Introduction
Osteoarthritis (OA) is a leading cause of disability
and is characterized by articular cartilage destruction,
subchondral bone changes and synovial tissue inflammation (1). In previous decades, the incidence of
OA has increased substantially in several countries and regions,
including the Nordic region (2)
and the United States (3).
Emerging evidence has revealed that OA is an inflammatory disease
and the increased secretion of various pro-inflammatory chemokines
is crucial in the pathogenesis of OA (4). In addition, signaling factors
involved in the proliferation, differentiation, senescence and
apoptosis of chondrocytes, which is the only cell type in mature
cartilage, have been shown to be partially responsible for OA
processes (5–7). However, the molecular mechanisms
contributing to the pathology and pathophysiology of OA remain to
be fully elucidated.
MicroRNAs (miRNAs) constitute a large family of
small non-coding RNAs (~22 nucleotides) that function as
post-transcriptional regulators of gene expression by recognizing
and binding to partially complementary sites in the 3′-untranslated
region (3′-UTR) of target genes for either the cleavage or
depression of translation in eukaryotic organisms (8). A number of miRNAs have been
implicated in regulating a wide range of complex biological
processes, including development, proliferation, differentiation,
apoptosis, and immune and inflammation responses (9). The aberrant or deregulated expression
of miRNAs is often associated with the biology and pathology of
human cancer (10), cardiovascular
disease (11) and OA (12), and this is a major target for
diagnostic, prognostic and therapeutic interventions. Nugent first
examined the differences in the levels of 157 miRNAs between human
cartilage and bone in post-mortem OA specimens from donors with no
previous joint pain (13). It was
found that 30 miRNAs were significantly dysregulated in the
cartilage and bone of diseased tissue compared with the normal
control (13). Alterations in
miRNA expression levels, including miRNA (miR)-9, miR-140, miR-27b
and miR-34a, are associated with OA processes (14–17).
miR-671-3p is known to be downregulated in the serum of patients
with OA, and it has been suggested to be involved in metabolic
processes that contribute to OA pathology (18). However, the biological function and
the underlying molecular mechanisms of miR-671-3p in OA remain to
be elucidated. Pathogen-associated molecular pattern-activated
Toll-like receptor (TLR) is important in innate immune responses,
cell apoptosis and the production of interferons and pro- and
anti-inflammatory cytokines (19).
As an indispensable component of signaling pathways triggered by
members of the tumor necrosis factor (TNF) receptor (TNFR) and TLR
families, TNFR-associated factor (TRAF) has been shown to function
as a negative regulator of nuclear factor-κB and mitogen-activated
protein kinases (19,20). TRAF-interacting protein is
important in regulating inflammatory process in rheumatoid
arthritis-proliferative fibroblast-like synoviocytes and is
considered a potential therapeutic target for human rheumatoid
arthritis (21). Therefore, TRAF
may be crucial in the development of OA.
In the present study, the expression of miR-671-3p
was detected in human knee OA tissues and normal cartilage tissues,
and in OA chondrocytes and normal chondrocytes. This in combination
with experiments involving the gain- and loss-of-function of
miR-671-3p in OA chondrocytes and a luciferase reporter assay, was
used to evaluate the implication of miR-671-3p and its association
with TRAF3 in the progression of OA. The results will clarify the
potential correlations between miR-671-3p/TRAF3 and the development
of OA, which may be conductive to exploitation of a novel strategy
for the treatment of OA.
Materials and methods
Tissue samples and chondrocyte
culture
The human knee OA cartilage samples were collected
from patients (n=15; six women and nine men, aged 50–79 years)
undergoing knee replacement surgery at Jingzhou Central Hospital
(Jingzhou, China; Table I). None
of the patients had received intra-articular steroid injections
within 3 months prior to surgery. All patients were diagnosed based
on the American College of Rheumatology criteria (22) and evaluated by a certified
rheumatologist. Matched healthy cartilage tissues were obtained
from traumatic amputees (n=15; eight men and seven women, aged
50–79 years) with no history of OA or joint pain. The collected
knee OA cartilage samples or healthy cartilage tissues were placed
in 0.25% trypsin-EDTA for digestion, followed by centrifugation at
800 × g for 30 min at 4°C for isolating the chondrocytes. All
participants signed informed consent forms and the study was
approved by the Ethics Committee of Jingzhou Central Hospital.
 | Table I.Clinical characteristics of patients
with knee OA and traumatic amputees. |
Table I.
Clinical characteristics of patients
with knee OA and traumatic amputees.
| Patient
characteristic | Patients with knee
OA | Traumatic
amputees |
|---|
| Sex |
|
Male | 9 | 8 |
|
Female | 6 | 7 |
| Average age
(years) |
|
50–59 | 8 | 6 |
|
60–69 | 5 | 6 |
|
70–79 | 2 | 3 |
| Occupation |
| Office
staff | 5 | 7 |
|
Technician | 4 | 4 |
|
Housewife | 2 | 1 |
|
Other | 4 | 3 |
Chondrocytes were released from the cartilage
samples as previously described (23). Following isolation, the
chondrocytes were cultured in Dulbecco's modified Eagle's medium
(DMEM, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.), streptomycin (100 mg/ml; Gibco; Thermo Fisher
Scientific, Inc.), and penicillin (100 units/ml; Gibco; Thermo
Fisher Scientific, Inc.) and maintained in an incubator at 37°C
with 5% CO2. During the culture period, the medium was
replaced every 2–3 days and cultured chondrocytes at 80% confluence
were used in the experiments.
Cell transfection
The human chondrocytes were transfected with
scramble miRNA mimics as the negative control, miR-671-3p mimics,
and miR-671-3p inhibitor, respectively (purchased from GenePharma,
Shanghai, China). For the rescue experiment, the full-length cDNA
sequence for human TRAF3 was obtained from the National Center for
Biotechnology Information database, amplified by PCR in OA
chondrocytes and cloned into the pcDNA3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.) to generate the pcDNA3.1-TRAF3
expression vector. The empty pcDNA3.1-vector was used as control.
All cell transfections were performed using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), following the
manufacturer's protocol. At 48 h post-transfection, the cells were
used for the following experiments.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNAs from the cartilage tissues and
chondrocytes were isolated using the miRNeasy Mini kit and reverse
transcribed with the miScript Reverse Transcription kit (Qiagen
GmbH, Dusseldorf, Germany) according to the manufacturer's
protocol. For RT-qPCR analysis, the TaqMan microRNA Assay kit was
used for miR-671-3p and U6 expression analysis. The 20-µl reaction
system included 5 µl cDNA, 10 µl 2X SYBR premix ex taq, 0.8 µl
forward (miR-671-3p, 5′-GCCGCCAAAGTGCTGTTC-3′; U6,
5′-CTCGCTTCGGCAGCACA-3′) and reverse (miR-671-3p,
5′-CAGAGCAGGGTCCGAGGTA-3′; U6, 5′-AACGCTTCACGAATTTGCGT-3′) primers
(2.5 µM) and 4.2 µl ddH2O. All reactions were performed
in triplicate on a 7900 Real-time system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with the following conditions: 95°C
for 10 min, followed by 40 cycles at 95°C for 15 sec, and 60°C for
1 min. The expression of U6 was used as endogenous control to
normalize the expression level of miR-671-3p. Relative gene
expression was calculated using the 2−ΔΔCq method
(24).
ELISA
The synovial fluid and cell supernatants were
collected from the 24-well plates. The concentrations of
inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-8
and TNF-α, were detected using ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Cell Counting Kit-8 (CCK-8)
The chondrocytes were seeded in 96-well plates at
the density of 1,000 cells per well. Following transfection, the
cells were cultured for another 24, 48, 72 or 96 h, respectively.
The supernatant was removed, and 10 µl of CCK-8 reagent (Beyotime
Institute of Biotechnology, Shanghai, China) was added to each well
to continue culture for 2 h at 37°C. The optical density (OD) value
was obtained at the wavelength of 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blotting
Total protein was extracted from the frozen
cartilage tissues and cultured cells using RIPA lysis buffer and
then quantified using the Pierce BCA kit (Thermo Fisher Scientific,
Inc.). Subsequently, ~30 µg of protein lysate was loaded onto a 10%
SDS denatured polyacrylamide gel and then transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Bedford, MA.
USA). After 2 h of blocking with 5% fat-free milk, the membranes
were then incubated with primary antibodies against collagen type
II α1 chain (COL2A1; 1:5,000; cat. no. ab34712; Abcam, Cambridge,
UK), aggrecan (ACAN; 1:2,000; cat. no. ab36861; Abcam), a
disintegrin and metalloproteinase with thrombospondin motifs
(ADAMTS)-5 (1:250; cat. no. ab41037; Abcam) and GAPDH (1:5,000;
cat. no. 10494-1-AP; Proteintech Group, Inc. Chicago, IL, USA)
overnight at 4°C, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
sc-2054; Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. The protein bands were detected using the
Supersignal® West Pico kit (Thermo Fisher Scientific,
Inc.). Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA) was used to quantify the gray value of the
bands.
Flow cytometry
Cell apoptosis was assayed by flow cytometry using
Annexin V-APC/7-AAD double staining. Briefly, the cells were washed
twice with PBS and then resuspended in binding buffer. The cells
were then subjected to Annexin V-APC/7-AAD double staining
according to the manufacturer's protocol (KeyGEN Biotech Co., Ltd.,
Nanjing, China). The stained cells were analyzed using a
FACScalibur (BD Biosciences, Franklin Lakes, NJ, USA).
Caspase-3 activity assay
The caspase-3 activity assay was performed using the
Caspase Colorimetric Assay kit (KeyGEN Biotech Co., Ltd.).
Following transfection of the cells for 48 h, the cells were
collected and disrupted in lysis buffer on ice for 20 min. The
lysates were then incubated with the caspase substrate at 37°C in
the reaction buffer for 4 h. The OD value at the wavelength of 405
nm was detected using a microplate reader (Spectra MAX Plus,
Molecular Devices, LLC). The relative caspase-3 activity was
assessed with the percentage of OD values in the treatment group
over that in the control group.
miRNA target prediction and
dual-luciferase reporter assay
The miRNAs targets were predicted using the
TargetScan (http://www.targetscan.org/), miRanda (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/) tools. Among putative
genes predicted by the three algorithms, TRAF3 was indicated as a
potential target gene of miR-671-3p.
The wild-type TRAF3-3′-UTR (Wt TRAF3) and mutant
TRAF3-3′-UTR (Mut TRAF3) containing the putative binding site of
miR-671-3p were constructed and cloned into pmirGLO dual luciferase
reporter vectors (Promega Corporation, Madison, WI, USA). For the
dual reporter luciferase assay, the reporter vectors containing the
Wt TRAF3 or Mut TRAF3 and miR-671-3p mimics or scramble were
co-transfected into 293T cells (ATCC, Manassas, VA, USA) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.).
Following 48 h of incubation, the luciferase activity was analyzed
with the Dual Luciferase Reporter Assay system (Promega
Corporation) according to the manufacturer's protocol.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 software (IBM Corp. Armonk, NY, USA), and graphs were
generated using GraphPad Prism 6 software (GraphPad Software, Inc.,
San Diego, CA, USA). Data are expressed as the mean ± standard
deviation of at least three experiments. Statistical significance
was evaluated using Student's t-test for two-group comparison and
one-way analysis of variance for multiple-group comparison.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-671-3p is
downregulated in knee OA clinical specimens
RT-qPCR analysis was used to evaluate the expression
levels of miR-671-3p in 15 knee OA cartilage tissues and 15 normal
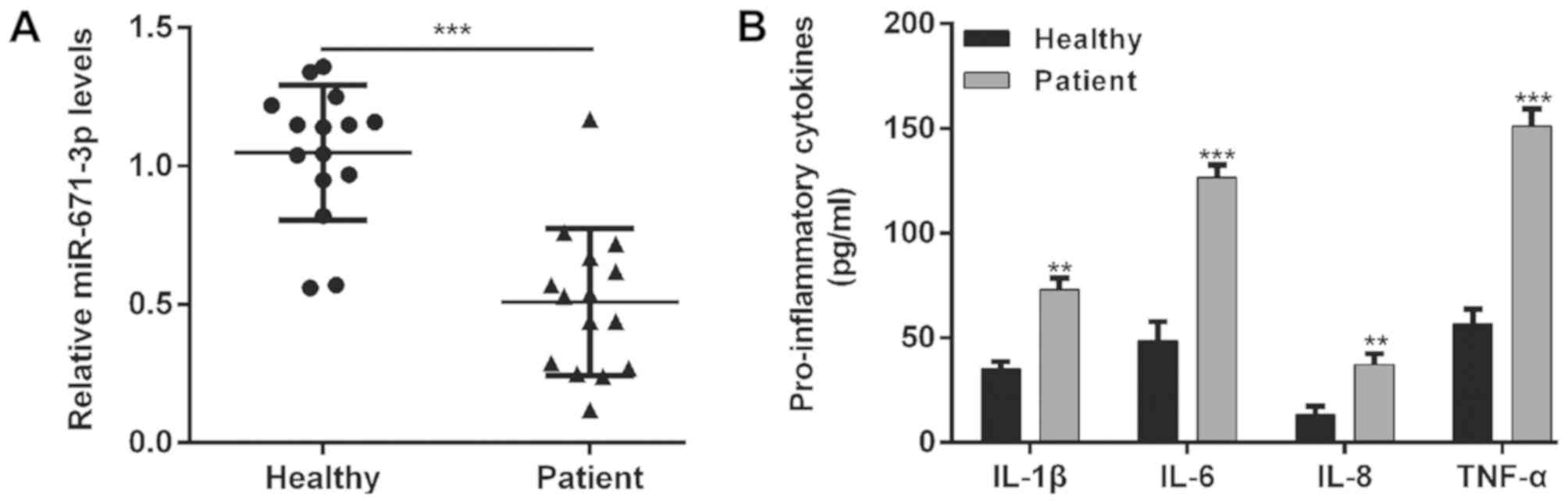
cartilage tissues. As shown in Fig.
1A, the expression of miR-671-3p in the knee OA cartilage
tissues was significantly lower than that in the normal tissues
(P<0.001). In addition, the concentration of several
pro-inflammatory cytokines was determined by ELISA. The results
showed increased levels of IL-1β (73.0±5.6 vs. 35.0±3.6,
P<0.01), IL-6 (126.7±6.1 vs. 48.7±9.1, P<0.001), IL-8
(37.3±5.0 vs. 13.3±4.2, P<0.01) and TNF-α (151.3±8.0 vs.
56.7±7.0, P<0.001) in the knee OA cartilage tissues compared
with the normal cartilage tissues (Fig. 1B).
Upregulation of miR-671-3p promotes
matrix synthesis and chondrocyte proliferation
To further confirm the regulation of miR-671-3p in
the pathogenesis of OA, its mRNA level was also analyzed in
chondrocytes from patients with OA and healthy participants, and it
was found that chondrocytes from patients with OA had lower
expression of miR-671-3p (Fig. 2A,
P<0.001), which suggested that miR-671-3p may function as a
suppressor of OA. Subsequently, scramble control, miR-671-3p
mimics, or miR-671-3p inhibitor was transfected into OA
chondrocytes, respectively. As shown in Fig. 2B, the miR-671-3p mimics increased
the expression of miR-671-3p (2.0±0.1 vs. 0.5±0.1, P<0.001) and
the miR-671-3p inhibitor decreased the expression of miR-671-3p
(0.13±0.01 vs. 2.0±0.1, P<0.01) in the OA chondrocytes. Using
gain-of-function and loss-of-function experiments, the effect of
the expression of miR-218-5p on cell proliferation was analyzed
using a CCK-8 assay. As shown in Fig.
2C, the proliferation of chondrocytes was significantly
increased when the cells were transfected with miR-671-3p mimics,
compared with the scramble group at 72 and 96 h (P<0.05 and
P<0.01). However, miR-671-3p inhibitor transfection suppressed
the proliferation of chondrocytes (P<0.05). Furthermore, the
effect of the expression of miR-671-3p on matrix synthesis
biomarkers, including COL2A1, ACAN, and ADAMTS-5, was analyzed in
chondrocytes following 48 h of transfection. The results of the
western blotting showed that matrix protein expression (COL2A1 and
ACAN) was increased, whereas the expression of catabolic factor
ADAMTS-5 was decreased in chondrocytes transfected with miR-671-3p
mimics compared with the scramble control- or miR-671-3p
inhibitor-transfected cells (Fig.
2D).
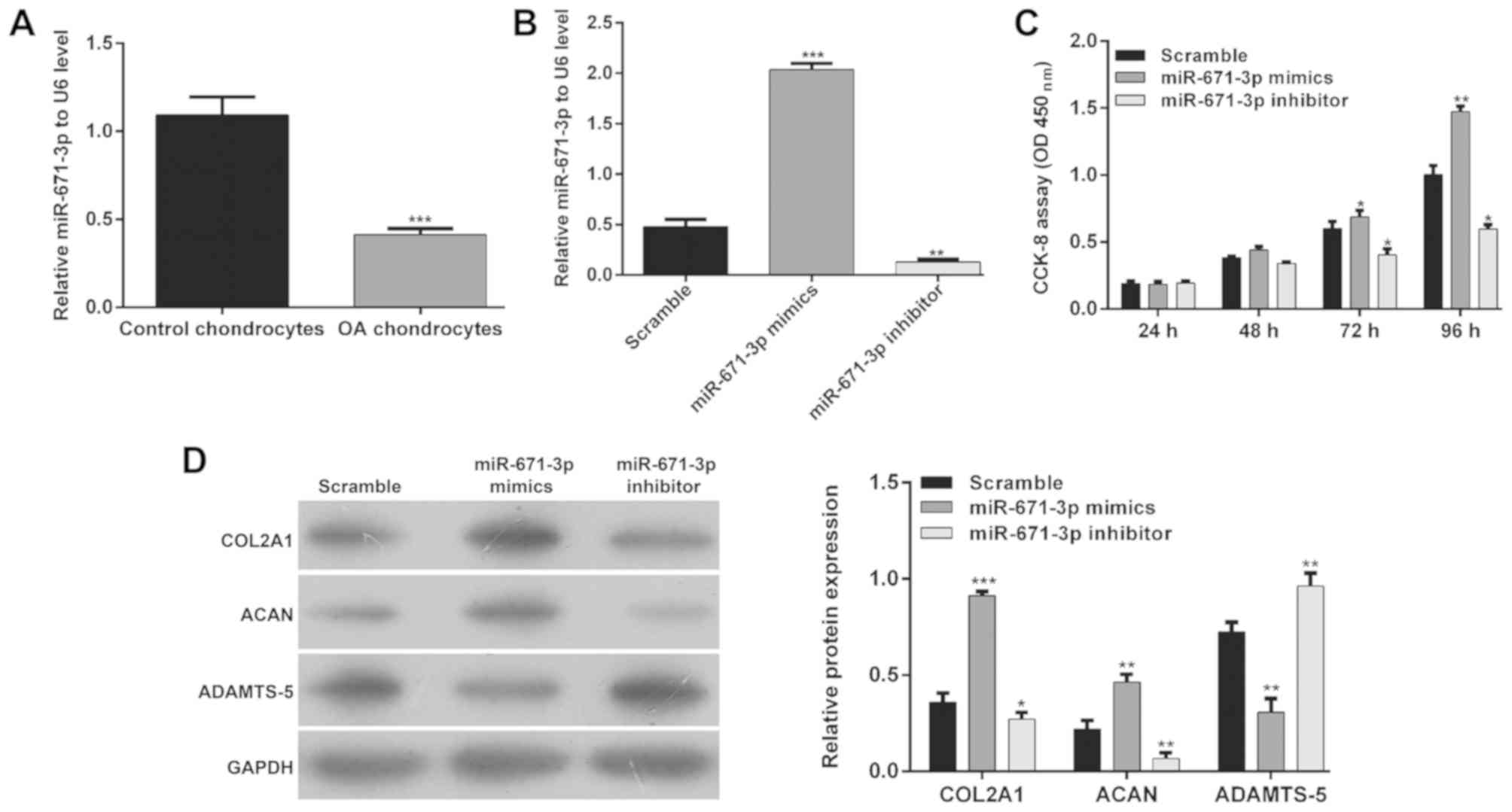 | Figure 2.Effects of the expression of
miR-671-3p on matrix synthesis and chondrocyte proliferation. (A)
Analysis of the expression of miR-671-3p in chondrocytes from
healthy participants and patients with OA by RT-qPCR analysis. The
miR-control, miR-671-3p mimics or miR-671-3p inhibitor were
transfected into human OA chondrocytes. At 48 h post-transfection,
the cells were used for the following experiments. (B) Transfection
efficiency of miR-671-3p was analyzed by RT-qPCR analysis. (C)
Chondrocyte proliferation was investigated in OA chondrocytes at
24, 48, 72 and 96 h, respectively. Data are presented as the mean ±
standard deviation from triplicate experiments. (D) Expression
levels of COL2A1, ACAN and ADAMTS-5 were detected by western blot
analysis. *P<0.05, **P<0.01 and ***P<0.001, compared with
the scramble group. miR, microRNA; OA, osteoarthritis; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction;
COL2A1, collagen type II α1 chain; ACAN, aggrecan ADAMTS-5, a
disintegrin and metalloproteinase with thrombospondin motifs 5;
CCK-8, Cell Counting Kit-8; OD, optical density. |
Upregulation of miR-671-3p inhibits
chondrocyte apoptosis and inflammation
Accumulating evidence indicates that miRNAs are
important in regulating apoptosis and inflammation associated with
OA. Therefore, the present study evaluated whether the altered
expression of miR-671-3p affected chondrocyte apoptosis and
inflammation. As shown in Fig. 3A,
the results of the flow cytometric analysis revealed that the
apoptotic rates of the chondrocytes transfected with miR-671-3p
mimics and scramble control were 14.0±0.4 and 25.7±2.9%,
respectively, with a significant difference (P<0.01). However,
the apoptotic rate in the miR-671-3p inhibitor group was 66.0±3.4%,
which was significantly higher than that in the scramble group
(P<0.001). In addition, the caspase-3 activity of chondrocytes
was significantly suppressed by miR-671-3p mimics transfection, but
enhanced by miR-671-3p inhibitor transfection when compared with
the scramble group (Fig. 3B,
P<0.01 and P<0.001, respectively). Additionally, the
pro-inflammatory cytokines IL-1β, IL-6, IL-8 and TNF-α were all
significantly decreased by the overexpression of miR-671-3p
(Fig. 3C, P<0.05 and
P<0.01). These results demonstrated that the overexpression of
miR-671-3p alleviated cell apoptosis and pro-inflammatory cytokine
production in the OA chondrocytes.
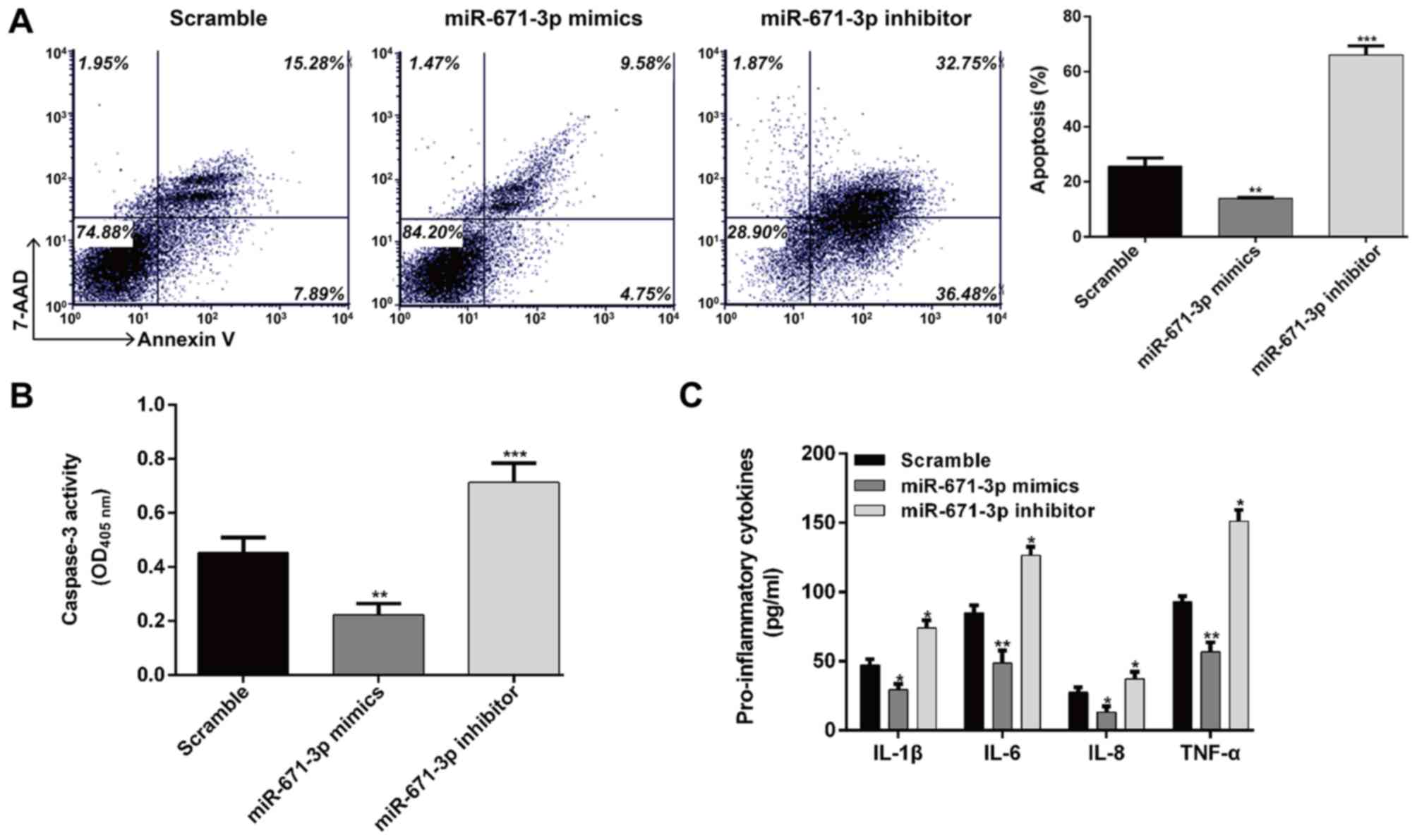 | Figure 3.Effects of the expression of
miR-671-3p on chondrocyte apoptosis and inflammation. The
miR-control, miR-671-3p mimics or inhibitor were transfected into
human osteoarthritis chondrocytes, respectively. At 48 h
post-transfection, the cells were used for the following
experiments. (A) Apoptotic ability of chondrocytes at 48 h
post-transfection was detected by the flow cytometric analysis. (B)
Cell apoptosis was detected using a caspase-3 activity assay. (C)
Production of IL-1β, IL-6, IL-8 and TNF-α in the cell culture
supernatants was detected using an enzyme-linked immunosorbent
assay. Data are presented as the mean ± standard deviation from
triplicate experiments. *P<0.05, **P<0.01 and ***P<0.001,
compared with the scramble group. miR, microRNA; IL, interleukin;
TNF, tumor necrosis factor; OD, optical density. |
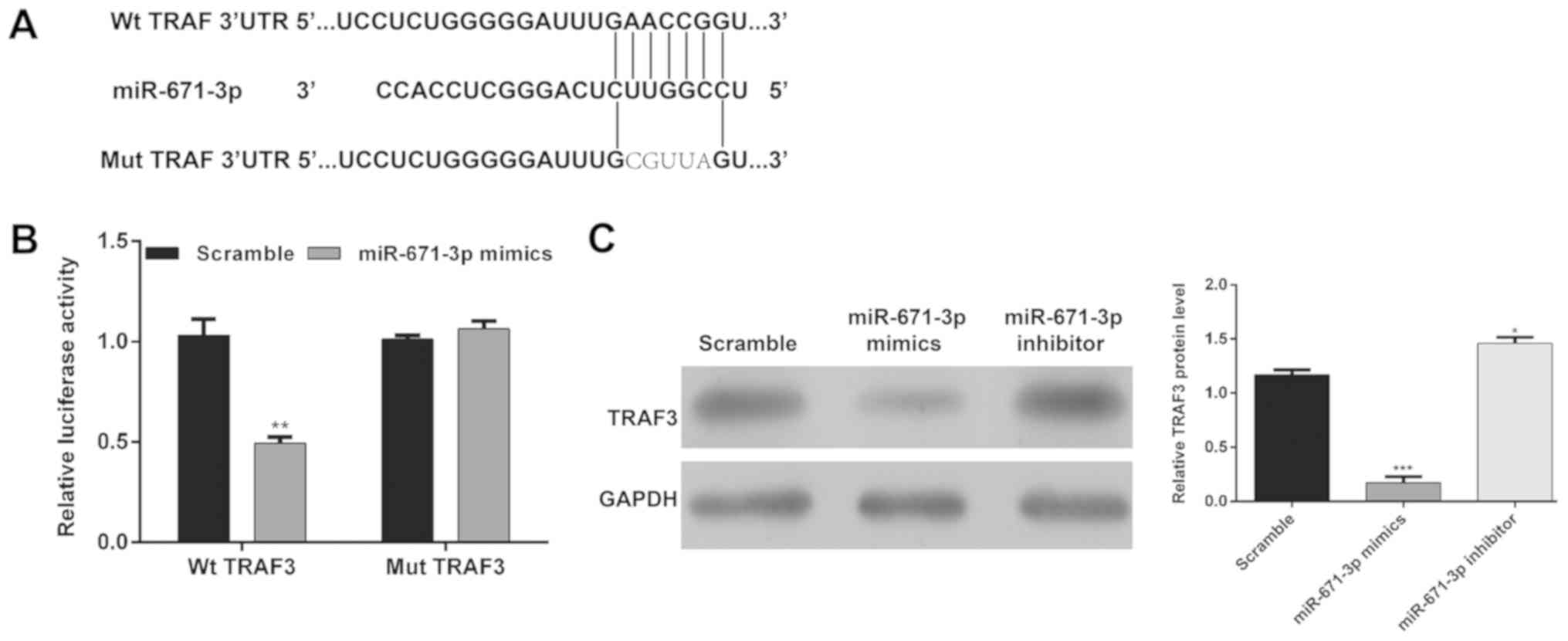
Identification of TRAF3 as a
functional target of miR-671-3p
To define the target by which miR-671-3p regulates
the pathogenesis of OA, the TargetScan, miRanda, PicTar databases
were used. The results predicted that TRAF3 was a target of
miR-671-3p, which contains a putative target seed sequence
(Fig. 4A). Subsequently, firefly
luciferase reporters containing the Wt 3′-UTR of TRAF3 or Mut
3′-UTR of TRAF3 were constructed and the luciferase reporter assay
was performed. As shown in Fig.
4B, miR-671-3p mimics transfection significantly inhibited the
luciferase expression of the reporter containing Wt TRAF3, but not
the Mut reporter gene (P<0.01). In addition, western blot
analysis revealed that the upregulation of miR-671-3p significantly
reduced the protein levels of TRAF3 and the downregulation of
miR-671-3p significantly increased the protein levels of TRAF3 in
OA chondrocytes (Fig. 4C). These
results suggested that miR-671-3p directly targeted TRAF3 and
negatively regulated the expression of TRAF3 by binding to its
3′-UTR.
Restoration of TRAF3 markedly
abrogates the effect of miR-671-3p in chondrocytes
To validate whether miR-671-3p regulates the
pathogenesis of OA through TRAF3, a rescue experiment was performed
by restoring the expression of TRAF3. The CCK-8 assay showed that
the restoration of TRAF3 significantly abrogated the enhanced
effect of miR-671-3p on the proliferation of OA chondrocytes
(Fig. 5A, P<0.05 and
P<0.01). In addition, the overexpression of TRAF3 partially
reversed the miR-671-3p-induced upregulation of COL2A1 and ACAN and
downregulation of ADAMTS-5 (Fig.
5B). Furthermore, the protective effects of miR-671-3p on
OA-induced apoptosis (Fig. 5C,
P<0.01 and P<0.001) and inflammation (Fig. 5D, P<0.05 and P<0.01) were
also eliminated by restoring the expression of TRAF3. These results
suggested that miR-671-3p protected chondrocytes from OA-induced
injury by inhibiting TRAF3.
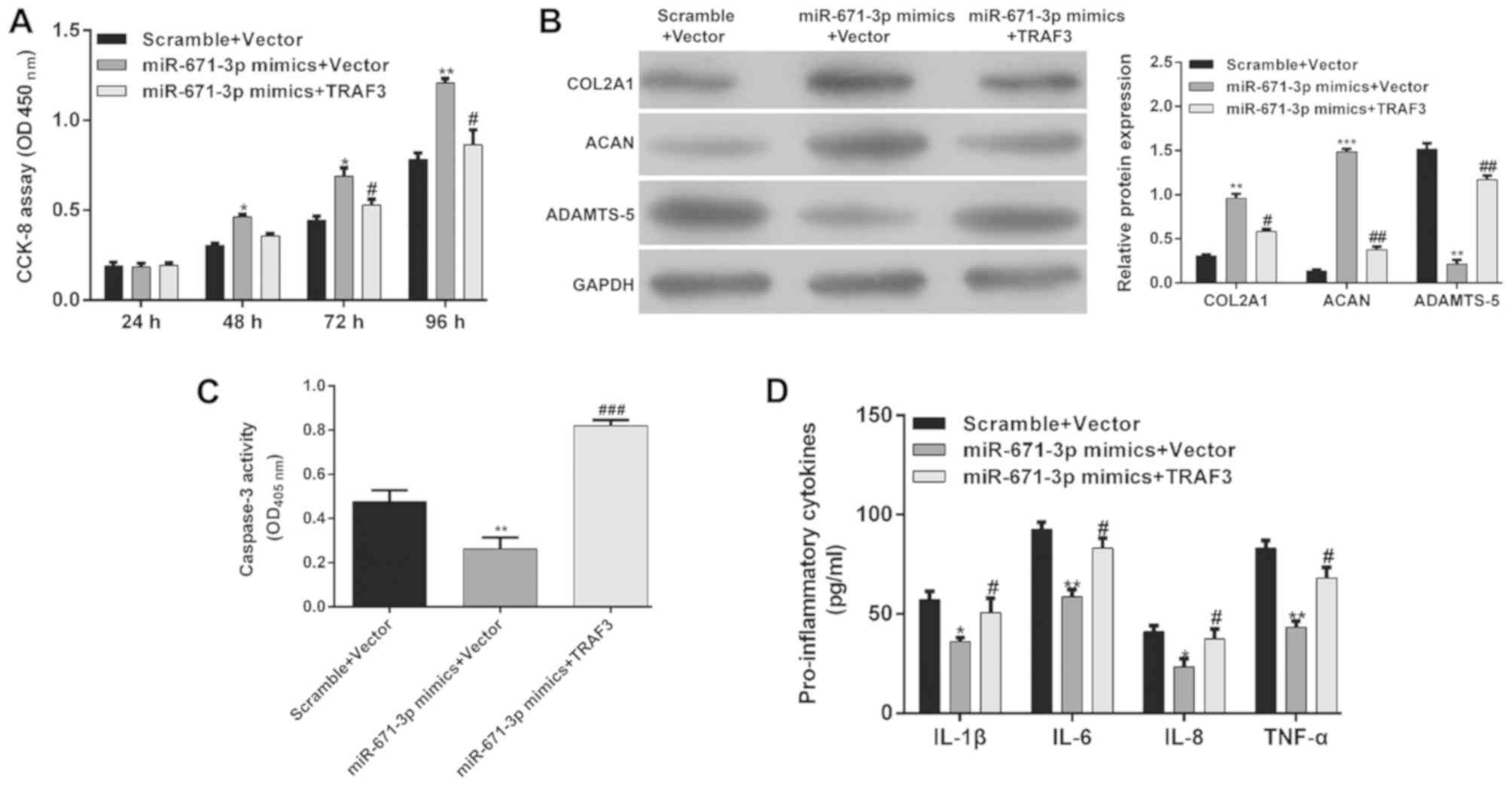 | Figure 5.Restoration of TRAF3 markedly
abrogates the effect of miR-671-3p. miR-671-3p-overexpressing
chondrocytes were transfected with TRAF3, or control vector. (A)
Chondrocyte proliferation was investigated in osteoarthritis
chondrocytes at 24, 48, 72 and 96 h, respectively. (B) Expression
levels of COL2A1, ACAN and ADAMTS-5 were detected by western blot
analysis. (C) Cell apoptosis was detected using a caspase-3
activity assay. (D) Production of IL-1β, IL-6, IL-8 and TNF-α in
the cell culture supernatants was detected by enzyme-linked
immunosorbent assay. Data are presented as the mean ± standard
deviation from triplicate experiments. *P<0.05, **P<0.01 and
***P<0.001, compared with the scramble + vector group;
#P<0.05, ##P<0.01 and
###P<0.001, compared with the miR-671-3p mimics +
vector group. miR, microRNA; TRAF3, tumor necrosis factor
receptor-associated factor; COL2A1, collagen type II α1 chain;
ACAN, aggrecan ADAMTS-5, a disintegrin and metalloproteinase with
thrombospondin motifs 5; CCK-8, Cell Counting Kit-8; OD, optical
density. |
Discussion
In recent years, miRNAs have attracted attention due
to their association with various human diseases and they provide
novel target for the investigation of specific therapeutic agents
and intervention tools (25). For
example, let-7 exhibits genomic alterations at a high frequency in
multiple types of human cancer and is involved in tumor progression
and metastasis (26). In addition,
Leidinger et al (27)
revealed that a 12-miRNA signature may affect 2,354 genes and be
involved in nervous system development, including Alzheimer's
disease and Parkinson's disease.
The present study investigated whether miR-671-3p
expression signatures may be involved in the pathogenesis of OA.
Substantial effort has been made in understanding the biological
function of miRNAs in OA, as reported by Miyaki and Asahara
(28). However, there is limited
literature reporting the role of miRNAs in the chondrocytes of
patients with OA. A study by Yin et al (29) reported that miR-26a and miR-26b
contribute to chondrocyte apoptosis in human OA. Zhang et al
(30) revealed that the enforced
expression of miR-21 depressed the process of chondrogenesis. The
results of the present study suggest that the role of miR-671-3p is
critical in the pathogenesis of OA. The expression of miR-671-3p
was markedly reduced in human knee OA tissues and chondrocytes.
Using TargetScan, PicTar and a luciferase reporter assay, TRAF3 was
confirmed as a target of miR-671-3p. The overexpression of
miR-671-3p promoted chondrocyte survival and proliferation, and
inhibited the synthesis of pro-inflammatory cytokines though
downregulating TRAF3.
As a complex and dynamic meshwork of proteins,
extracellular matrix (ECM) is an important component of
multicellular organisms that provides mechanical functions for the
orchestration of cellular and tissue organization and function
(31). Cartilage in which the
catabolism of ECM prevails over its anabolism leads to the
development and progression of OA (32). Inflammation is known to be
associated with the risk of cartilage loss and progression, and
with the clinical characteristics of OA (4). Pro-inflammatory cytokines are
considered to be catabolic factors and to control the degeneration
of articular cartilage matrix, which indicates their potential as
therapeutic targets (33). In the
present study, miR-671-3p mimics inhibited the production of
pro-inflammatory cytokines IL-1β, IL-6, IL-8 and TNF-α. In
addition, the overexpression of miR-671-3p enhanced matrix protein
expression (COL2A1 and ACAN) and suppressed the levels of catabolic
factor ADAMTS-5, suggesting that miR-671-3p reduced ECM catabolism
and inflammation and increased ECM production, thus being involved
in the inhibition of OA development.
TRAF3 is an essential signaling adaptor downstream
of multiple TNFR and TLR pathways (34). Pathogen-associated molecular
pattern-activated TLRs have been shown to induce the secretion of
interferons, pro- and anti-inflammatory cytokines (19). Xiao et al (35) revealed that that the degradation of
TRAF3 appeared to be responsible for the promotion of
microglia-mediated central nervous system inflammation that was
induced by Peli1. In a previous study, TRAF3 was shown to be
expressed in myeloid cells and function to attenuate inflammation
and tumor progression in mice (36). In the present study, TRAF3 was
identified as a direct target of miR-671-3p, and the results
further showed that the expression level of TRAF3 was inversely
correlated with that of miR-671-3p in chondrocytes. The restoration
of TRAF3 significantly abrogated the protective effect of
miR-671-3p on cell proliferation, caspase-3 mediated apoptosis,
matrix production and inflammation. These results demonstrated that
miR-671-3p is pivotal in the pathogenesis of OA though directly
targeting TRAF3. Further investigations are required to further
examine the molecular mechanism of miR-671-3p in OA. There were
several limitations to the present study, as follows: i) Relatively
small sample size; ii) no in vivo animal experiments; iii)
additional miRNA transfection methods require validation for
transfection of miRNA mimics with strict concentration control
(37); iv) mRNA expression levels
of the ACAN, COL2A1 and ADAMTS-5 transcripts require determination
if conditions permit; v) collection of additional tissue samples is
required to confirm the expression levels of ILs in vitro
correlated with the patients' samples.
In conclusion, the present study revealed that
miR-671-3p was downregulated in OA tissues and chondrocytes. The
overexpression of miR-671-3p promoted cell proliferation and
suppressed cell apoptosis and inflammation by targeting TRAF3 in OA
chondrocytes. These results may provide clues for examining novel
therapeutic strategies for preventing inflammation and cartilage
destruction in OA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HP made substantial contributions to study
conception and design. ZJL performed the experiments. SGC made
substantial contributions to acquisition of data. YZY and SJL were
involved in collection and analysis of the data XMZ was involved in
drafting and revising the manuscript critically for important
intellectual content. BH checked the accuracy and integrity of the
work and ensured that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All participants signed informed consent forms and
the study was approved by the Ethics Committee of Jingzhou Central
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Johnson VL and Hunter DJ: The epidemiology
of osteoarthritis. Best Pract Res Clin Rheumatol. 28:5–15. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiadaliri AA, Lohmander LS, Moradi-Lakeh
M, Petersson IF and Englund M: High and rising burden of hip and
knee osteoarthritis in the Nordic region, 1990–2015. Acta Orthop.
89:177–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Showery JE, Kusnezov NA, Dunn JC, Bader
JO, Belmont PJ Jr and Waterman BR: The rising incidence of
degenerative and posttraumatic osteoarthritis of the knee in the
United States Military. J Arthroplasty. 31:2108–2114. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor M, Martel-Pelletier J, Lajeunesse
D, Pelletier JP and Fahmi H: Role of proinflammatory cytokines in
the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 7:33–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
van der Kraan PM, Blaney Davidson EN, Blom
A and van den Berg WB: TGF-beta signaling in chondrocyte terminal
differentiation and osteoarthritis: Modulation and integration of
signaling pathways through receptor-Smads. Osteoarthritis
Cartilage. 17:1539–1545. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin JA and Buckwalter JA: Aging,
articular cartilage chondrocyte senescence and osteoarthritis.
Biogerontology. 3:257–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashimoto S, Ochs RL, Komiya S and Lotz M:
Linkage of chondrocyte apoptosis and cartilage degradation in human
osteoarthritis. Arthritis Rheum. 41:1632–1638. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tüfekci KU, Meuwissen RL and Genç S: The
role of microRNAs in biological processes. Methods Mol Biol.
1107:15–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Van Peer G, Mets E, Claeys S, De Punt I,
Lefever S, Ongenaert M, Rondou P, Speleman F, Mestdagh P and
Vandesompele J: A high-throughput 3′UTR reporter screening
identifies microRNA interactomes of cancer genes. PLoS One.
13:e01940172018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romaine SP, Tomaszewski M, Condorelli G
and Samani NJ: MicroRNAs in cardiovascular disease: An introduction
for clinicians. Heart. 101:921–928. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vicente R, Noël D, Pers YM, Apparailly F
and Jorgensen C: Deregulation and therapeutic potential of
microRNAs in arthritic diseases. Nat Rev Rheumatol. 12:211–220.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nugent M: MicroRNAs: Exploring new
horizons in osteoarthritis. Osteoarthritis Cartilage. 24:573–580.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akhtar N, Rasheed Z, Ramamurthy S,
Anbazhagan AN, Voss FR and Haqqi TM: MicroRNA-27b regulates the
expression of matrix metalloproteinase 13 in human osteoarthritis
chondrocytes. Arthritis Rheum. 62:1361–1371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abouheif MM, Nakasa T, Shibuya H, Niimoto
T, Kongcharoensombat W and Ochi M: Silencing microRNA-34a inhibits
chondrocyte apoptosis in a rat osteoarthritis model in vitro.
Rheumatology (Oxford). 49:2054–2060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tardif G, Hum D, Pelletier JP, Duval N and
Martel-Pelletier J: Regulation of the IGFBP-5 and MMP-13 genes by
the microRNAs miR-140 and miR-27a in human osteoarthritic
chondrocytes. BMC Musculoskelet Disord. 10:1482009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Le LT, Swingler TE and Clark IM: Review:
The role of microRNAs in osteoarthritis and chondrogenesis.
Arthritis Rheum. 65:1963–1974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ntoumou E, Tzetis M, Braoudaki M, Lambrou
G, Poulou M, Malizos K, Stefanou N, Anastasopoulou L and Tsezou A:
Serum microRNA array analysis identifies miR-140-3p, miR-33b-3p and
miR-671-3p as potential osteoarthritis biomarkers involved in
metabolic processes. Clin Epigenetics. 9:1272017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Häcker H, Redecke V, Blagoev B,
Kratchmarova I, Hsu LC, Wang GG, Kamps MP, Raz E, Wagner H, Häcker
G, et al: Specificity in Toll-like receptor signalling through
distinct effector functions of TRAF3 and TRAF6. Nature.
439:204–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vallabhapurapu S, Matsuzawa A, Zhang W,
Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL and Karin M:
Nonredundant and complementary functions of TRAF2 and TRAF3 in a
ubiquitination cascade that activates NIK-dependent alternative
NF-kappaB signaling. Nat Immunol. 9:1364–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kong QZ, Guo LT, Yang JN, Wang YF, Zhao
JX, Kong SH, Zhang M, Yan S and Jin Y: Anti-Inflammatory effects of
TRAF-interacting protein in rheumatoid arthritis fibroblast-like
synoviocytes. Mediators Inflamm. 2016:39061082016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Altman R, Alarcón G, Appelrouth D, Bloch
D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Feldman D,
et al: The American College of Rheumatology criteria for the
classification and reporting of osteoarthritis of the hip.
Arthritis Rheum. 34:505–514. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hautier A, Salentey V, Aubert-Foucher E,
Bougault C, Beauchef G, Ronzière MC, De Sobarnitsky S, Paumier A,
Galéra P, Piperno M, et al: Bone morphogenetic protein-2 stimulates
chondrogenic expression in human nasal chondrocytes expanded in
vitro. Growth Factors. 26:201–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kota J, Chivukula RR, O'Donnell KA,
Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P,
Torbenson M, Clark KR, et al: Therapeutic microRNA delivery
suppresses tumorigenesis in a murine liver cancer model. Cell.
137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Helland Å, Anglesio MS, George J, Cowin
PA, Johnstone CN, House CM, Sheppard KE, Etemadmoghadam D, Melnyk
N, Rustgi AK, et al: Deregulation of MYCN, LIN28B and LET7 in a
molecular subtype of aggressive high-grade serous ovarian cancers.
PLoS One. 6:e180642011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leidinger P, Backes C, Deutscher S,
Schmitt K, Mueller SC, Frese K, Haas J, Ruprecht K, Paul F, Stähler
C, et al: A blood based 12-miRNA signature of Alzheimer disease
patients. Genome Biol. 14:R782013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyaki S and Asahara H: Macro view of
microRNA function in osteoarthritis. Nat Rev Rheumatol. 8:543–552.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin X, Wang JQ and Yan SY: Reduced miR26a
and miR26b expression contributes to the pathogenesis of
osteoarthritis via the promotion of p65 translocation. Mol Med Rep.
15:551–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, Jia J, Yang S, Liu X, Ye S and
Tian H: MicroRNA-21 controls the development of osteoarthritis by
targeting GDF-5 in chondrocytes. Exp Mol Med. 46:e792014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naba A, Clauser KR, Ding H, Whittaker CA,
Carr SA and Hynes RO: The extracellular matrix: Tools and insights
for the ‘omics’ era. Matrix Biol. 49:10–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rahmati M, Nalesso G, Mobasheri A and
Mozafari M: Aging and osteoarthritis: Central role of the
extracellular matrix. Ageing Res Rev. 40:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goldring MB: Osteoarthritis and cartilage:
The role of cytokines. Curr Rheumatol Rep. 2:459–465. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Häcker H, Tseng PH and Karin M: Expanding
TRAF function: TRAF3 as a tri-faced immune regulator. Nat Rev
Immunol. 11:457–468. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Y, Jin J, Chang M, Chang JH, Hu H,
Zhou X, Brittain GC, Stansberg C, Torkildsen Ø, Wang X, et al:
Peli1 promotes microglia-mediated CNS inflammation by regulating
Traf3 degradation. Nat Med. 19:595–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lalani AI, Moore CR, Luo C, Kreider BZ,
Liu Y, Morse HC III and Xie P: Myeloid cell TRAF3 regulates immune
responses and inhibits inflammation and tumor development in mice.
J Immunol. 194:334–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jin HY, Gonzalez-Martin A, Miletic AV, Lai
M, Knight S, Sabouri-Ghomi M, Head SR, Macauley MS, Rickert RC and
Xiao C: Transfection of microRNA mimics should be used with
caution. Front Genet. 6:3402015. View Article : Google Scholar : PubMed/NCBI
|