Introduction
Insulin resistance is implicated in the development
of metabolic syndromes such as obesity, type 2 diabetes mellitus
and hyperlipidemia (1). Lipid
metabolism disorders result in excessive lipid accumulation and
adipocyte hypertrophy. Typically, adipocytes increase insulin
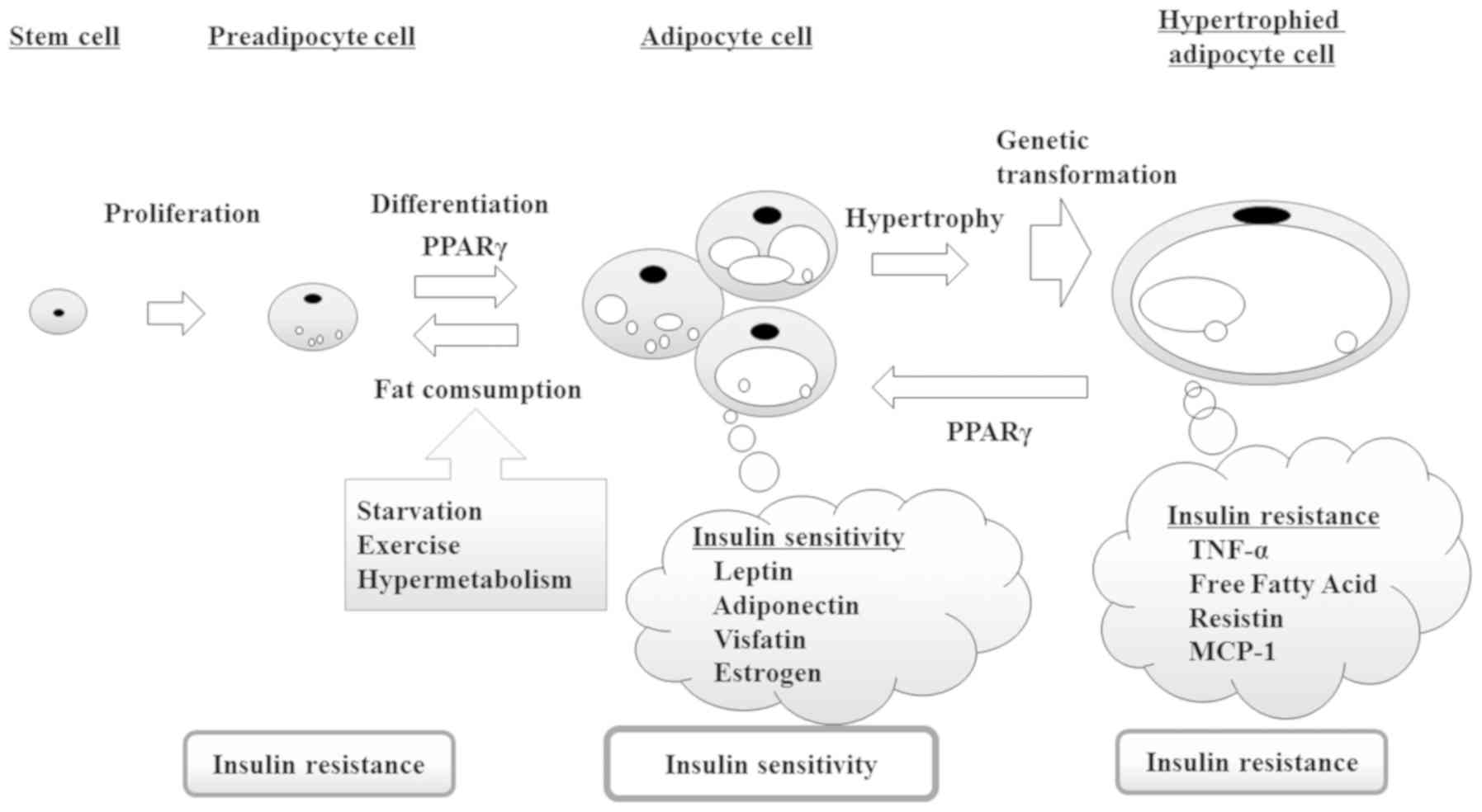
sensitivity and maintain glucose homeostasis (2). However, as shown in Fig. 1, insulin resistance caused by
adipocyte dysfunction impairs glucose homeostasis, further
enhancing insulin resistance through a feedback loop (3). Accordingly, an increase in the
production of insulin-sensitive adipocytes promotes mature
adipocyte differentiation.
The peroxisome proliferator associated receptor γ
(PPAR γ) is a ligand-activated transcription factor that belongs to
the nuclear receptor superfamily, and is associated with adipocyte
cell differentiation and glucose and lipid metabolism (4). As PPARγ regulates the differentiation
of pre-adipocytes, it also increases the number of small
insulin-sensitive adipocytes. Moreover, the activation of PPARγ in
mature adipocytes regulates several target genes involved in the
insulin signaling cascade and glucose and lipid metabolism
(5). Consequently, PPARγ serves as
a therapeutic target for antidiabetic drugs regulating glucose and
lipid metabolism.
The thiazolidinediones, including rosiglitazone and
pioglitazone, are a group of anti-diabetic drugs that strongly
activate PPARγ (6). The activation
of PPARγ leads to the trapping of free fatty acids in adipocytes
and increased hormone secretion, which both contribute to insulin
sensitivity (7). However, while
thiazolidinediones exhibit excellent antidiabetic effects, they are
also associated with undesirable side effects, such as heart
failure, weight gain and osteopenia, which restrict their clinical
use (8).
Polygonum tinctorium, a widely-known indigo
plant, is an annual herbaceous plant belonging to the Polygonaceae
family. It has been used in traditional Chinese medicinal and as an
indigo-blue dye since ancient times in Asian countries. Extracts
from Polygonum tinctorium have been reported to exert
biological effects, including anti-inflammatory, antibacterial and
antitumor activities. Furthermore, this plant has also been
reported to improve hyperlipidemia (9). This activity may contribute to the
enhancement of insulin sensitivity through the modulation of
adipocyte function by PPARγ. The present study investigated the
effect of indirubin on adipocyte differentiation and glucose
regulation in mature 3T3-L1 adipocytes.
Materials and methods
Chemicals and Reagents
The antibiotic-antimycotic solution was obtained
from Sigma-Aldrich Co., Ltd. Indirubin was purchased from Beijing
Putian Tongchuang Biotechnology Co., Ltd. All other chemicals were
purchased from Wako Pure Chemical Industry Ltd. unless otherwise
stated.
PPARγ binding assay
Cyclic AMP response element binding protein (CREB)
binding protein (CBP) was immobilized in plastic wells at 37°C for
1 h. PPARγ was incubated for 24 h at 4°C after preincubation of 5%
skim milk for 1 h at 37°C as a blocking reagent. Pioglitazone and
indirubin were added to an individual well and incubated for 1 h at
37°C. PPARγ antibody (rabbit polyclonal, Bio-Rad) and alkaline
phosphatase conjugated IgG antibody (goat anti-rabbit IgG, Bio-Rad)
were diluted and incubated for 1 h at 37°C, respectively.
p-Nitrophenyl phosphate (SIGMAFAST™
p-Nitrophenyl phosphate tablets; Sigma-Aldrich Co., LLC.)
was used as the substrate for alkaline phosphatase. After the
developing process (shaking at 600 rpm in dark site), the
absorbance of the solution in each well was measured at 405 nm.
When the absorbance of the sample well was greater than that of the
blank well (no sample well), the sample was deemed to contain PPARγ
agonist. The indirubin and pioglitazone were dissolved in 100% DMSO
and diluted with the D-PBS to obtain the each concentrations. The
final concentration of DMSO was equal (0.5%) in all groups.
Cell culture
The 3T3-L1 adipocytes were purchased from the JCRB
Cell Bank (Osaka, Japan) and were cultured at 37°C under 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% heat-inactivated fetal bovine serum
(Gibco-BRL) and antibiotic-antimycotic solution (100 IU/ml of
penicillin and 25 µg/ml of amphotericin B). After the preadipocytes
reached confluence, differentiation for adipocyte was induced with
or without samples in a mixture containing 0.25 mM
isobutyl-methylxanthine, 1 µM dexamethasone, and 1.7 µM insulin.
The indirubin and rosiglitazone were dissolved 100% DMSO and
diluted with the culture medium to obtain the needed each reaction
concentrations (10 µM-1 nM). The final concentration of DMSO was
equal (0.5%) in all groups. When the PPARγ antagonist GW9662 and
samples (indirubin and rosiglitazone) were treated together, the
samples was added after treatment with its antagonist.
Cell viability assay
Cell viability was determined by MTT assay. The
3T3-L1 cell suspension 100 µl (5.00×103 cells) was added
to each well of a 96-well plate and the medium was preincubated in
a CO2 incubator for 24 h. Each well was added with 10 µl
of each concentration of the prepared indirubin and rosiglitazone
and incubated the medium for 24 h in CO2 incubator. Each
plate was added 10 µl of Cell Count Reagent SF (Nacalai tesque) and
incubated the medium for 1 h in CO2 incubator. Reduction
of MTT to formazan was measured by a microplate reader (Immuno Mini
NJ-2300; Biotech Ltd.) at 450 nm. Viability was calculated
considering vehicle control (vehicle with cells) as a control.
Fig. S1 shows that native control
and vehicle control have the same background.
Oil-Red-O staining
After differentiation in 5 days with indirubin and
rosiglitazone, the 3T3-L1 cells were harvested. Then, cells were
washed with D-PBS(−) and immobilized with 10% formaldehyde for 1 h
at room temperature. Cells were stained with the ORO working
solution (3 mg/ml in 60% (v/v) 2-propanol) for 1 h at 37°C and
examined by an optical microscope ×200 (Nikon ECLIPSE Ts2; Olympus
CKX31) to evaluate lipid accumulation. Lipid droplet (LD) were
extracted from the cells using 2-propanol for 5 min and quantified
using a microplate reader at 510 nm. The results were expressed
relative to the differentiated cell control group. In addition,
PPARγ is required for mature adipocyte-survival, therefore
adipocytes only survive for a few days after selective ablation of
PPARγ in mature adipocytes of mice (10). GW9662 would inhibit mature
adipocytes-survival completely, followed by these cell death. Thus,
in this experiment using mature cell, we used only the samples
without GW9662.
Glucose uptake assay
Completely differentiated 3T3-L1 adipocytes in
96-well plates were incubated in the presence of 4 concentrations
of indirubin (1 µM-1 nM) and rosiglitazone (1 µM-1 nM) for 4 days.
After the cells were washed by D-PBS(−), they were incubated with
DMEM for another 3 h and determined the glucose concentrations at 3
points over time. The glucose concentrations in the cell culture
medium were determined by the Glucose Assay kit (Cell Biolabs,
Inc.).
Measurement of estradiol in
adipocytes
Fully differentiated 3T3-L1 adipocytes in 96-well
plates were incubated in the presence of 4 concentrations of
indirubin (1 µM-1 nM) and rosiglitazone (1 µM-1 nM) for 4 days.
Supernatants were taken from these plates on the 4th day of
differentiation. The estradiol levels were determined by the
Estradiol ELISA kit (Cayman Chemical, Inc.) and the concentrations
were calculated following the manufacturer's instructions.
Statistical analysis
Statistical analysis was performed by one-way ANOVA
followed by Dunnett's test for multiple group comparisons using
Sigma Stat statistical software ver. 2.03 (SPSS, Inc.). All data
are presented as the mean ± standard deviation. Experiments were
repeated three times. P<0.05 was considered to indicate a
statistically significant difference.
Results
PPARγ ligand activity
The present study investigated whether indirubin
served as a PPARγ ligand. Compared with 50 nM pioglitazone, 5 and
50 nM indirubin exhibited a 1.35- and 1.80-fold increased activity,
respectively (P<0.001; Fig.
2A), indicating that indirubin exerted a stronger PPARγ
ligand-binding activity than pioglitazone. In addition, the effects
of a PPARγ antagonist, GW9662, on indirubin-binding activity were
examined. As shown in Fig. 2B,
treatment with 10 nM GW9662 significantly suppressed the activity
of indirubin in a dose-dependent manner. Therefore, these results
suggested that indirubin was a PPARγ agonist by directly binding to
the PPARγ ligand-binding domain.
Indirubin promotes cell
differentiation of 3T3-L1 cells via PPARγ
An MTT assay was performed to evaluate the
cytotoxicity of indirubin and rosiglitazone in pre-adipocytes. As
shown in Fig. 3, >10 µM of
either indirubin or rosiglitazone impaired cell viability.
Therefore, a concentration range of 1 nM-1 µM was used for further
experimentation. As indirubin and rosiglitazone exhibited PPARγ
ligand activity, their effect on adipogenesis and cell
differentiation was investigated. 3T3-L1 pre-adipocytes were
exposed to indirubin and rosiglitazone in the presence or absence
of insulin for seven days. Accumulated lipid droplets were
subsequently detected by Oil Red O (ORO) staining and microscopy.
3T3-L1 pre-adipocytes become mature adipocytes via PPARγ signaling,
and accumulate lipid droplets. Therefore, mature cells are stained
red following ORO staining.
Effects of indirubin-treatment on
pre-adipocytes
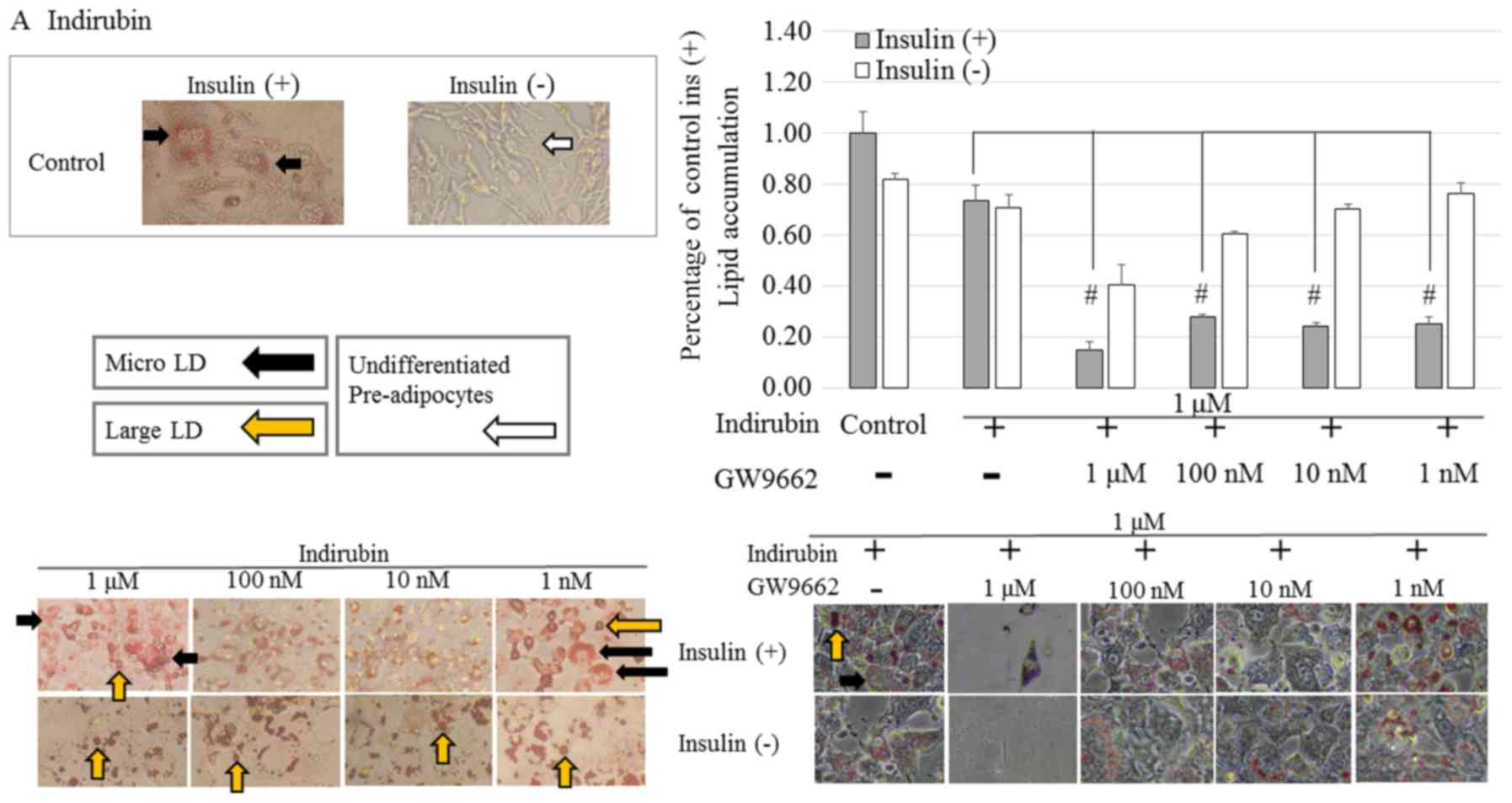
As shown in Fig.
4A, indirubin-treated cells in the presence or absence of
insulin were stained red compared with the control cells. However,
clear dose-dependency was not observed. Furthermore, in the
presence of insulin, indirubin-treated pre-adipocytes, exhibited a
morphological change from spindle-like to a more rounded shape
following differentiation. Moreover, it was observed that in the
differentiated adipocytes, large LD fragments had dispersed into
smaller micro LD. In the absence of insulin, indirubin promoted
adipocyte differentiation compared with the control group, but to a
lesser extent than the indirubin-treated cells in the presence of
insulin. This result suggested that indirubin promoted the
differentiation of pre-adipocytes.
In the presence of GW9662, the effects
of indirubin-treatment on pre-adipocytes
3T3-L1 pre-adipocytes were induced to differentiate
for seven days by culturing them in 1 µM indirubin in the absence
and presence of GW9662 (1 µM-1 nM). In the presence of insulin, the
addition of 1 µM GW9662 significantly reduced lipid accumulation
and adipocyte differentiation compared with the control group for
indirubin-treated cells. Furthermore, a dose-dependent effect in
cells treated with or without insulin was observed.
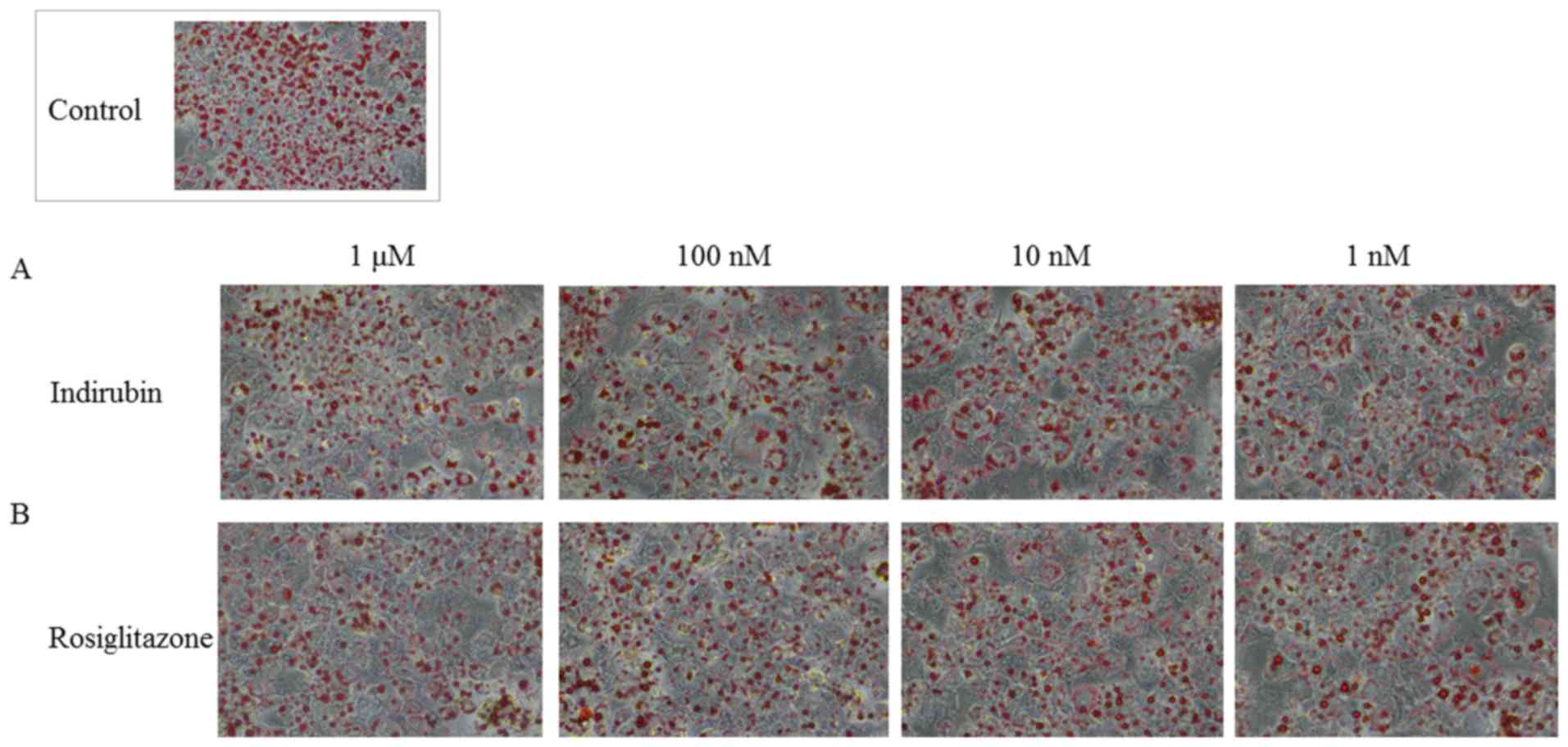
Effects of rosiglitazone-treatment on
pre-adipocytes
As shown in Fig.
4B, rosiglitazone-treated cells in the presence or absence of
insulin were stained red compared with the control cells. However,
clear dose-dependency was not observed. Furthermore, in the
presence of insulin, rosiglitazone-treated pre-adipocytes,
exhibited a morphological change from spindle-like to a more
rounded shape following differentiation as with indirubin.
Moreover, it was observed that in the differentiated adipocytes,
large LD fragments had dispersed into smaller micro LD. In the
absence of insulin, rosiglitazone promoted adipocyte
differentiation compared with the control group, but to a lesser
extent than the indirubin-treated cells.
In the presence of GW9662, the effects
of rosiglitazone-treatment on pre-adipocytes
In the presence of insulin, the addition of 1 µM
GW9662 significantly reduced lipid accumulation and adipocyte
differentiation compared with the control group for
rosiglitazone-treated cells. Furthermore, undifferentiated
pre-adipocytes were observed in the absence of insulin. Insulin
activates the PI3K/Akt signaling pathway, which plays an important
role in cell differentiation and lipid metabolism. Therefore, the
presence or absence of insulin affects the regulation of PPARγ,
which is downstream of this pathway. The lipid accumulation rate
was higher in the presence of insulin compared with cells cultured
without insulin. This suggested that the selectivity of the target
gene affected by indirubin may be different depending on the
presence or absence of insulin. In other words, the presence of
insulin may activate lipid metabolism-related genes while the
absence of insulin may result in the activation of genes that
promote cell differentiation. Therefore, indirubin may regulate the
expression of PPARγ to optimize cell growth.
Indirubin reduces lipid droplet size
and accumulation in mature adipocytes via PPARγ activation
Mature 3T3-L1 adipocytes were obtained by culturing
pre-adipocytes in MDI medium for four days and maintenance medium
for six days. The cells were subsequently cultured with various
concentrations of indirubin and rosiglitazone for an additional
five days. The effect of these treatments was examined using ORO
staining. PPARγ affects the function of both pre-adipocytes and
mature adipocytes. In addition to its role in cell differentiation
and lipid metabolism, PPARγ is also important for regulating
glucose and lipid metabolism, and increases the expression of
glucose transporter 4 (GLUT4) and c-Cbl-associated protein (CAP).
Moreover, PPARγ regulates the expression of various factors
secreted from mature adipocytes, such as leptin, adiponectin and
estrogen, which also affect insulin resistance. As shown in
Fig. 5, indirubin and
rosiglitazone interfered with adipocyte differentiation and lipid
accumulation compared with the control group, as indicated by the
decrease in ORO staining. It was also observed that larger LD had
accumulated in treated cells compared with the control group.
Although, treatment with 1 nM-1 µM indirubin reduced LD size,
several micro LD were observed. The absorbance quantified at a
wavelength of 510 nm also indicated that indirubin dose-dependently
decreased lipid accumulation. Considering that these effects were
likely mediated through distinct signaling pathways and targets,
several signaling pathways may be involved in reducing lipid
accumulation in adipocytes.
Indirubin increases insulin-stimulated
glucose uptake in mature adipocytes
PPARγ is an important transcription factor in
insulin sensitivity and adipogenesis. It has been reported that
activation of this factor stimulates the expression of downstream
genes involved in glucose metabolism. Therefore, the present study
examined the effects of indirubin and rosiglitazone on glucose
consumption in mature 3T3-L1 adipocytes. As shown in Fig. 6A, treatment with 1 µM indirubin
resulted in a decrease in the medium glucose concentration, which
was 13.7% lower than the control group. The 1 µM-10 nM
rosiglitazone treated groups resulted in a decrease in the medium
glucose concentration (Fig. 6B).
However, there was no definite dose-dependence as
insulin-stimulated glucose uptake was enhanced in mature
adipocytes.
Indirubin promotes estrogen
biosynthesis by activating aromatase in mature adipocytes
After menopause, adipocytes become the major source
of estrogens. It is known that estrogen is biosynthesized from
androgens by aromatase cytochrome P450 in mature adipocytes
(11). Mature 3T3-L1 adipocyte
cells were treated with 1 nM-1 µM indirubin to determine its effect
on estrogen biosynthesis in adipocytes. As shown in Fig. 7, estrogen biosynthesis exhibited a
1.64-fold increase following treatment with 1 µM indirubin compared
with the control group. However, this effect was not significantly
different at 1–100 nM. Since undifferentiated cells do not
participate in the biosynthesis of estrogen, the results observed
in the present study suggested that the pre-adipocytes had
differentiated into mature cells. Therefore, the 1 µM
indirubin-treated group may have higher insulin sensitivity
compared with the control group.
Discussion
Impaired adipocyte differentiation is associated
with insulin resistance and type 2 diabetes in obesity-related
diseases. As shown in Fig. 1,
adipocytokines secreted from adipocytes, such as adiponectin and
leptin, regulate insulin resistance. The ligand activity of PPARγ
is important in adipocyte differentiation and plays a role in
increasing with the expression of their gene. Consequently,
promoting adipocyte differentiation improves insulin resistance.
Furthermore, after the binding of insulin to its receptor, several
cellular signaling pathways are activated. Specifically, the
PI3K/Akt signaling pathway has been implicated in a variety of
insulin-dependent cellular processes such as cell survival, glucose
transport and adipocyte differentiation. Insulin stimulates
cellular differentiation, however, in the present study, cellular
differentiation and activation of the PPARγ signaling pathway were
also observed in indirubin-treated pre-adipocytes in the absence of
insulin. Therefore, indirubin may promote the differentiation of
pre-adipocytes into mature cells via the activation of PPARγ
regardless of insulin stimulation, followed by the restoration of
insulin sensitivity.
The present study revealed that whereas a low
concentration of indirubin induced a strong PPARγ ligand activity,
this effect was inhibited at high concentrations. Therefore,
indirubin has a biphasic effect on PPARγ ligand activity and the
activation of PPARγ may be involved in multiple mechanisms. It was
reported that PPAR ligands activate the ERK pathway via PPARγ in a
dose-dependent manner (12). On
the other hand, it is also reported that PPARγ-specific agonists
activate AMP-activated kinase (AMPK) in a PPARγ dose-independent
manner (13). Thus, the biphasic
effect of indirubin may be due to both its PPARγ-dependent and
independent mechanisms, suggesting that the optimal concentration
should be evaluated in vivo in future experiments.
The development of insulin resistance is
characterized by the impairment of glucose uptake mediated by GLUT4
in adipocytes (14). The result
showed that indirubin increased insulin-stimulated glucose uptake
in mature adipocytes. PPARγ ligands are reported to increase
glucose transport in adipocytes by regulating the expression of
several target genes directly involved in glucose metabolism
(15). Therefore, indirubin may
promote adipocyte glucose utilization through the upregulation of
GLUT4.
In the process of differentiation of pre-adipocytes
into mature adipocytes, morphological and biochemical
characteristics are determined by hormone-sensitive metabolic
processes. Besides promoting glucose metabolism, adipocytes also
acquire the ability to perform lipolysis and lipogenesis and to
secrete adipokines (3,16). Perilipin, which is a type of
adipophilin, is located on the surface of triglycerides (TG)
droplets in mature adipocytes. In the present study, small LD was
observed in mature cells, similarly to pre-adipocytes. Dispersion
of LD is considered to affect the localization of perilipin and
hormone-sensitive lipase, which is one of the main regulators of
lipid metabolism. The smaller LD exhibit higher lipolytic activity
compared with the larger LD (17).
Lipolytic activity affects the surface-volume ratio of LD, which
results in more efficient degradation of stored TG by several lipid
metabolic enzymes and perilipin. These findings suggested that
treatment of cells with indirubin significantly enhanced adipocyte
differentiation and lipolysis. Therefore the intracellular
distribution of TG and the generation of micro LDs may enhance
lipid metabolism and improve insulin resistance.
Estrogen is produced in mature adipocytes and its
deficiency leads to numerous metabolic disturbances, including
insulin resistance (18). The
present study revealed that indirubin promoted estrogen
biosynthesis in 3T3-L1 cells, suggesting that it may directly
and/or indirectly regulate the activity of aromatase and generate
estradiol via PPARγ. The mitogen-activated protein kinase (MAPK)
and AMPK are regulated by different metabolic pathways, such as
PPARγ and C/EBPα, which are required to maintain adipocyte function
(19,20). Therefore, indirubin may result in
the enhancement of aromatase activity by directly and/or indirectly
acting on the mitogen-activated protein kinase (MAPK) or PI3K/Akt
signaling pathways. Thus, indirubin may serve as a novel modulator
of estrogen biosynthesis that can act locally in adipose
tissues.
The PPARγ and PI3K/Akt signaling pathway regulate
adipocyte differentiation and lipid metabolism. It was reported
that hyperglycemia in 3T3-L1 adipocytes was ameliorated by the
upregulation of PPARγ and PI3K/Akt and promoting fat accumulation
(21). On the other hand, it was
revealed that water-extracted plum (Prunus salicina L. cv. Soldam)
attenuates adipogenesis in murine 3T3-L1 adipocyte cells through
the PI3K/Akt signaling pathway (22). These studies suggested that the
activities of anti-adipogenic and/or lipolysis via enhancing cell
differentiation without cytotoxicity were enhanced. The results of
the present study revealed that indirubin, an extract from
Polygonum tinctorium, exhibited PPARγ ligand-binding ability
and enhanced adipocyte differentiation by increasing its
transcriptional activity. In mature adipocytes, indirubin was
involved in the promotion of glucose uptake, reduction of TG
accumulation and lipid size and enhancement of estrogen
biosynthesis. Therefore, indirubin may serve as a novel agent in
the treatment of diabetes mellitus by improving insulin
resistance.
Finally, there are some limitations to this study.
These assays were performed assuming that insulin resistance in
patients with type 2 diabetes was improved by the use of
thiazolidinedione. In other words, this study attempted to
elucidate the mechanism in which indirubin improves insulin
resistance in the presence of insulin. In addition, inhibition of
PPARγ by GW9662 has a critical effect on the survival of mature
adipocytes. Therefore, it should be demonstrated whether indirubin
improves insulin resistance without insulin after proving its
detailed mechanism in the presence of insulin.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the current
study are included in this published article.
Authors' contributions
TK and KS conceived and designed the study. TK wrote
the manuscript and KS revised the manuscript. TK, KK and TM
performed and analyzed the experiments. TK, KS, KK and TM
interpreted experiment data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
LD
|
lipid droplet
|
|
C/EBP
|
CCAAT-enhancer-binding protein
|
|
GLUT
|
glucose transporter
|
|
TG
|
triglyceride
|
|
AMPK
|
AMP-activated protein kinase
|
References
|
1
|
Lanktree MB and Hegele RA: Metabolic
syndrome. Genomic Precis Med Prim Care Third Ed. 365:283–299. 2017.
View Article : Google Scholar
|
|
2
|
Reuter S and Mrowka R: Obesity, adipocytes
and insulin resistance-friends for life? Acta Physiol(Oxl).
225:e132582019.
|
|
3
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lehrke M and Lazar MA: The many faces of
PPARγ. Cell. 123:993–999. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siersbæk R, Nielsen R and Mandrup S: PPARγ
in adipocyte differentiation and metabolism-Novel insights from
genome-wide studies. FEBS Lett. 584:3242–3249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schoonjans K, Staels B and Auwerx J: The
peroxisome proliferator activated receptors (PPARs) and their
effects on lipid metabolism and adipocyte differentiation. Biochim
Biophys Acta. 1302:93–109. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peters JM, Latruffe N, Passilly P,
Motojima K and Gonzalez FJ: Expression of putative fatty acid
transporter genes are regulated by peroxisome
proliferator-activated receptor α and γ activators in a tissue- and
inducer-specific manner. J Biol Chem. 273:16710–16714. 2002.
|
|
8
|
Cariou B, Charbonnel B and Staels B:
Thiazolidinediones and PPARγ agonists: Time for a reassessment.
Trends Endocrinol Metab. 23:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Masakia N, Fukudaa S, Ikeda M and Kurimoto
M: Improvement of high fat diet-induced hyperlipidemia by
Polygonum tinctorium Lour. J Nat Med. 54:261–264. 2000.
|
|
10
|
Imai T, Takakuwa R, Marchand S, Dentz E,
Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W,
et al: Peroxisome proliferator-activated receptor γ is required in
mature white and brown adipocytes for their survival in the mouse.
Proc Natl Acad Sci USA. 101:4543–4547. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mauvais-Jarvis F, Clegg DJ and Hevener AL:
The role of estrogens in control of energy balance and glucose
homeostasis. Endocr Rev. 34:309–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bolden A, Bernard L, Jones D, Akinyeke T
and Stewart LV: The PPAR gamma agonist troglitazone regulates Erk ½
Phosphorylation via a PPARγ-Independent, MEK-dependent pathway in
human prostate cancer cells. PPAR Res. 2012:9290522012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lebrasseur NK, Kelly M, Tsao T, Farmer SR,
Saha AK, Ruderman NB and Tomas E: Thiazolidinediones can rapidly
activate AMP-activated protein kinase in mammalian tissues. Am J
Physiol Endocrinol Metab. 291:E175–E181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slca T and Lucia M: SLC2A4 gene: A
promising target for pharmacogenomics of insulin resistance E
ditorial. Pharmacogenomics. 14:847–850. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kudo M, Sugawara A, Uruno A, Takeuchi K
and Ito S: Transcription suppression of peroxisome
proliferator-activated receptor γ2 gene expression by tumor
necrosis factor α via an inhibition of CCAAT/enhancer-binding
protein δ during the early stage of adipocyte differentiation.
Endocrinology. 145:4948–4956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marrow B, Secreted S and Protect C:
Vitamin B12 as a modulator of gut microbial ecology. Cell Metab.
71:3831–3840. 2014.
|
|
17
|
Paar M, Jüngst C, Steiner NA, Magnes C,
Sinner F, Kolb D, Lass A, Zimmermann R, Zumbusch A, Kohlwein SD and
Wolinski H: Remodeling of lipid droplets during lipolysis and
growth in adipocytes. J Biol Chem. 287:11164–11173. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma G, Mauvais-Jarvis F and Prossnitz
ER: Roles of G protein-coupled estrogen receptor GPER in metabolic
regulation. J Steroid Biochem Mol Biol. 176:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bost F, Aouadi M, Caron L and Binétruy B:
The role of MAPKs in adipocyte differentiation and obesity.
Biochimie. 87:51–56. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burns KA and Vanden Heuvel JP: Modulation
of PPAR activity via phosphorylation. Biochim Biophys Acta.
1771:952–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Balakrishnan BB, Krishnasamy K and Choon
K: Moringa concanensis Nimmo ameliorates hyperglycemia in 3T3-L1
adipocytes by upregulating PPAR-γ, C/EBP-α via Akt signaling
pathway and STZ-induced diabetic rats. Biomed Pharmacother.
103:719–728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choe WK, Kang BT and Kim SO: Attenuates
adipogenesis in murine 3T3-L1 adipocyte cells through the PI3K/Akt
signaling pathway. Exp Ther Med. 1608–1615. 2018.PubMed/NCBI
|