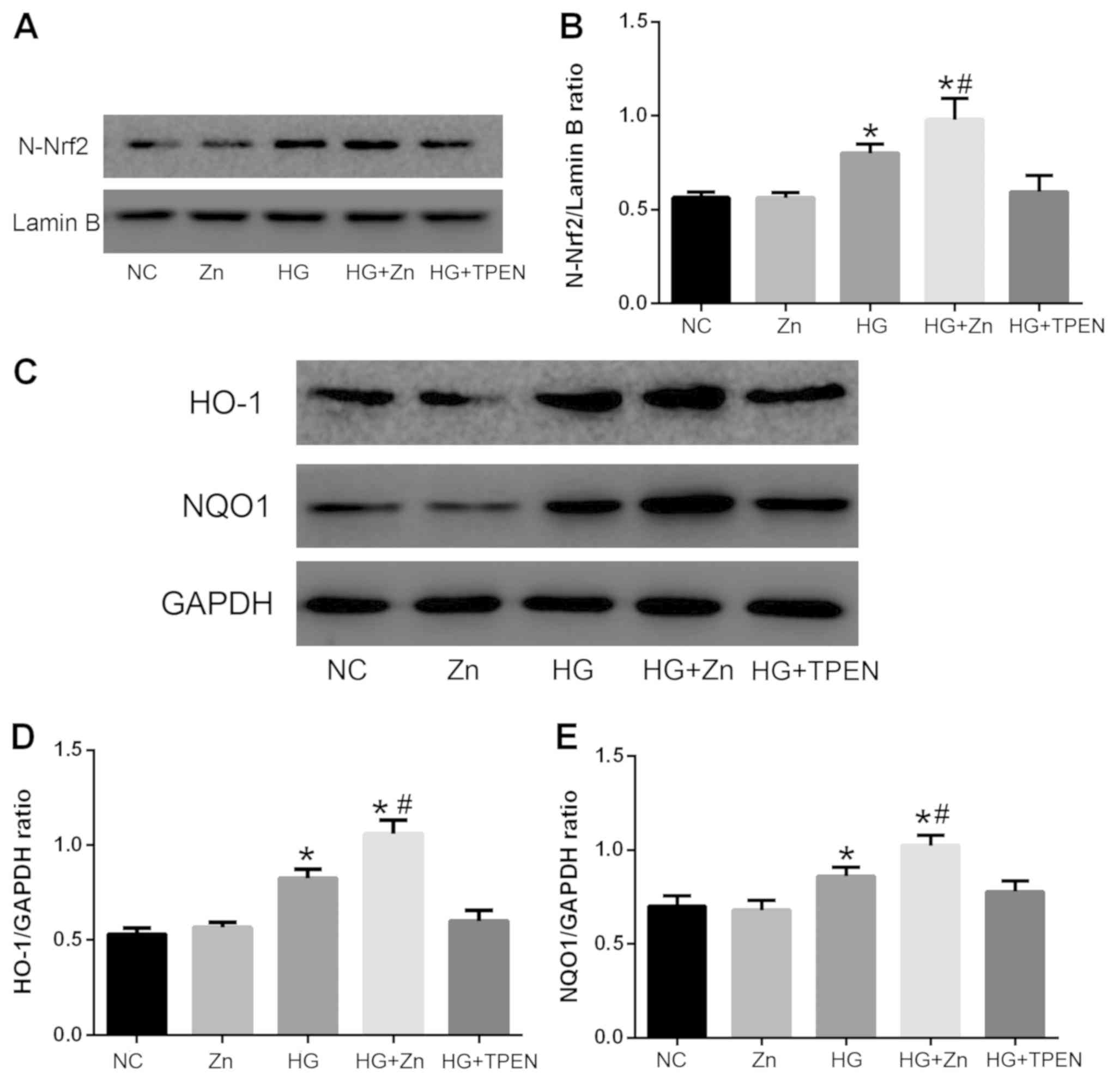 | Figure 7.Effects of Zn on the activity of the
Nrf2 antioxidant pathway in HPMCs stimulated with HG. HPMCs were
co-treated with HG, and TPEN or ZnSO4 for 48 h. Expression levels
of (A and B) N-Nrf2, and (C-E) HO-1 and NQO1, target proteins of
the Nrf2 antioxidant pathway, were analyzed via western blotting.
Data are presented as the mean ± standard deviation. P-values were
determined using ANOVA with Tukey's post hoc test. *P<0.05 vs.
NC; #P<0.05 vs. HG. NC, negative control; HG, high glucose;
TPEN, N,N,N',N'-tetrakis (2-pyridylmethyl) ethylenediamine; Zn,
ZnSO4; HPMC, human peritoneal mesothelial cell; N-, nuclear; Nrf2,
nuclear factor-like 2; HO-1, heme oxygenase-1; NQO1, NAD(P)H
quinone dehydrogenase 1. |
Discussion
In the present study, a rat model of PF was induced
by a 4.25% glucose dialysis solution. The HG group exhibited the
induction of EMT and characteristics of PF compared with the
control group, indicating that this model is a reliable animal
model for studying PD-induced PF. Zn deletion employed by CQ
further promoted HG-induced EMT in the rat PF model. Notably, Zn
supplementation was able to significantly inhibit the EMT by
promoting the expression of E-cadherin, inhibiting the expression
of vimentin and decreasing the peritoneum thickness, thus improving
peritoneal function. Various studies (29,30)
have demonstrated the capacity of CQ to target chelatable Zn. There
is evidence that mice injected intraperitoneally with CQ exhibited
notable reductions in chelatable Zn in the brain, testes and
pancreas (31,32). CQ has also been reported to be a
therapeutic agent due to its ability to reduce Zn accumulation in
neuritic plaques of Alzheimer's disease (33). Intraperitoneally injected CQ is
considered safe (32). The
lipophilic nature of CQ suggests that it can pass directly into the
peritoneum (32). Therefore, CQ
was selected in the present study for the in vivo
experiments to inhibit the effects of endogenous Zn.
TPEN [Kd=2.6×10−16 M (34)], another Zn chelator, exhibited an
increased affinity for Zn2+ compared with CQ
[Kd=~1×10−7 M (34)]. Previous studies (35,36)
indicated TPEN was an effective tool for inhibiting Zn in cell
models, so it was selected for the in vitro experiments.
A concentration of 126 mM glucose was used to induce
EMT, based on our previous in vitro study (20,23).
EMT not only serves an important role in certain physiological
processes, such as embryogenesis, but also is involved in the
pathophysiological process of tissue fibrosis (37).
The EMT of PMCs has been reported to be a crucial
mechanism underlying PF, and increasing evidence has indicated that
inhibition of the EMT may alleviate PF (38,39).
Numerous genes are involved in regulating EMT, including
E-cadherin, occludin, desmoplakin, α-smooth muscle actin,
N-cadherin, vimentin, fibronectin, collagen I and snail (40,41).
In the present study, the EMT markers vimentin and E-cadherin were
selected for analysis. Consistent with our previous study (20), Zn supplementation significantly
protected against HG-induced EMT in HPMCs. The acquisition of
increased migration potential is a typical feature of cells
undergoing EMT (42). Therefore,
the migratory ability of cells was evaluated using a Transwell cell
assay. The data revealed that the increase in migration induced by
HG was suppressed by Zn supplementation.
Previous studies indicated that high concentrations
of glucose induce ROS production in HPMCs (43,44),
which may be involved in peritoneal EMT induced by HG and
glucose-based PD solution. In addition, accumulating evidence has
demonstrated that Zn inhibition can result in oxidative stress,
leading to cell component damage and alterations in cellular
functions (45,46). Conversely, Zn supplementation has
been reported to decrease ROS generation (47,48).
In the present study, Zn supplementation reduced ROS generation in
the HG-stimulated HPMCs, whereas Zn depletion increased ROS
generation, indicating that Zn supplementation may inhibit the EMT
of HPMCs by reducing ROS generation.
The Nrf2 pathway exerts an important role in
antioxidative processes. Under normal conditions, Nrf2 interacts
with Kelch-like ECH-associated protein 1 (Keap1) to form a compound
complex in the cytoplasm, which is degraded by the
ubiquitin-proteasome pathway (49). On exposure to excessive ROS, Nrf2
can dissociate from Keap1 and accumulate in the nucleus,
subsequently binding with antioxidant responsive element sequences
and activating antioxidant-associated genes, including NAD(P)H
dehydrogenase, NQO-1 and HO-1 (50,51).
The present findings revealed that HG induced the expression of
nuclear Nrf2 and the target genes of the Nrf2 pathway, indicating
that HG may activate the Nrf2 pathway by inducing ROS production.
Of note, Zn supplementation further promoted the expression of
nuclear Nrf2 and target genes of the Nrf2 pathway, suggesting that
Zn supplementation may inhibit the EMT of HPMCs by activating the
Nrf2 pathway and subsequently decreasing oxidative stress. Kim and
Vaziri (52) reported that severe
oxidative stress and inflammation decreased Nrf2 activity, whereas
the Nrf2 repressor, Keap1 was upregulated, and the products of Nrf2
target genes decreased, consistent with the present findings. The
HG + TPEN group also demonstrated increased ROS, and a decrease in
the expression of nuclear Nrf2 and target proteins of the Nrf2
pathway; however, Nrf2-associated protein expression was not
significantly different compared with that in the HG group.
Our previous studies (20,23,24)
revealed that Zn attenuated the HG-induced EMT of peritoneal
mesothelial cells by downregulating TGF-β expression and inhibiting
mitogen-activated protein kinase (MAPK), NF-κB, and TGF-β/Smad
pathways. In the present study, Zn was reported to activate the
Nrf2 pathway. Therefore, it was hypothesized that Nrf2 regulates
the MAPK, NF-κB and TGF-β/Smad pathways by regulating the
expression of TGF-β1. The potential associations between these
pathways will be investigated by silencing TGF-β1 and Nrf2 in
future experiments.
In conclusion, the present findings demonstrated
that Zn reversed HG-induced EMT by activating the Nrf2 pathway. Zn
supplementation may be effective in preventing HG-induced PF;
however, further investigation is required to reveal whether Nrf2
pathway activation is the primary mechanism by which Zn alleviates
PF induced by a glucose-based PD solution, and whether patients
will benefit from Zn supplementation to prevent PF in PD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370865).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM designed the study. LG, YF, XZ, LY, WH, TH, ML
and SD performed the experiments. LG and YF analyzed the data and
prepared the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Ethics Committee of China Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Prasad AS: Discovery of human zinc
deficiency: Its impact on human health and diseas. Adv Nutr.
4:176–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krediet RT and Struijk DG: Peritoneal
changes in patients on long-term peritoneal dialysis. Nat Rev
Nephrol. 9:419–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang CY, Chau YP, Chen A, Lee OK, Tarng DC
and Yang AH: Targeting cannabinoid signaling for peritoneal
dialysis-induced oxidative stress and fibrosis. World J Nephrol.
6:111–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holmes CJ and Faict D: Peritoneal dialysis
solution biocompatibility: Definitions and evaluation strategies.
Kidney Int Suppl. S50–S56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yáñez-Mó M, Lara-Pezzi E, Selgas R,
Ramírez-Huesca M, Domínguez-Jiménez C, Jiménez-Heffernan JA,
Aguilera A, Sánchez-Tomero JA, Bajo MA, Alvarez V, et al:
Peritoneal dialysis and Epithelial-To-Mesenchymal transition of
mesothelial cells. N Engl J Med. 348:403–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu J, Xing C, Zhang L, Mao H, Chen X,
Liang M, Wang F, Ren H, Cui H, Jiang A, et al: Autophagy promotes
fibrosis and apoptosis in the peritoneum during long-term
peritoneal dialysis. J Cell Mol Med. 22:1190–1201. 2018.PubMed/NCBI
|
|
7
|
Kim YL: Update on mechanisms of
ultrafiltration failure. Perit Dial Int. 29 (Suppl 2):S123–S127.
2009.PubMed/NCBI
|
|
8
|
Ha H, Yu MR and Lee HB: High
glucose-induced PKC activation mediates TGF-beta 1 and fibronectin
synthesis by peritoneal mesothelial cells. Kidney Int. 59:463–470.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao Q, Pawlaczyk K, Ayala ER, Styszynski
A, Breborowicz A, Heimburger O, Qian JQ, Stenvinkel P, Lindholm B
and Axelsson J: The role of the TGF/Smad signaling pathway in
peritoneal fibrosis induced by peritoneal dialysis solutions.
Nephron Exp Nephrol. 109:e71–e78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ksiazek K, Breborowicz A, Jorres A and
Witowski J: Oxidative stress contributes to accelerated development
of the senescent phenotype in human peritoneal mesothelial cells
exposed to high glucose. Free Radic Biol Med. 42:636–641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Noh H, Kim JS, Han KH, Lee GT, Song JS,
Chung SH, Jeon JS, Ha H and Lee HB: Oxidative stress during
peritoneal dialysis: Implications in functional and structural
changes in the membrane. Kidney Int. 69:2022–2028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wellinghausen N and Rink L: The
significance of zinc for leukocyte biology. J Leukoc Biol.
64:571–577. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beyersmann D and Haase H: Functions of
zinc in signaling, proliferation and differentiation of mammalian
cells. Biometals. 14:331–341. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cousins RJ, Blanchard RK, Moore JB, Cui L,
Green CL, Liuzzi JP, Cao J and Bobo JA: Regulation of zinc
metabolism and genomic outcomes. J Nutr. 133 (Suppl 1):1521S–1526S.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Honscheid A, Rink L and Haase H:
T-lymphocytes: A target for stimulatory and inhibitory effects of
zinc ions. Endocr Metab Immune Disord Drug Targets. 9:132–144.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahashi M, Saito H, Higashimoto M and
Hibi T: Possible inhibitory effect of oral zinc supplementation on
hepatic fibrosis through downregulation of TIMP-1: A pilot study.
Hepatol Res. 37:405–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Zhou Z, Saari JT and Kang YJ:
Alcohol-induced myocardial fibrosis in metallothionein-null mice:
Prevention by zinc supplementation. Am J Pathol. 167:337–344. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandhi MS, Deshmukh PA, Kamalov G, Zhao T,
Zhao W, Whaley JT, Tichy JR, Bhattacharya SK, Ahokas RA and Sun Y:
Causes and consequences of zinc dyshomeostasis in rats with chronic
aldosteronism. J Cardiovasc Pharmacol. 52:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Van Biervliet S, Vande Velde S, Van
Biervliet JP and Robberecht E: The effect of zinc supplements in
cystic fibrosis patients. Ann Nutr Metab. 52:152–156. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Wang J, Fan Y, Yang L, Wang L and
Ma J: Zinc supplementation attenuates high glucose-induced
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells. Biol Trace Elem Res. 150:229–235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duman S, Sen S, Gunal AI, Asci G, Akcicek
F and Basci A: How can we standardize peritoneal thickness
measurements in experimental studies in rats? Perit Dial Int. 21
(Suppl 3):S338–S341. 2001.PubMed/NCBI
|
|
22
|
Rougier JP, Moullier P, Piedagnel R and
Ronco PM: Hyperosmolality suppresses but TGF beta 1 increases MMP9
in human peritoneal mesothelial cells. Kidney Int. 51:337–347.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Liang D, Guo B, Sun L, Chi ZH,
Cai Y, Wang L and Ma J: Zinc transporter 7 induced by high glucose
attenuates epithelial-to-mesenchymal transition of peritoneal
mesothelial cells. Biol Trace Elem Res. 151:138–147. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Liang D, Guo B, Yang L, Wang L
and Ma J: Zinc inhibits high glucose-induced apoptosis in
peritoneal mesothelial cells. Biol Trace Elem Res. 150:424–432.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiang S, Li M, Xie X, Xie Z, Zhou Q, Tian
Y, Lin W, Zhang X, Jiang H, Shou Z and Chen J: Rapamycin inhibits
epithelial-to-mesenchymal transition of peritoneal mesothelium
cells through regulation of Rho GTPases. FEBS J. 283:2309–2325.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kovac S, Angelova PR, Holmstrom KM, Zhang
Y, Dinkova-Kostova AT and Abramov AY: Nrf2 regulates ROS production
by mitochondria and NADPH oxidase. Biochim Biophys Acta.
1850:794–801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanlaya R, Khamchun S, Kapincharanon C and
Thongboonkerd V: Protective effect of epigallocatechin-3-gallate
(EGCG) via Nrf2 pathway against oxalate-induced epithelial
mesenchymal transition (EMT) of renal tubular cells. Sci Rep.
6:302332016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oyama TM, Ishida S, Okano Y, Seo H and
Oyama Y: Clioquinol-induced increase and decrease in the
intracellular Zn2+ level in rat thymocytes. Life Sci. 91:1216–1220.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Priel T, Aricha-Tamir B and Sekler I:
Clioquinol attenuates zinc-dependent beta-cell death and the onset
of insulitis and hyperglycemia associated with experimental type I
diabetes in mice. Eur J Pharmacol. 565:232–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JH, Jang BG, Choi BY, Kwon LM, Sohn M,
Song HK and Suh SW: Zinc chelation reduces hippocampal neurogenesis
after pilocarpine-induced seizure. PLoS One. 7:E485432012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nitzan YB, Sekler I, Frederickson CJ,
Coulter DA, Balaji RV, Liang SL, Margulis A, Hershfinkel M and
Silverman WF: Clioquinol effects on tissue chelatable zinc in mice.
J Mol Med (Berl). 81:637–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang T, Wang CY, Shan ZY, Teng WP and Wang
ZY: Clioquinol reduces zinc accumulation in neuritic plaques and
inhibits the amyloidogenic pathway in AβPP/PS1 transgenic mouse
brain. J Alzheimers Dis. 29:549–559. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cherny RA, Atwood CS, Xilinas ME, Gray DN,
Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, et
al: Treatment with a copper-zinc chelator markedly and rapidly
inhibits beta-amyloid accumulation in Alzheimer's disease
transgenic mice. Neuron. 30:665–676. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang M, Zhao L, Ren M, Deng M and Li C:
Reduced metallothionein expression induced by Zinc deficiency
results in apoptosis in hepatic stellate cell line LX-2. Int J Clin
Exp Med. 8:20603–20609. 2015.PubMed/NCBI
|
|
36
|
Yang H, Keen CL and Lanoue L: Influence of
intracellular zinc on cultures of rat cardiac neural crest cells.
Birth Defects Res B Dev Reprod Toxicol. 104:11–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lopez-Cabrera M: Mesenchymal conversion of
mesothelial cells is a key event in the pathophysiology of the
peritoneum during peritoneal dialysis. Adv Med. 2014:4731342014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu J, Zeng L, Zhao Y, Zhu B, Ren W and Wu
C: Selenium suppresses lipopolysaccharide-induced fibrosis in
peritoneal mesothelial cells through inhibition of
epithelial-to-mesenchymal transition. Biol Trace Elem Res.
161:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strippoli R, Moreno-Vicente R, Battistelli
C, Cicchini C, Noce V, Amicone L, Marchetti A, Del Pozo MA and
Tripodi M: Molecular mechanisms underlying peritoneal EMT and
fibrosis. Stem Cells Int. 2016:35436782016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Aroeira LS, Aguilera A, Sanchez-Tomero JA,
Bajo MA, del Peso G, Jimenez-Heffernan JA, Selgas R and
Lopez-Cabrera M: Epithelial to mesenchymal transition and
peritoneal membrane failure in peritoneal dialysis patients:
Pathologic significance and potential therapeutic interventions. J
Am Soc Nephrol. 18:2004–2013. 2004. View Article : Google Scholar
|
|
43
|
Hsieh HL, Chi PL, Lin CC, Yang CC and Yang
CM: Up-regulation of ROS-dependent matrix metalloproteinase-9 from
high-glucose-challenged astrocytes contributes to the neuronal
apoptosis. Mol Neurobiol. 50:520–533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee HB, Yu MR, Song JS and Ha H: Reactive
oxygen species amplify protein kinase C signaling in high
glucose-induced fibronectin expression by human peritoneal
mesothelial cells. Kidney Int. 65:1170–1179. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oteiza PI, Olin KL, Fraga CG and Keen CL:
Zinc deficiency causes oxidative damage to proteins, lipids and DNA
in rat testes. J Nutr. 125:823–829. 1995.PubMed/NCBI
|
|
46
|
Ho E and Ames BN: Low intracellular zinc
induces oxidative DNA damage, disrupts p53, NFkappa B, and AP1 DNA
binding, and affects DNA repair in a rat glioma cell line. Proc
Natl Acad Sci USA. 99:16770–16775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Prasad AS: Clinical, immunological,
anti-inflammatory and antioxidant roles of zinc. Exp Gerontol.
43:370–377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bao B, Prasad AS, Beck FW, Snell D, Suneja
A, Sarkar FH, Doshi N, Fitzgerald JT and Swerdlow P: Zinc
supplementation decreases oxidative stress, incidence of infection,
and generation of inflammatory cytokines in sickle cell disease
patients. Transl Res. 152:67–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1-Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McMahon M, Lamont DJ, Beattie KA and Hayes
JD: Keap1 perceives stress via three sensors for the endogenous
signaling molecules nitric oxide, zinc, and alkenals. Proc Natl
Acad Sci USA. 107:18838–18843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang R, Paul VJ and Luesch H: Seaweed
extracts and unsaturated fatty acid constituents from the green
alga Ulva lactuca as activators of the cytoprotective Nrf2-ARE
pathway. Free Radic Biol Med. 57:141–153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kim HJ and Vaziri ND: Contribution of
impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in
chronic renal failure. Am J Physiol Renal Physiol. 298:F662–F671.
2010. View Article : Google Scholar : PubMed/NCBI
|