Introduction
Scars are the products of wound healing by the human
body. Among them, hypertrophic scars (HS) and keloids are known as
pathological scars, which are characterized by sustained
hyperactive growth, irregular scar shape, and an uneven and tough
texture (1). Pathological scars
are the result of hypertrophy of scar tissue in the process of
tissue repair following trauma. Pathological scars have serious
aesthetic effects and result in tissue dysfunction due to
deformities of the scar contractures, particularly at the joints.
Certain patients experience pain, itching, ulceration and the
development of cancerous cells (2,3).
Although pathological scars are recognized as harmful, their
etiology and pathogenesis are not fully understood.
Ubiquitin-specific protease 4 (USP4), also known as
UnpEL or Unph, is encoded by the proto-oncogene USP4. As an
important member of the deubiquitinase family, USP4 was initially
reported to bind to the tumor suppressor retinoblastoma, and
associated pocket proteins retinoblastoma-like protein 1,
retinoblastoma-like protein 2 and E3 ubiquitin (4). It has been hypothesized that USP4 and
E3 ubiquitin-protein ligase TRIM21 may regulate their substrates by
forming heterodimers via ubiquitination/deubiquitination pathways
(5). The transforming growth
factor-β (TGF-β) superfamily members are important functional
biological molecules (6). TGF-β is
closely associated with extracellular matrix deposition, fibrosis
and wound healing, which promotes formation of pathological scars
(7).
Smads are intracellular molecules of the TGF-β
signal transduction pathway (8).
TGF-β activates the pathway through membrane receptors TGF-β
receptor I (TβRI) and TβRII, which rapidly phosphorylate Smad
proteins activating C-terminal serine residues, forming
heterologous complexes that in turn activate the transcription of
target nuclear genes (9,10). USP4 has been reported to directly
deubiquitylate TβRI (11).
However, the mechanisms regarding USP4-TβRI in pathological
scarring are not completely clear. In the present study, the role
of USP4 in the formation of pathological scars in BALB/c nude mice
inoculated with keloid cells was examined. In addition, the
specific molecular mechanism by which USP4 regulates pathological
scar formation was assessed.
Materials and methods
Construction of a USP4 lentiviral
interference vector
Based on data from the National Center for
Biotechnology Information, 3 short hairpin RHA (shRNA) sequences
were designed by Santa Cruz (Santa Cruz Biotechnology, Inc.)
(Table I).
 | Table I.shRNA sequences for silencing USP4
expression. |
Table I.
shRNA sequences for silencing USP4
expression.
| shRNA molecule | Sequence (5′-3′) |
|---|
| sh-USP4-F1 |
GATCCGGAAGAAGTATGTGGGCTTTGCTTCCTGTCAGACAAAGCCCACATACTTCTTCCTTTTTG |
| sh-USP4-R1 |
AATTCAAAAAGGAAGAAGTATGTGGGCTTTGTCTGACAGGAAGCAAAGCCCACATACTTCTTCCG |
| sh-USP4-F2 |
GATCCGCCTTTCTTCTAGATGGATTGCTTCCTGTCAGACAATCCATCTAGAAGAAAGGCTTTTTG |
| sh-USP4-R2 |
AATTCAAAAAGCCTTTCTTCTAGATGGATTGTCTGACAGGAAGCAATCCATCTAGAAGAAAGGCG |
| sh-USP4-F3 |
GATCCGCTGAACATGTCCGAGTTTGTCTTCCTGTCAGAACAAACTCGGACATGTTCAGCTTTTTG |
| sh-USP4-R3 |
AATTCAAAAAGCTGAACATGTCCGAGTTTGTTCTGACAGGAAGACAAACTCGGACATGTTCAGCG |
Annealing conditions were as follows:
Pre-denaturation at 95°C for 5 min, 85°C for 5 min, 75°C for 5 min
and 70°C for 5 min, and then maintenance at 4°C. The annealing
product (1 µl) was diluted 200-fold and the final concentration was
4.4 ng/l, which was used for subsequent reactions. At 37°C, a
PLVshRNA-EGFP (2A) Puro vector (VL3103; Beijing Yingmao Shengye
Biotechnology Co., Ltd.) was digested by BamH I/EcoRI
and subjected to 1% agarose gel electrophoresis. The 7,861 bp
fragments were collected. Lentiviruses encoding shUSP4 were
established as described previously (12).
Cell transfection
Human keloid fibroblasts (KFs) were purchased from
the American Type Culture Collection (cat. no. CRL-1762) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Biological Industries) and 100 U/ml penicillin-streptomycin
(P1400; Beijing Solarbio Science & Technology, Co., Ltd.) in 5%
CO2 at 37°C. Cells at 80% confluence were transfected
with the aforementioned lentiviruses (Shanghai GenePharma Co. Ltd.)
encoding USP4 shRNA (1×106 IFU/PFU/ml) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 6 h, the medium was replaced with fresh
DMEM medium containing 10% FBS and cells cultured in a 5%
CO2 incubator at 37°C for 24 h. Experiments were
performed 48 h after transfection. Expression of USP4 was verified
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The experimental groups were as follows: Control;
shUSP4; vector; vector + TGF-β (48 h after transfection, 10 ng/ml
TGF-β was applied for an additional 48 h); and shUSP4 +TGF-β (48 h
after transfection, 10 ng/ml TGF-β was applied for an additional 48
h).
MTT assay
Following treatment, the cells (3×105/ml)
were digested and suspended in 96-well plates. The cell numbers at
0 and 48 h were assessed by an MTT assay. MTT (50 µl) was added to
each well, and the cells cultured at 37°C for 4 h in a 5%
CO2 incubator. After 4 h incubation, supernatants were
discarded and dimethyl sulfoxide (150 µl) added to each well to
dissolve the formazan. Absorption was measured at 550 nm with an
enzyme labeling instrument.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Following transfection and treatment for 48 h, mRNA
from the different treatment groups was extracted using a TRIzol
assay kit (Baosheng Science & Technology Innovation, Co. Ltd.).
mRNA was transcribed into cDNA using a reverse transcription kit
(cat. no. 639522, Takara Biotechnology Co., Ltd.) at 37°C using the
following thermocycler conditions: 25°C for 10 min, 37°C for 120
min and 85°C for 5 min. RT-qPCR was used to detect the expression
level of the target genes with SYBR Green (HY-K0501;
MedChemExpress). The thermocycling conditions were as follows:
Initial denaturation at 95°C for 10 min, followed by 40 cycles of
PCR at 95°C for 10 sec, 60.3°C for 30 sec and 72°C for 30 sec. The
2−ΔΔCq method was used to quantify the results as
previously described (13,14). The primers (5′-3′) used are listed
in Table II.
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Genes | Primers | Primer length,
bp | Product length,
bp | Annealing
temperature, °C |
|---|
| TβRI F |
GACGGCGTTACAGTGTTTCT | 20 | 318 | 56.47 |
| TβRI R |
CCTTTGCCAATGCTTTCTT | 19 |
|
|
| Smad 7 F |
CCAAAGGTCACCACCATCC | 19 | 105 | 58.82 |
| Smad 7 R |
TCAGTTTCTTGAGCACCGAGT | 21 |
|
|
| GAPDH F |
GAAGGTCGGAGTCAACGGAT | 20 | 224 | 58.3 |
| GAPDH R |
CCTGGAAGATGGTGATGGG | 19 |
|
|
Immunoprecipitation (IP)
IP experiments were performed using a Pierce
Crosslink IP kit (Thermo Fisher Scientific, Inc.). As described in
the manufacturer's instructions, the cells of different groups were
homogenized in IP lysis/wash buffer containing proteasome inhibitor
MG-132 (10 mM; Sigma-Aldrich; Merck KGaA) and complete EDTA-free
Protease Inhibitor Cocktail (Sigma-Aldrich; Merck KGaA). The
supernatants were collected after centrifugation at 15,000 × g for
10 min at 4°C. Samples (2 mg) were added to anti-ubiquitin
antibody-cross-linked Protein A/G Plus Agarose, and then incubated
at 4°C overnight. Following incubation, nonspecific binding was
eliminated by repeated washing with IP lysis/wash buffer. Eluted IP
products were used for western blot analysis to detect
ubiquitinated TRβI with an anti-TrβI antibody (1:1,000; cat. no.
ab92486; Abcam).
Western blot analysis
Following transfection and treatment for 48 h,
proteins were extracted from cell lines for western blot analysis
using a ReadyPrep protein isolation kit (GE Healthcare Life
Sciences) as described previously (15). Protein levels were quantified with
a bicinchoninic acid protein assay kit. Proteins (25 µg/lane) were
separated via 12% SDS-PAGE and transferred onto nitrocellulose
membranes. The membranes were blocked with 5% skim milk for 2 h at
room temperature and incubated with the following primary
antibodies overnight at 4°C: Anti-TRβI (1:1,000; cat. no. ab92486;
Abcam); and anti-Smad7 (1:1,000; cat. no., ab190987; Abcam). The
anti-rabbit IgG horseradish peroxidase-conjugated secondary
antibody (1:100; cat. no. ab131368; Abcam) was added and incubated
with the membranes for 2 h at room temperature. An enhanced
chemiluminescence reagent (cat. no. RPN2133; GE Healthcare Life
Sciences) was added to the membranes. The membranes were visualized
using a gel imaging system (Bio-Rad Laboratories, Inc.).
Densitometry was performed using Quantity One v1.4.6 (Bio-Rad
Laboratories, Inc.). Experiments were repeated 3 times.
Establishment of an in vivo tumor
model
All animal experiments were approved by the Ethics
Committee of Nanchang University (Nanchang, China). A total of 24
male BALB/c nude mice (4-week-old) were purchased from Hunan
Shanghai Laboratory Animal Center, Jingda Laboratory Animal Co.,
Ltd., License No. SCXK 2016-0002) and housed in specific
pathogen-free conditions that were automatically maintained at a
temperature of 23±2°C, a relative humidity of 45–65%, and with a
controlled 12 h light/dark cycle. Mice implanted with the tumor
cell lines were randomly divided into four groups (n=5 in each
group): A control cell group; a control cells + vialinin A (USP
inhibitor, HY-13814, MedChemExpress LLC) group; a
shUSP4-transfected cell group; and a vector transfected cell group.
Animals were injected with KFs in the logarithmic growth phase
(1×106). For the control group, KFs were diluted in 0.2
ml PBS and administrated through subcutaneous injection into the
nude mice. For the vector control group, KFs transfected with
control vector were diluted in 0.2 ml PBS and injected. For the
shUSP4 group, KFs transfected with viral USP4 shRNA were diluted in
0.2 ml PBS and injected. For the control cells + vialinin A group,
KFs were treated with vialinin A (5 µM, 48 h) and diluted in 0.2 ml
PBS and injected. For the shUSP4 group, KFs transfected with viral
USP4 shRNA (Santa Cruz Biotechnology, Inc.) were diluted in 0.2 ml
PBS and injected. The nude mice were sacrificed at 14, 28 and 42
days after inoculation. All animals presented with a single
subcutaneous tumor in the forelimb armpit and the longest diameter
was <8 mm. Following complete anesthesia with 5% isoflurane, the
tumors were excised and stored at −80°C for western blot analysis,
RT-qPCR, and fixed with 10% formalin overnight at 4°C for
hematoxylin and eosin staining.
Hematoxylin and eosin (H&E)
staining
The tissues were then embedded in paraffin for
tissue sectioning and sectioned into 5 µm-thick sections. Then, the
sections were stained with hematoxylin (3%) and eosin (3%) at room
temperature for 3 min and observed by light microscopy
(magnification, ×200, BX51, Olympus Corporation).
Statistical analysis
Data are presented as mean ± standard error of the
mean. Analyses were performed using SPSS version 19.0 (IBM Corp.).
Significant differences were determined using one-way analysis of
variance followed by the Student-Newman-Keuls post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
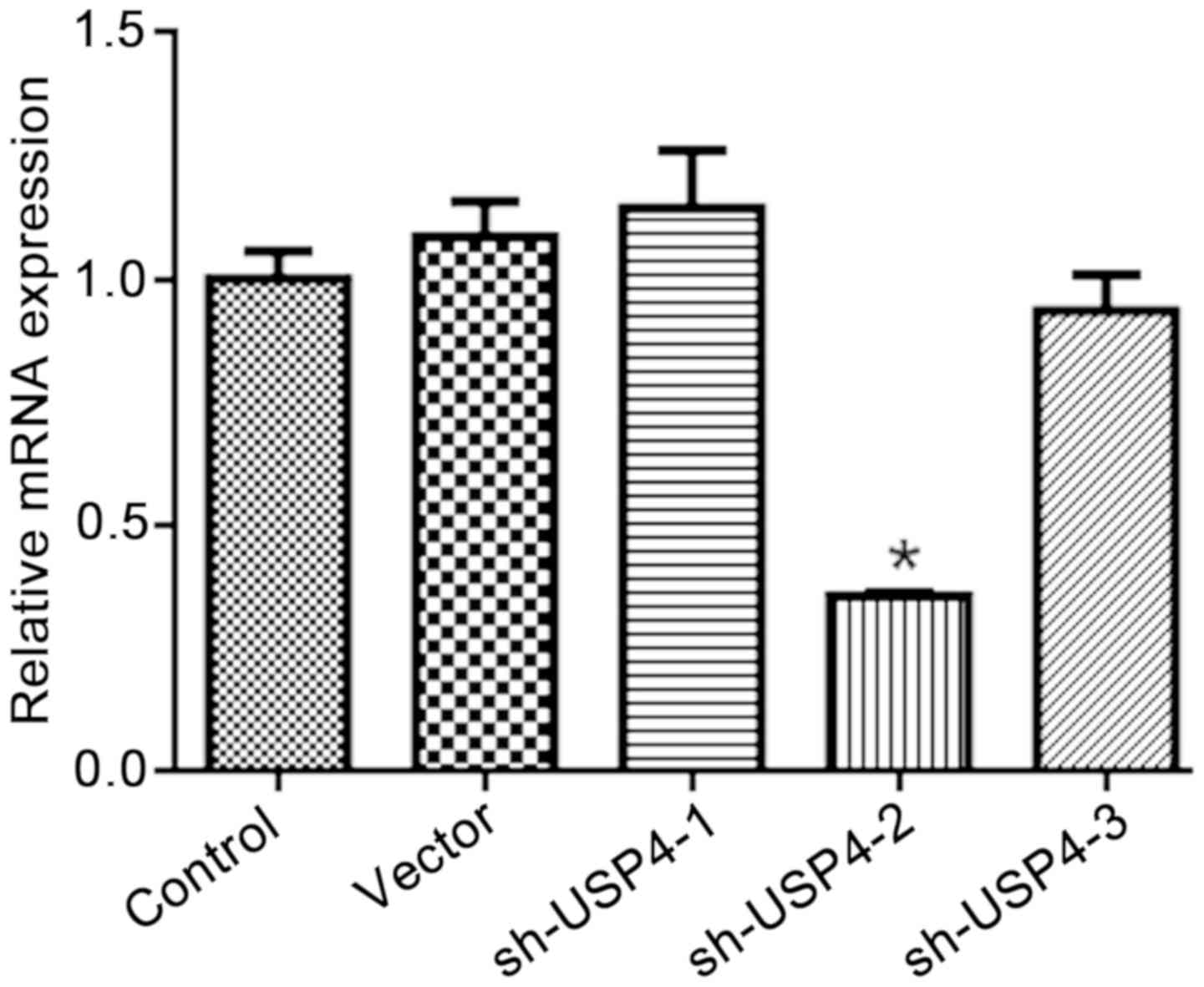
Confirmation of USP4 silencing
As demonstrated in Fig.
1, 3 interference sequences for shUSP4 were designed, with
shUSP4-2 producing a significant interference effect. Therefore,
shUSP4-2 was selected for subsequent experiments.
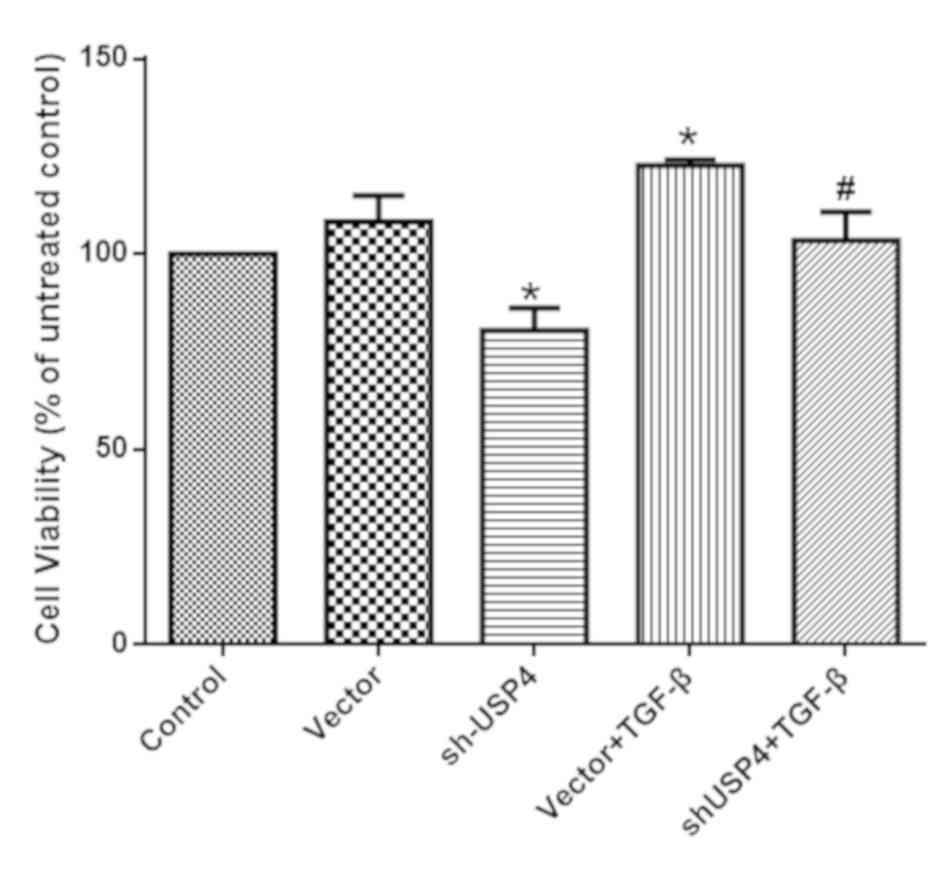
shUSP4 decreases cell viability
As presented in Fig.
2, the cell viability of the shUSP4 group was significantly
decreased compared with the control group. By contrast, TGF-β
incubation significantly increased cell viability, which was
decreased by shUSP4 interference (P<0.05). These data suggest
that USP4 silencing decreased the proliferation of KFs in the
negative control, following TGF-β-treatment.
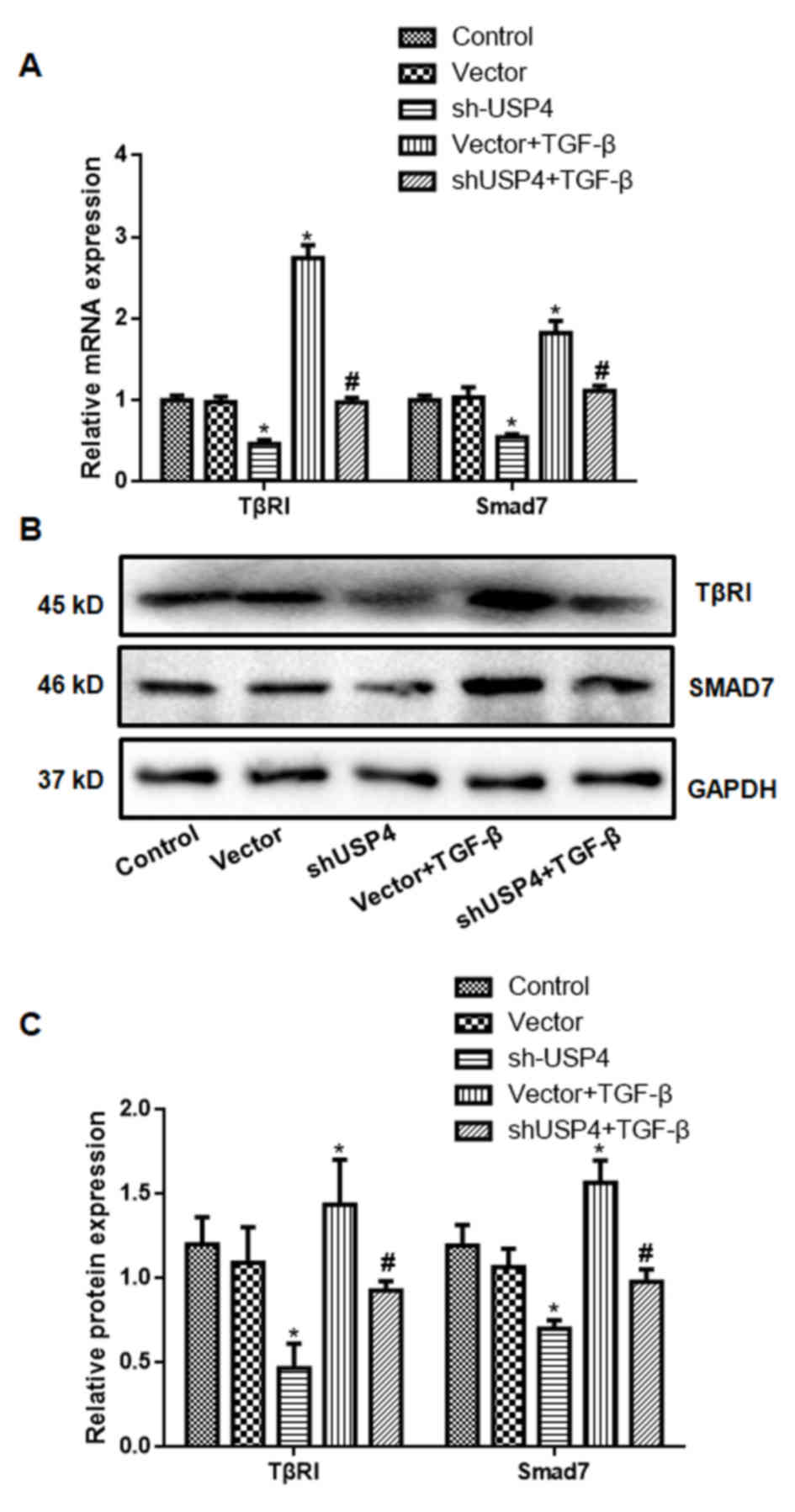
shUSP4 decreases TβRI and Smad7
expression
The expression levels of TβRI and Smad7 for each
group are indicated in Fig. 3.
Compared with the control group, the expression of TβRI and Smad7
by the shUSP4 group was significantly decreased, while the
expression levels of TβRI and Smad7 in the Vector + TGF-β group was
significantly increased; this was attenuated by shUSP4 (P<0.05).
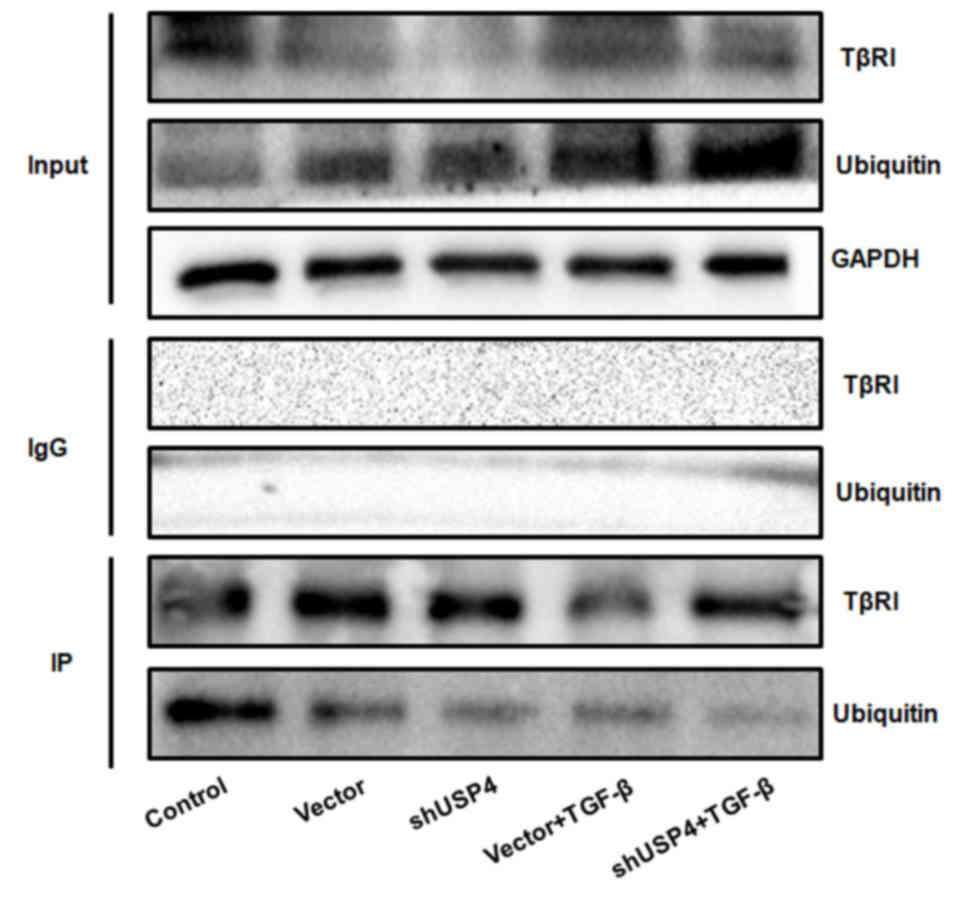
As demonstrated by the IP analysis data, ubiquitination of TβRI was
identified in each group (Fig. 4),
indicating a direct correlation of TβRI with ubiquitin.
shUSP4 facilitates necrotic death of
tumor tissue and decreases the expression of TβRI and Smad7
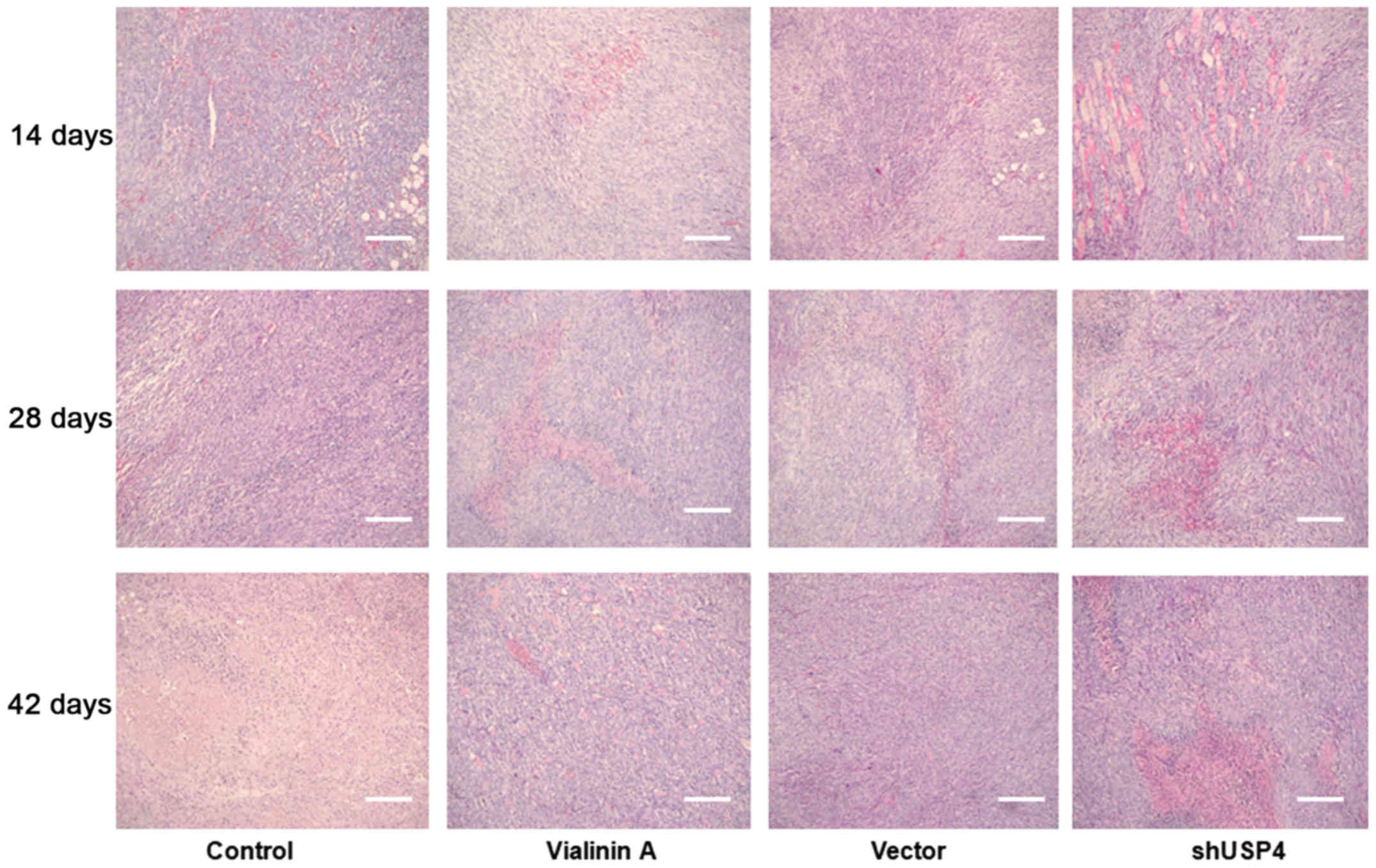
As demonstrated in Fig.
5, at day 14 there was no remarkable difference in the
histological structures of the KF among the groups. At days 28 and
42, marked necrotic scarring was observed in the shUSP4 and
vialinin A groups compared with the control group.
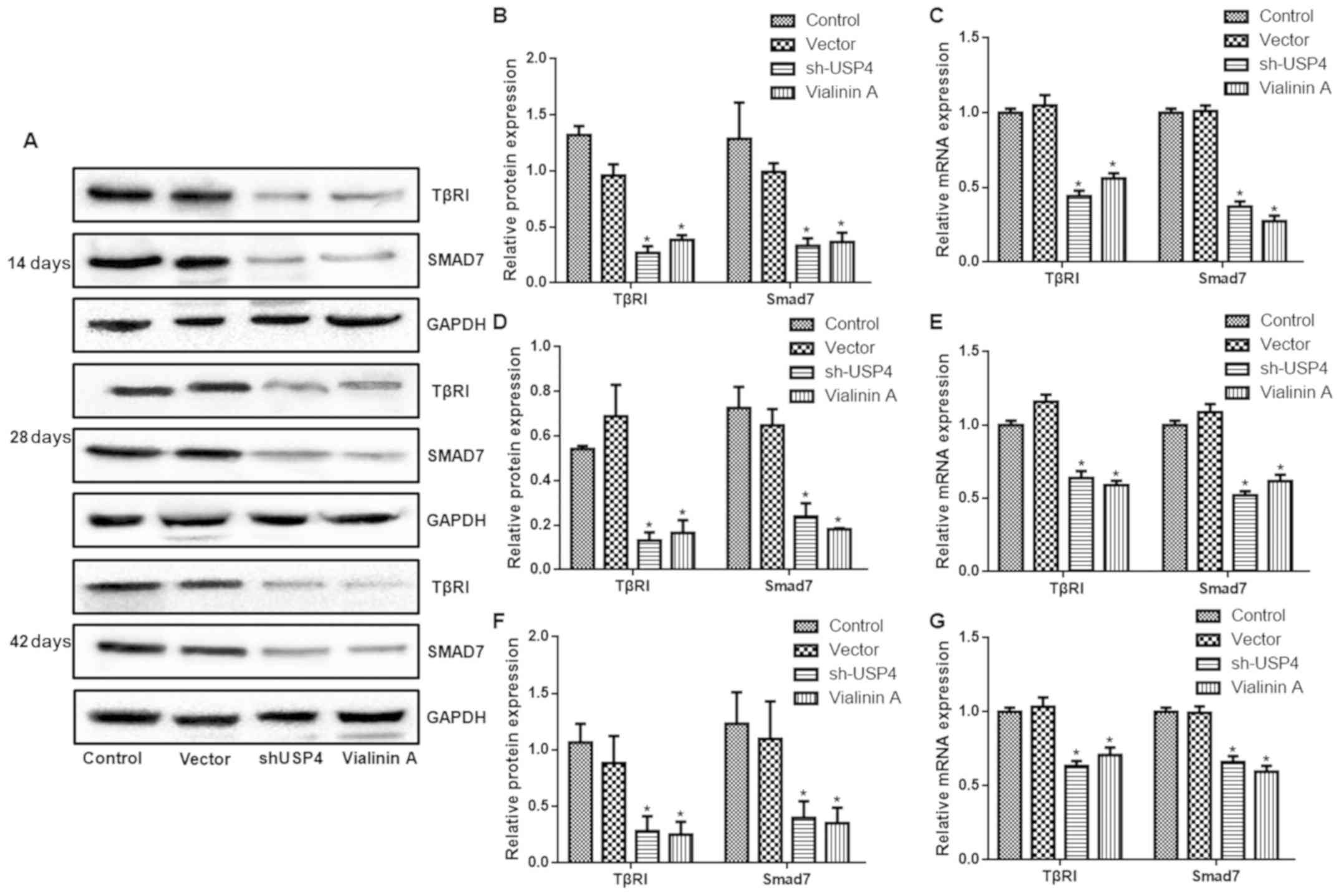
As presented in Fig.
6, the expression levels of TβRI and Smad7 in the shUSP4 and
vialinin A groups were significantly decreased compared with those
of the control group at days 14, 28 and 42 (P<0.05).
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that silencing or inhibition of USP4
prevented the TGF-β/Smad signaling pathway, which inhibited the
formation of pathological scars. USP4 may bind TβRI to inhibit the
Smad7-mediated signaling pathway, preventing the ubiquitination of
TβRI, which maintains a high level of TβRI and sustained
stimulation of TGF-β in tissues of the skin.
The localization and stability of TβRI determines
the activity of the TGF-β signaling pathway (16), while the Smad7/Smurf 2 complex
targets and degrades TβRI through the ubiquitination pathway
(17). Using genome-wide
functional screening, Zhang et al (11) identified that USP4 enhanced the
TGF-β signaling pathway. USP4 interacts directly with TβRI and
inhibits its ubiquitination pathway, thereby maintaining a high
level of TβRI at the cell membrane surface (11). Removal of USP4 attenuates the
process of epithelial mesenchymal transition mediated by the TGF-β
pathway (11). In the present
study, cellular and animal experiments revealed that the expression
of TβRI and Smad7 decreased following USP4 silencing, which
indicated that USP4 affected the TGF-β signaling pathway. The
tumorigenesis experiment demonstrated that USP4 inhibition improved
pathological scarring. These results suggested that USP4 may serve
a regulatory role in the formation of pathological scars by
regulating the TGF-β signaling pathway.
USP4 is also involved in the negative regulation of
the Wnt signaling pathway (18),
deubiquitination of adenosine A2a (19) and regulation of the cell cycle
(20). Zhang et al
(21) identified that USP4
enhanced its stability by ubiquitinating E3 ubiquitin-protein
ligase HUWE1, in turn downregulating P53 expression. Fan et
al (22) reported that USP4
downregulated TNF-α-induced NF-κB activation by deubiquitinating
mitogen-activated protein kinase kinase kinase 7. In the present
study, following the downregulation of USP4, the ubiquitination
level of TβRI was markedly decreased and pathological scarring
improved. These results imply an association between pathological
scarring and the ubiquitination level of TβRI.
The TGF-β/Smad pathway serves a major regulatory
role in the formation of pathological scar formation (23,24).
The expression of TGF-β1, TGF-β2 and TβRI in keloid keratinocytes
was increased compared with that of normal skin, suggesting an
association between TGF-β1, TGF-β2 and TβRI in KFs. Fibroblasts
have been induced to express more TGF-β1 and TGF-β2 by paracrine
action (25). The expression
levels of TGF-β1/Smad3 differ during stages of human growth and
development; TGF-β/Smad3 are not expressed at all during the fetal
period. This may, in part, explain the mechanistic basis for the
presence of scarless wounds during the fetal period (26,27).
Chin et al (28)
demonstrated that the expression levels of TβRI and Smad3 proteins
in KFs were significantly increased compared with normal skin,
indicating that these proteins exhibited positive feedback effects
on the TGF-β/Smad pathway, promoting the formation of keloids.
Bock et al (29) revealed that in KFs, the expression
of TβRI was high and the expression of TβRII was low, and that the
proliferation of KF was enhanced when stimulated with TGF-β1.
Goldberg et al (30)
suggested that the overexpression of TβRII inhibited the
proliferation of fibroblasts. Bran et al (31) also demonstrated that the expression
levels of TGF-β1 and TGF-β2 in cultured KFs were increased compared
with that in normal fibroblasts, while the expression of TβRII was
significantly decreased compared with that in normal fibroblasts.
The TGF-β/Smad signal transduction pathway affects the healing
process (32). This pathway
functions at multiple levels, from the release of early
inflammatory mediators, to wound healing, to subsequent
pathological scarring (33).
The results of the in vitro and animal
experiments suggest that the inhibition of the TGF-β/Smad signaling
pathway may effectively decrease the deposition of extracellular
matrix, and the levels of tissue fibrosis and pathological scarring
during wound healing (34).
Ubiquitination is a prominent strategy for post-translational
modification, which regulates a number of biological functions,
including: Cell cycle; apoptosis; DNA damage repair; cellular
immunity; and neuronal degeneration (35–37).
The results of the present study demonstrate that ubiquitin had a
direct association with TβRI, which in turn regulated downstream
signal transduction and pathological scarring.
There were several limitations of the present study.
Firstly, to the best of our knowledge, the study was the first to
use an in vivo xenograft tumor model to examine pathological
scarring. The similarity between these in vitro and in
vivo experiments requires further confirmation. Secondly,
whether USP4 may be a target for the treatment of pathological
scarring requires additional pharmacological data.
In summary, the inhibition of USP4 attenuates the
TGF-β/Smad signaling pathway and decreases the formation of
pathological scars. The present study provides the foundation for a
novel method to prevent and treat pathological scars of the
skin.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81460295 and
81660322) and Jiangxi Provincial Science Foundation for
Distinguished Young Scholars (grant no. 20171BCB23090).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, SN, SP, SL, YX, QJ, ZL, JY and ZC performed the
experiments and analyzed the data. JZ and ZC designed the study and
wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of Nanchang University (Nanchang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song H, Tan J, Fu Q, Huang L and Ao M:
Comparative efficacy of intralesional triamcinolone acetonide
injection during early and static stage of pathological scarring. J
Cosmet Dermatol. Jun 22–2018.(Epub ahead of print).
|
|
2
|
Liao N, Lu F, Zhao W, Zeng WS, Li YT, Wang
SJ and Gao JH: Relationship between gene p53 codon 72 polymorphism
and pathological scar formation after caesarean section. Zhonghua
Zheng Xing Wai Ke Za Zhi. 29:206–210. 2013.(In Chinese). PubMed/NCBI
|
|
3
|
Wu WY, Zhang LT, Zheng ZF, Zhu SZ and Wang
ZY: Expression and significance of mRNA and protein of eIF4E,
p-eIF4E and MCl-1 in pathological scar. Zhonghua Zheng Xing Wai Ke
Za Zhi. 28:360–365. 2012.(In Chinese). PubMed/NCBI
|
|
4
|
DeSalle LM, Latres E, Lin D, Graner E,
Montagnoli A, Baker RT, Pagano M and Loda M: The de-ubiquitinating
enzyme Unp interacts with the retinoblastoma protein. Oncogene.
20:5538–5542. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada K and Kamitani T: UnpEL/Usp4 is
ubiquitinated by Ro52 and deubiquitinated by itself. Biochem
Biophys Res Commun. 342:253–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan X, Liao H, Cheng M, Shi X, Lin X, Feng
XH and Chen YG: Smad7 protein interacts with receptor-regulated
Smads (R-Smads) to inhibit transforming growth factor-β
(TGF-β)/Smad signaling. J Biol Chem. 291:382–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong X, Zhao B, Iacob RE, Zhu J, Koksal
AC, Lu C, Engen JR and Springer TA: Force interacts with
macromolecular structure in activation of TGF-β. Nature. 542:55–59.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Q, Guo S, Wang CC, Sun X, Wang D, Xu
N, Jin SF and Li KZ: Cross-talk between TGF-β/Smad pathway and
Wnt/β-catenin pathway in pathological scar formation. Int J Clin
Exp Pathol. 8:7631–7639. 2015.PubMed/NCBI
|
|
9
|
Beanes SR, Dang C, Soo C and Ting K: Skin
repair and scar formation: The central role of TGF-beta. Exp Rev
Mol Med. 5:1–22. 2003. View Article : Google Scholar
|
|
10
|
Penn JW, Grobbelaar AO and Rolfe KJ: The
role of the TGF-β family in wound healing, burns and scarring: A
review. Int J Burns Trauma. 2:18–28. 2012.PubMed/NCBI
|
|
11
|
Zhang L, Zhou F, Drabsch Y, Gao R,
Snaar-Jagalska BE, Mickanin C, Huang H, Sheppard KA, Porter JA, Lu
CX and ten Dijke P: USP4 is regulated by AKT phosphorylation and
directly deubiquitylates TGF-β type I receptor. Nat Cell Biol.
14:717–726. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li W, Tao S, Wu Q, Wu T, Tao R and Fan J:
Glutamine reduces myocardial cell apoptosis in a rat model of
sepsis by promoting expression of heat shock protein 90. J Surg
Res. 220:247–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu G, Li J, He L, Wang X and Hong X:
MPTP-induced changes in hippocampal synaptic plasticity and memory
are prevented by memantine through the BDNF-TrkB pathway. Br J
Pharmacol. 172:2354–2368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song Z, Chen H, Xu W, Wu S and Zhu G:
Basolateral amygdala calpain is required for extinction of
contextual fear-memory. Neurobiol Learn Mem. 155:180–188. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong G, Huang Z, Jiang H, Pan Z, Xie J
and Wang S: Inhibition of microRNA-21 decreases the invasiveness of
fibroblast-like synoviocytes in rheumatoid arthritis via TGFβ/Smads
signaling pathway. Iran J Basic Med Sci. 19:787–793.
2016.PubMed/NCBI
|
|
17
|
Xu X, Xu C, Saud SM, Lu X, Liu L, Fang L,
Zhang X, Hu J and Li W: Effect of kuijie granule on the expression
of TGF-β/Smads signaling pathway in patients with ulcerative
colitis. Evid Based Complement Alternat Med. 2016:26018302016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao B, Schlesiger C, Masucci MG and
Lindsten K: The ubiquitin specific protease 4 (USP4) is a new
player in the Wnt signalling pathway. J Cell Mol Med. 13:1886–1895.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Milojevic T, Reiterer V, Stefan E, Korkhov
VM, Dorostkar MM, Ducza E, Ogris E, Boehm S, Freissmuth M and
Nanoff C: The ubiquitin-specific protease Usp4 regulates the cell
surface level of the A2A receptor. Mol Pharmacol. 69:1083–1094.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wijnhoven P, Konietzny R, Blackford AN,
Travers J, Kessler BM, Nishi R and Jackson SP: USP4
auto-deubiquitylation promotes homologous recombination. Mol Cell.
60:362–373. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Berger FG, Yang J and Lu X: USP4
inhibits p53 through deubiquitinating and stabilizing ARF-BP1. EMBO
J. 30:2177–2189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan YH, Yu Y, Mao RF, Tan XJ, Xu GF, Zhang
H, Lu XB, Fu SB and Yang J: USP4 targets TAK1 to downregulate
TNFα-induced NF-κB activation. Cell Death Differ. 18:1547–1560.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Zhang L, Lei R, Shen Y, Shen H, Wu
Z and Xu J: Effects of blocking two sites of transforming growth
factor-β/Smads signaling on the formation of scar-related proteins
in human skin fibroblasts. Zhonghua Shao Shang Za Zhi. 31:372–377.
2015.(In Chinese). PubMed/NCBI
|
|
24
|
Nong Q, Li S, Wu Y and Liu D: LncRNA
COL1A2-AS1 inhibits the scar fibroblasts proliferation via
regulating miR-21/Smad7 pathway. Biochem Biophys Res Commun.
495:319–324. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia W, Phan TT, Lim IJ, Longaker MT and
Yang GP: Complex epithelial-mesenchymal interactions modulate
transforming growth factor-beta expression in keloid-derived cells.
Wound Repair Regen. 12:546–556. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Emami A, Halim AS, Salahshourifar I,
Yussof SJ, Khoo TL and Kannan TP: Association of TGFβ1 and SMAD4
variants in the etiology of keloid scar in the Malay population.
Arch Dermatol Res. 304:541–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ward SV, Cadby G, Heyworth JS, Fear MW,
Wallace HJ, Cole JM, Wood FM and Palmer LJ: Association of TGFβ1
and clinical factors with scar outcome following melanoma excision.
Arch Dermatol Res. 304:343–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chin GS, Liu W, Peled Z, Lee TY,
Steinbrech DS, Hsu M and Longaker MT: Differential expression of
transforming growth factor-beta receptors I and II and activation
of Smad 3 in keloid fibroblasts. Plast Reconstr Surg. 108:423–429.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bock O, Yu H, Zitron S, Bayat A, Ferguson
MW and Mrowietz U: Studies of transforming growth factors beta 1–3
and their receptors I and II in fibroblast of keloids and
hypertrophic scars. Acta Derm Venereol. 85:216–220. 2005.PubMed/NCBI
|
|
30
|
Goldberg HJ, Huszár T, Mózes MM, Rosivall
L and Mucsi I: Overexpression of the type II transforming growth
factor-beta receptor inhibits fibroblasts proliferation and
activates extracellular signal regulated kinase and c-Jun
N-terminal kinase. Cell Biol Int. 26:165–174. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bran GM, Goessler UR, Schardt C, Hormann
K, Riedel F and Sadick H: Effect of the abrogation of TGF-beta1 by
antisense oligonucleotides on the expression of TGF-beta-isoforms
and their receptors I and II in isolated fibroblasts from keloid
scars. Int J Mol Med. 25:915–921. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li SC, Ma LN, Chen J and Li YK: Effect of
allicin on myocardial fibrosis after myocardial infarction in rats
and its relationship with TGFβ/Smads signal transduction. Zhongguo
Zhong Yao Za Zhi. 41:2517–2521. 2016.(In Chinese). PubMed/NCBI
|
|
33
|
Hu ZC, Shi F, Liu P, Zhang J, Guo D, Cao
XL, Chen CF, Qu SQ, Zhu JY and Tang B: TIEG1 Represses
Smad7-mediated activation of TGF-β1/Smad signaling in keloid
pathogenesis. J Invest Dermatol. 137:1051–1059. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
D'Arpino MC, Fuchs AG, Sánchez SS and
Honoré SM: Extracellular matrix remodeling and TGF-β1/Smad
signaling in diabetic colon mucosa. Cell Biol Int. 42:443–456.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu Y, Kang J, Zhang L, Liang Z, Tang X,
Yan Y, Qian H, Zhang X, Xu W and Mao F: Ubiquitination regulation
of inflammatory responses through NF-κB pathway. Am J Transl Res.
10:881–891. 2018.PubMed/NCBI
|
|
37
|
Cheng YF, Zhu GQ, Wang M, Cheng H, Zhou A,
Wang N, Fang N, Wang XC, Xiao XQ, Chen ZW and Li QL: Involvement of
ubiquitin proteasome system in protective mechanisms of Puerarin to
MPP(+)-elicited apoptosis. Neurosci Res. 63:52–58. 2009. View Article : Google Scholar : PubMed/NCBI
|