Introduction
Acute promyelocytic leukemia (APL) is the M3 subtype
of acute myeloid leukemia (AML) and is characterized by an
accumulation of abnormal promyelocytes in the bone marrow and a
severe bleeding tendency (1). Five
decades ago, APL was the most fatal type of acute leukemia and was
considered to be essentially untreatable (2). With the application of
anthracyclines, retinoic acid (ATRA), arsenic trioxide (ATO) and
other drugs, the APL complete remission rate has been significantly
improved (3–5). However, between 20 and 30% of newly
diagnosed APL patients treated with regimens based on the use of
ATRA or ATO will develop disease recurrence or drug resistance.
With the rapid development of molecular biology, molecular-targeted
therapy has become the hotspot of cancer prevention.
Prohibitin (PHB), a potential tumor suppressor
protein, has attracted increasing attention due to its relationship
with the occurrence and development of tumors. Research has shown
that PHB may play a marked anti-tumor effect by regulating cell
proliferation, differentiation, apoptosis, and hormone or receptor
activity. However, the impact of PHB on the acute promyelocytic
leukemia cell line and the exact mechanism involved remain
controversial. RNA interference (RNAi) is a cellular pathway for
post-transcriptional gene silencing. The ability to manipulate RNAi
in the laboratory is being exploited to develop novel therapies for
human disease (6). Unlike
traditional plasmid vectors, lentiviral vector-mediated RNAi
achieves the ultimate goal of long-term inhibition of target genes
through combining the efficient infection and integration
characteristics of a lentiviral vector with RNA inhibition of
specific homologous gene expression. This provides a powerful
research tool for endogenous gene function investigation (7). In this study, we have generated
shRNAs that targeted against PHB in order to observe the effect on
the retinoic acid-resistant acute promyelocytic leukemia cell line
NB4-R1 and to try to elucidate the mechanism involved.
Materials and methods
Cell culture
The retinoic acid-resistant acute promyelocytic
leukemia cell line NB4-R1 was a gift from the Shanghai Second
Medical College. The cells were cultured in RPMI-1640 (Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% heated-inactivated fetal
bovine serum at 37°C in a humidified incubator containing 5%
CO2. Cell viability was evaluated by the trypan blue dye
exclusion assay and only cell suspensions that presented more than
95% viability were used.
Design and cloning of lentiviral shRNA
vectors
The target shRNAs against the human PHB gene
(GeneBank accession number NM_002634) for RNAi were designed using
an internet application system (Invitrogen, Carlsbad, CA, USA) as
follows: 5′-GAGTTCACAGAAGCGGTGGAA3′. A shRNA which had no
significant homology to any known human genes
(5′-TTCTCCGAACGTGTCACGT-3′) was used as a negative control
(8). Oligos were heated at 95°C
for 5 min and then annealed at 37°C for 1 h. The annealed sequence
was ligated into the AgeI and EcoRI sites of
pGCSIL-GFP (containing human U6 promoter) to generate a
pGCSIL-GFP-PHB vector (Shanghai Genechem Co., Ltd., China), which
was then transformed into E. coli. Positive recombinant
clones were selected by PCR (upstream primer:
5′-CCTATTTCCCATGATTCCTTCATA-3′; downstream primer:
5′-GTAATACGGTTATCCACGCG-3′) and DNA sequencing. The new recombinant
lentivirus vector was produced by co-transfecting 293T cells with
the lentivirus expression plasmid and packaging plasmids (pHelper
1.0 and pHelper 2.0) with Lipofectamine 2000 (Invitrogen).
Infectious lentivirus vector was harvested at 48 h
post-transfection and then concentrated. The infectious titer was
determined using the hole by hole dilution method to determine the
GFP-tagged positive rate in 293T cells.
Lentiviral vector infection
Cells were divided into 3 groups: CON (uninfected
NB4-R1 cells), RNAi-NC (infection with negative control lentiviral
vector) and RNAi-PHB (infection with pGCSIL-GFP-PHB lentiviral
vector). Cells were cultured at a density of 6×105/well
in 6-well plates and infected with specific/negative control
lentiviral vectors and 8 μg/ml polybrene (Sigma, St. Louis, MO,
USA), at the multiplicity of infection (MOI) 20, according to the
pre-experimental results. After incubation for 48 h, cells were
observed under the fluorescence microscope. The knockdown
efficiency of PHB was analyzed by real-time quantitative PCR and
western blotting.
Measurement of PHB by real-time
quantitative PCR
After infection with lentiviruses for 4 days, the
total RNA from each group of cells was extracted in TRIzol
(Invitrogen) and quantified by an ultraviolet spectrophotometer
(UVP, Upland, CA, USA) at a wavelength of 260 nm. The reverse
transcription (RT) reaction was performed with a RevertAid First
Strand cDNA Synthesis kit according to the manufacturer’s
instructions. A total of 20 μl of a solution containing 10 μl
SYBR-Green Real-time PCR Master mix (Takara, Japan), 2 μl cDNA, 7.2
μl water and 0.2 μmol/l sense and antisense primers was used. PCR
cycles were as follows: initial denaturation at 94°C for 5 min, the
reaction was repeated for 40 cycles, each cycle consisted of
denaturing at 94°C for 30 sec, annealing at 61°C for 45 sec, and
synthesis at 72°C for 45 sec. The primers for amplifying PHB and
GAPDH were as follows: GAPDH forward, 5′-CACCCTGTTGCTGTAGCCAAA-3′,
and reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′; PHB forward,
5′-CTGCCGTCCATCACAACTG-3′ and reverse, 5′-TCTGTAAGGTCGTCGCTCAC-3′.
The cutoff point (Ct) of each sample was plotted on a standard
curve and the mRNA copy numbers were calculated. The GAPDH gene was
used as an endogenous control. The relative PHB mRNA levels were
expressed as a ratio of PHB to GAPDH.
Measurement of PHB by western
blotting
After infection with lentiviruses for 7 days, the
cells were lysed in RIPA buffer in the presence of a proteinase
inhibitor cocktail (Sigma). Protein concentration was determined
using a BCA assay kit (Thermo, Waltham, MA, USA). Protein (30 μg)
was separated using 10% SDS-PAGE and transferred to nitrocellulose
membranes. Membranes were blocked in 5% skimmed milk for 2 h,
incubated with primary antibodies against PHB (mouse monoclonal,
1:700, Abcam, Cambridge, MA, USA) and GAPDH (mouse monoclonal,
1:10000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at
room temperature for 2 h and 4°C overnight, washed with TBS
containing 0.1% Tween-20 (TBST) followed by an incubation of 1 h in
mouse anti-mouse secondary antibody conjugated with HRP. After the
final wash with TBST, the membranes were developed using
chemiluminescence and exposed to X-ray films. The immunoblots were
quantified with Quantity One version 4.6.2 software. The expression
of PHB in each sample was internally normalized to GAPDH and levels
were given relative to the expression in control groups.
Measurement of apoptosis
Flow cytometric analysis
To measure the numbers and the ratio of apoptotic
cells, the Annexin V-FITC Apoptosis Detection kit (BD Biosciences,
Franklin Lakes, NJ, USA) was utilized. The cells were seeded at
1×105/well in 24-well plates, and on the next day, they
were transfected with RNAi-PHB or RNAi-NC, respectively. Seven days
after transfection, the cells were harvested, washed twice with
cold PBS, and resuspended in Annexin V binding buffer. The cell
suspension was then transferred to a 5-ml centrifuge tube, and
stained with 5 μl Annexin V-Fitc at room temperature in the dark
for 15 min. Finally, the stained cells were run immediately on a
FACSort Flow Cytometer (Becton-Dickinson) and evaluated with the
CELLQuest software system (BD Biosciences).
Western blot analysis of caspase-3
protein level
The western blot analysis of caspase-3 levels was
performed according to the caspase-3 (Mouse monoclonal, 1:1000;
Cell Signaling Technology, Inc., USA) antibody description.
Statistical analysis
All the experiments were carried out in triplicate,
and quantitative data in this study were expressed as χ̄ ± s.
Statistical differences between the groups were compared using a
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Construction and package of the
lentiviral vector
The target shRNAs against human PHB for RNAi were
constructed and connected with lentiviral frame plasmids. Positive
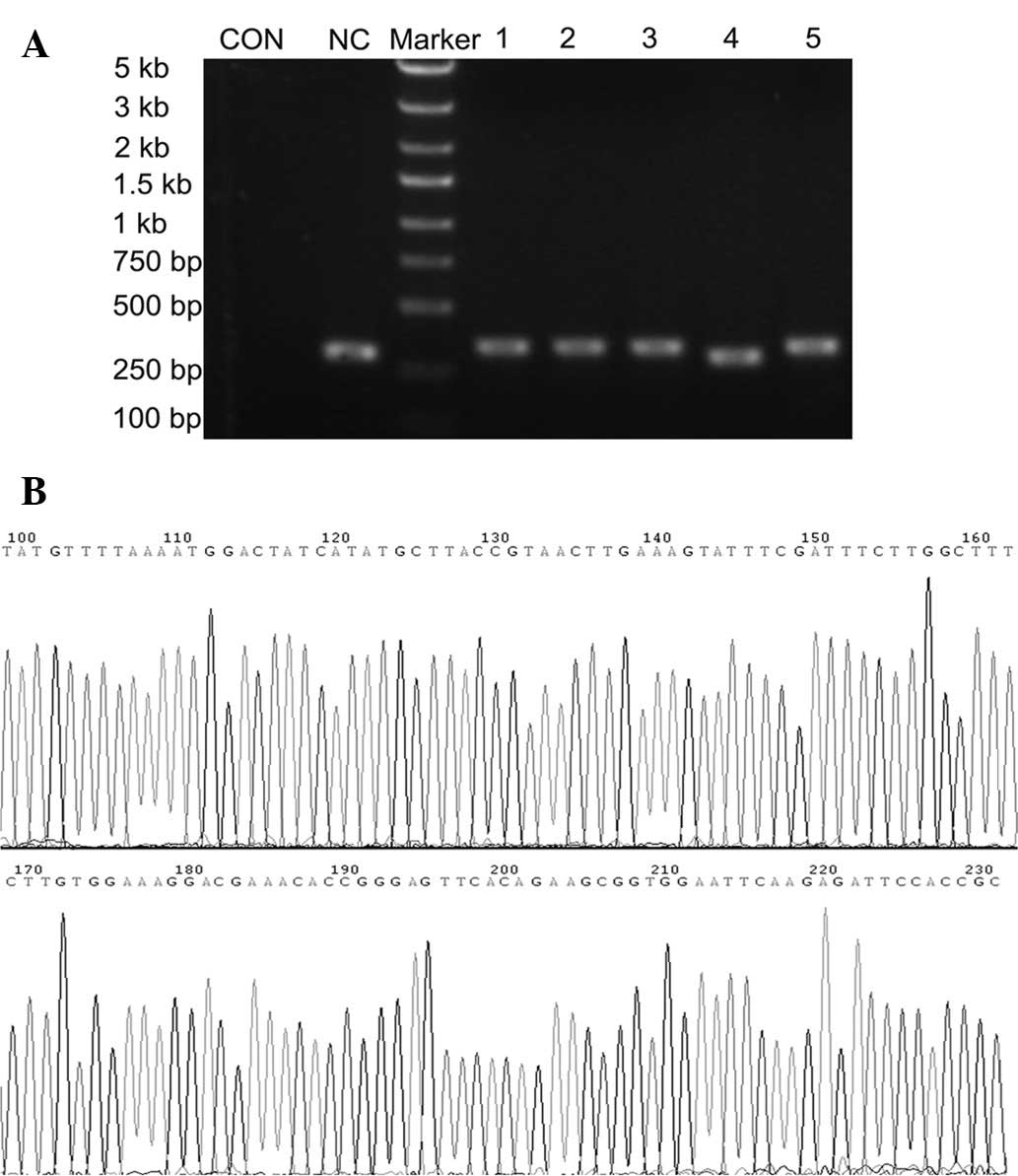
recombinant clones were selected and identified with PCR. The sizes
of PCR amplified fragments of recombinant clones and unconnected
shRNA empty vectors were 343 and 306 bp, respectively (Fig. 1A). DNA sequencing analysis
confirmed that the inserted PHB shRNA sequences were correct
(Fig. 1B). The recombinant
lentiviral vectors were applied to co-transfect 293T cells and
virus titers were determined as 1×108 (pGCSIL-GFP-PHB
lentiviral vector) and 5×108 (negative control
lentiviral vector), respectively.
Infection efficiency of human NB4-R1
cells with lentivirus
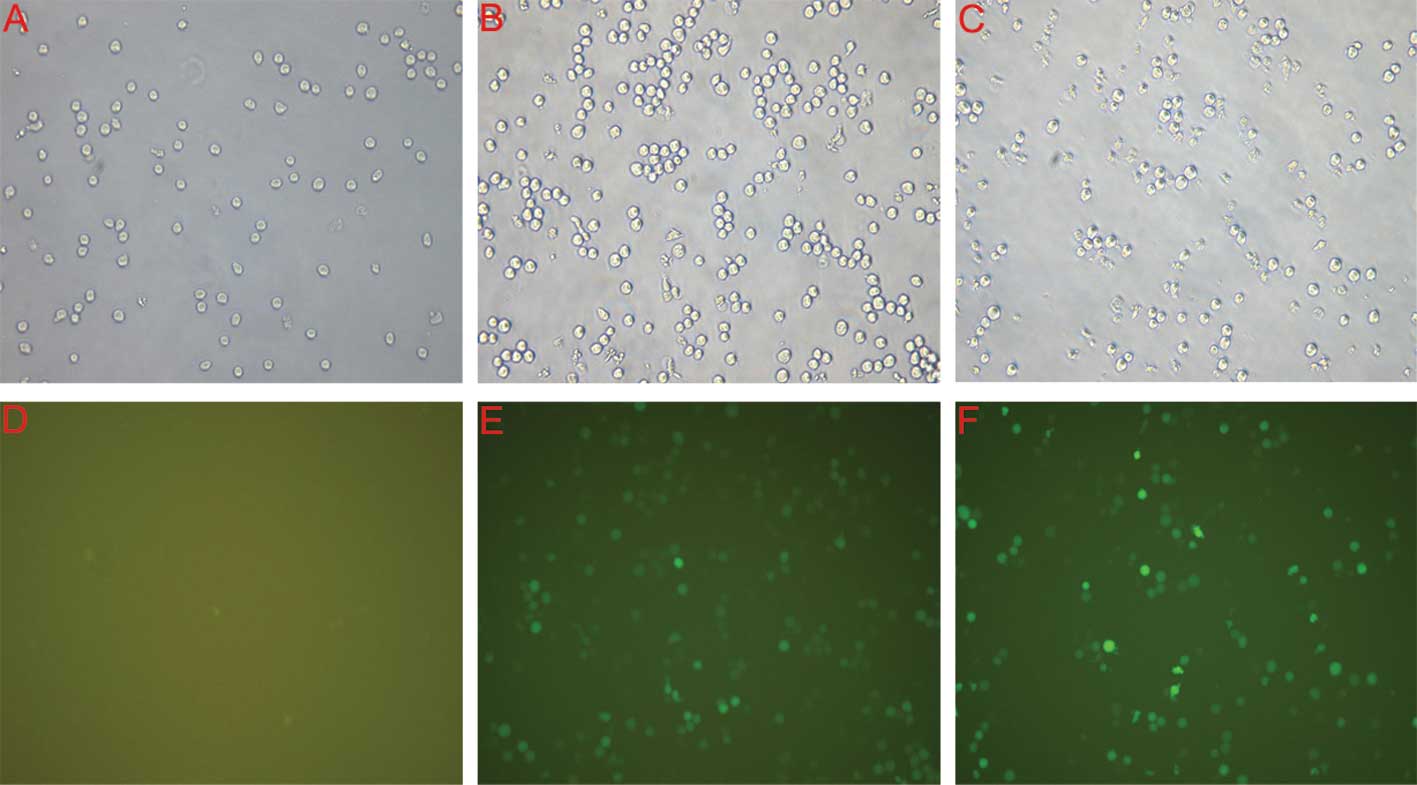
Assessment of the infection rate was accomplished
with the determination of the positive expression rate of GFP under
the fluorescent microscope 4 days after infection. As shown in
Fig. 2, the infection efficiency
of lentiviral RNAi vectors of PHB and negative controls in NB-R1
cells was >80%.
Silencing efficacy of PHB shRNA
lentivirus
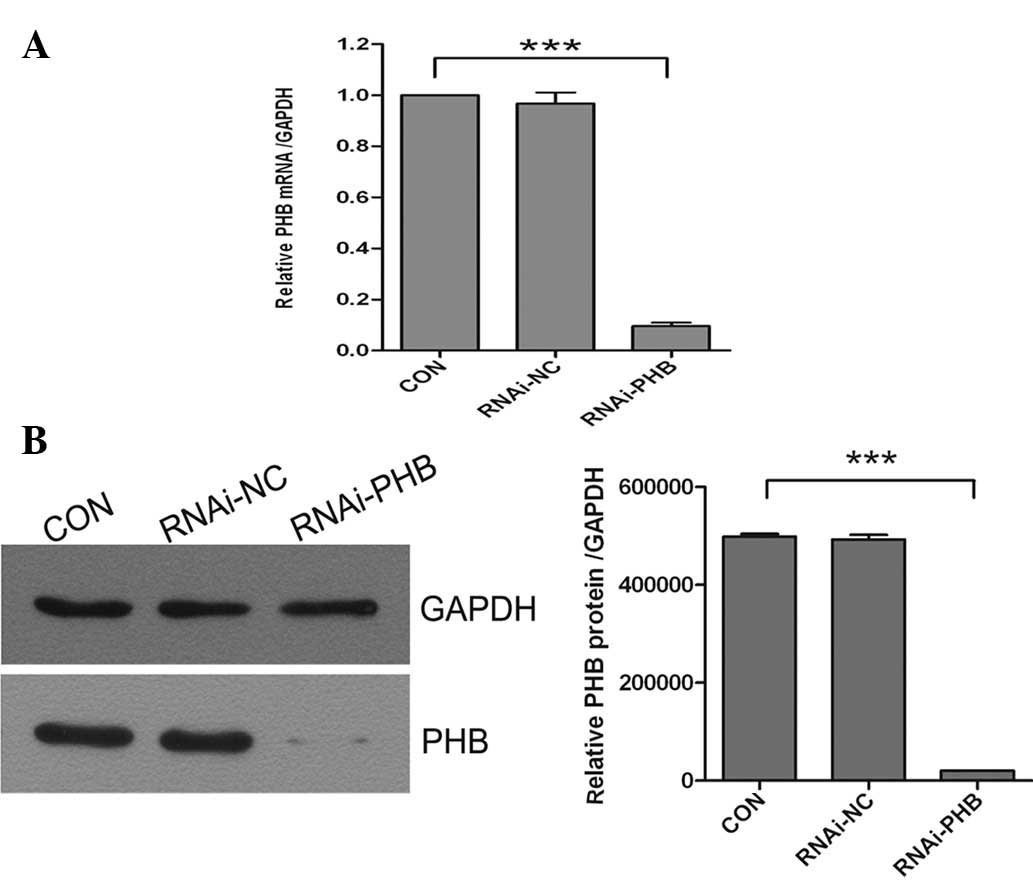
The silencing efficacy of PHB shRNA lentivirus at
mRNA and protein levels in NB4-R1 cells was determined by real-time
quantitative PCR and western blotting in CON, RNAi-NC and RNAi-PHB
groups, respectively. The results showed that the expression levels
of PHB in RNAi-PHB were markedly reduced by 90.3% (mRNA, Fig. 3A) and 95.8% (protein, Fig. 3B) compared with those in the
control group. Both real-time quantitative PCR and western blotting
demonstrated that lentiviral vectors were effective for PHB
silencing.
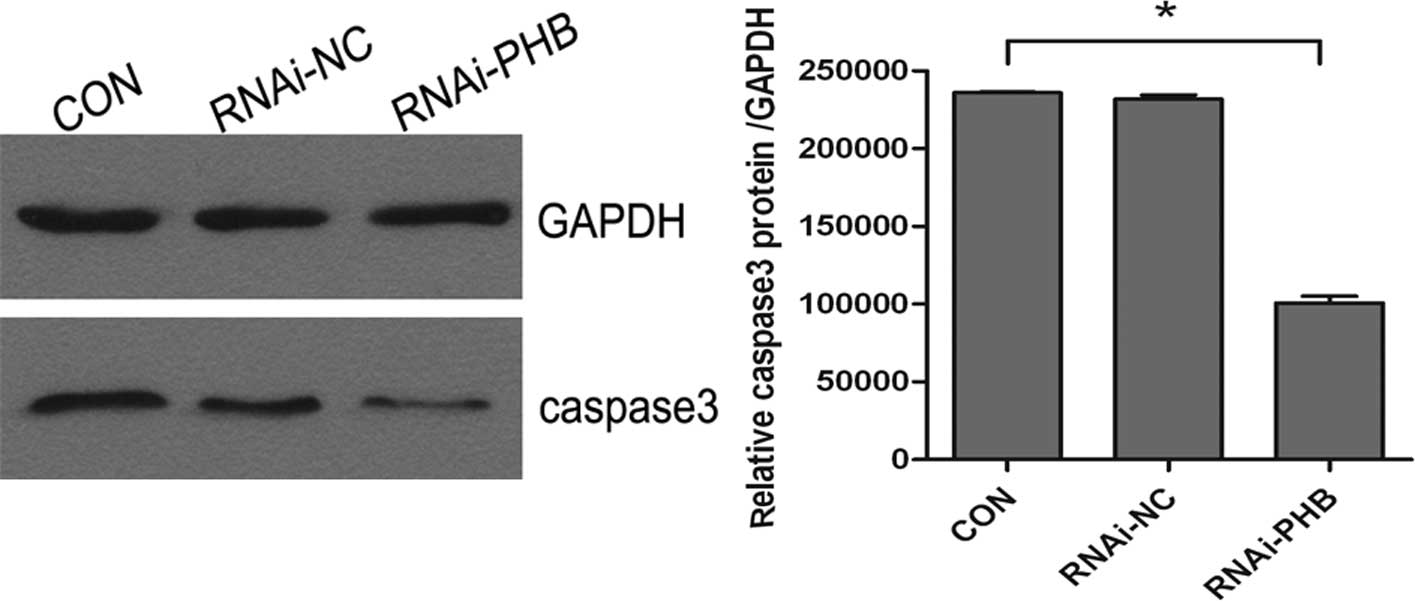
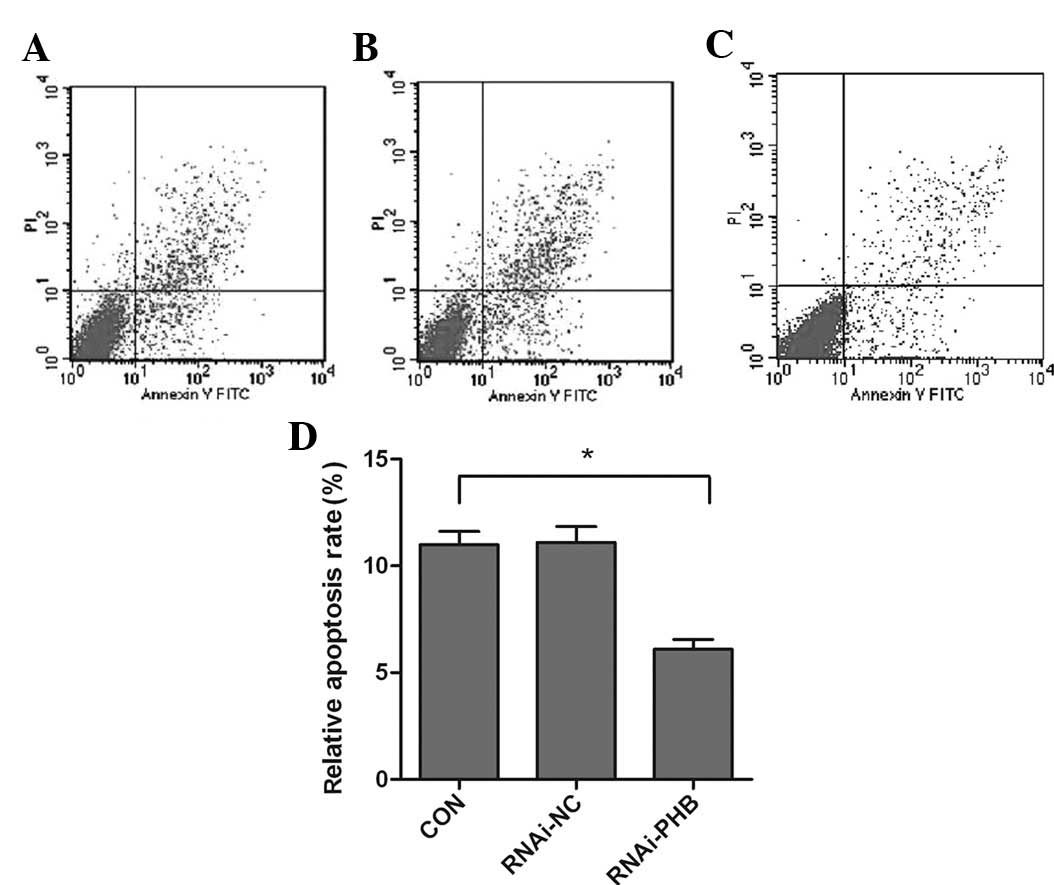
PHB gene silencing inhibits cell
apoptosis
The effect of PHB silencing on the apoptosis of
NB4-R1 cells was determined by FCM and western blot analysis of
caspase-3. As shown in Fig. 4,
when compared to the control group, the activity of caspase-3
decreased significantly, which showed a 57.3% downregulation, and
the rate of apoptosis was reduced by approximately 44.6% (Fig. 5; P<0.05).
Discussion
PHBs are ubiquitous, evolutionarily conserved
proteins, which comprise two highly homologous subunits, termed
PHB1 and PHB2. These two proteins assemble into a ring-like
macromolecular structure and are implicated in diverse cellular
processes, from mitochondrial biogenesis and function to cell
cycle, signaling, proliferation, apoptosis and transcriptional
control (9–11). In humans, the PHB gene has been
located at q21 of chromosome 17 (10), and has been associated with various
types of disease states, such as inflammation (12,13),
obesity and diabetes mellitus (14), Parkinson disease (15) and cancer (16,17).
Among them, PHB anti-tumor mechanisms are the focus of attention.
At present, many studies have confirmed that human breast cancer
(18), cervical and ovarian cancer
(19), bladder cancer (20), rectum cancer, leukemia (21) and other solid tumor or non-solid
tumor tissue cells have high expression of PHB. The biochemical
function and specific mechanism, however, have remained largely
elusive. Earlier reports showed that PHB, as a potential tumor
suppressor with various biological activity, was attributed to the
3′-UTR of mRNA. McClung et al found that wild-type 3′-UTR
mRNA was capable of inhibiting the proliferation of tumor cells,
while the mutant cells lose this activity (22). Other investigations have
demonstrated the anti-tumor activities and evolutionary
conservation of PHB, which has attracted the attention of
researchers, resulting in the discovery of the association between
PHB and the E2F pathway (23,24).
E2F activity is essential for the expression of critical cellular
genes. By combining with E2F, PHB may collect a variety of
transcription inhibitors and then suppress the transcriptional
activity of the tumor, so that the cell cycle is arrested in the
G1/S phase, achieving the purpose of inhibiting cell proliferation
and inducing cell apoptosis (25,26).
Additional diverse functions of PHB were recently reported, which
link this evolutionary highly-conserved gene to the induction of
cancer cell apoptosis through the Raf-MEK-ERK cascade (27), p53 (28,29)
and estrogen receptor (30). There
is significant evidence to indicate that PHB may be a promising
tumor-specific marker and drug therapy target.
RNAi is one of the most significant discoveries in
the field of gene regulation and has broad development prospects in
the research of tumor pathogeny, immune mechanisms and therapy
(31,32). Delivering siRNA into cells is the
greatest impediment to RNAi therapy. There are two main mechanisms
to achieve RNAi: delivering chemically synthesized siRNA into
cells, which directly or continuously produce siRNAs by
transcription in cells. Chemically synthesized siRNA could be
introduced into cells via traditional delivery strategies; however,
it has short gene silencing effects and cannot be passed to cell
progeny. Therefore, in recent years, the attention of researchers
has turned to expression vectors, which include retroviral,
lentivirus and others, in order to synthesize siRNA in mammalian
cells. In the past few years, shRNA has been widely used to silence
the expression of many target genes due to its high specificity and
apparent nontoxicity (33).
Since the lentivirus vectors have high efficiency
for gene delivery, low immune reactions in vivo, and may be
integrated into the non-dividing cell genome to achieve stable
long-term expression (34), we
constructed a lentiviral shRNA vector of the PHB gene. In this
study, we found an effective lentiviral vector-mediated RNAi had
been performed successfully. Moreover, accompanied by the
downregulation of PHB, reduced expression of caspase-3 and lower
apoptosis rates were observed. Caspases are crucial mediators of
programmed cell death (apoptosis). Among them, caspase-3 is a
frequently activated death protease, catalyzing the specific
cleavage of many key cellular proteins. Caspase-3 is essential for
major apoptotic scenarios in a remarkable tissue-, cell type- or
death stimulus-specific manner. It is also indispensable for
apoptotic chromatin condensation and DNA fragmentation in all cell
types examined (35). Although the
defined relationship and mechanism between PHB and NB4-R1 cell
apoptosis remain largely unknown, our research may provide
substantial information on the function of the PHB gene in APL and
provide a new target of leukemia therapy.
Acknowledgements
This study was supported by the Natural Science
Foundation of China (no. 30701133), the Fundamental Research Funds
for the Central Universities and the Shaanxi Province Science and
Technology Development Fund, China (2010K01-135, 2012KTCL03-12).
The authors express their gratitude to Dr Xinyang Wang and Dr Wen
Wen for their technological assistance.
References
|
1
|
Hu J, Liu YF, Wu CF, et al: Long-term
efficacy and safety of all-trans retinoic acid/arsenic
trioxide-based therapy in newly diagnosed acute promyelocytic
leukemia. Proc Natl Acad Sci USA. 106:3342–3347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hillestad LK: Acute promyelocytic
leukemia. Acta Med Scand. 159:189–194. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernard J, Weil M, Boiron M, Jacquillat C,
Flandrin G and Gemon MF: Acute promyelocytic leukemia: results of
treatment by daunorubicin. Blood. 41:489–496. 1973.PubMed/NCBI
|
|
4
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sanz MA: Arsenic trioxide in the
management of APL: proceed with caution. Oncology (Williston Park).
25:743–746. 2011.PubMed/NCBI
|
|
6
|
Gonzalez-Alegre P, Bode N, Davidson BL and
Paulson HL: Silencing primary dystonia: lentiviral-mediated RNA
interference therapy for DYT1 dystonia. J Neurosci. 25:10502–10509.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye X, Liu T, Gong Y, Zheng B, Meng W and
Leng Y: Lentivirus-mediated RNA interference reversing the
drug-resistance in MDR1 single-factor resistant cell line
K562/MDR1. Leuk Res. 33:1114–1119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rodriguez M, Aladowicz E, Lanfrancone L
and Goding CR: Tbx3 represses E-cadherin expression and enhances
melanoma invasiveness. Cancer Res. 68:7872–7881. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Artal-Sanz M and Tavernarakis N:
Prohibitin couples diapause signalling to mitochondrial metabolism
during ageing in C. elegans. Nature. 461:793–797. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Merkwirth C and Langer T: Prohibitin
function within mitochondria: essential roles for cell
proliferation and cristae morphogenesis. Biochim Biophys Acta.
1793:27–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Týc J, Faktorová D, Kriegová E, Jirků M,
Vávrová Z, Maslov DA and Lukes J: Probing for primary functions of
prohibitin in Trypanosoma brucei. Int J Parasitol. 40:73–83.
2010.PubMed/NCBI
|
|
12
|
Sharma A and Qadri A: Vi polysaccharide of
Salmonella typhi targets the prohibitin family of molecules
in intestinal epithelial cells and suppresses early inflammatory
responses. Proc Natl Acad Sci USA. 101:17492–17497. 2004.
|
|
13
|
Theiss AL, Idell RD, Srinivasan S,
Klapproth JM, Jones DP, Merlin D and Sitaraman SV: Prohibitin
protects against oxidative stress in intestinal epithelial cells.
FASEB J. 21:197–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kolonin MG, Saha PK, Chan L, Pasqualini R
and Arap W: Reversal of obesity by targeted ablation of adipose
tissue. Nat Med. 10:625–632. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrer I, Perez E, Dalfó E and Barrachina
M: Abnormal levels of prohibitin and ATP synthase in the substantia
nigra and frontal cortex in Parkinson’s disease. Neurosci Lett.
415:205–209. 2007.PubMed/NCBI
|
|
16
|
Mengwasser J, Piau A, Schlag P and Sleeman
JP: Differential immunization identifies PHB1/PHB2 as blood-borne
tumor antigens. Oncogene. 23:7430–7435. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gamble SC, Chotai D, Odontiadis M, Dart
DA, Brooke GN, Powell SM, Reebye V, Varela-Carver A, Kawano Y,
Waxman J and Bevan CL: Prohibitin, a protein downregulated by
androgens, represses androgen receptor activity. Oncogene.
26:1757–1768. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng X, Mehta R, Wang S, Chellappan S and
Mehta RG: Prohibitin is a novel target gene of vitamin D involved
in its antiproliferative action in breast cancer cells. Cancer Res.
66:7361–7369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gregory-Bass RC, Olatinwo M, Xu W,
Matthews R, Stiles JK, Thomas K, Liu D, Tsang B and Thompson WE:
Prohibitin silencing reverses stabilization of mitochondrial
integrity and chemoresistance in ovarian cancer cells by increasing
their sensitivity to apoptosis. Int J Cancer. 122:1923–1930. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu TF, Wu H, Wang YW, Chang TY, Chan SH,
Lin YP, Liu HS and Chow NH: Prohibitin in the pathogenesis of
transitional cell bladder cancer. Anticancer Res. 27:895–900.
2007.PubMed/NCBI
|
|
21
|
Qi J, He P, Chen W, Wang H, Wang X and
Zhang M: Comparative proteome study of apoptosis induced by As4S4
in retinoid acid resistant human acute promyelocytic leukemia
NB4-R1 cells. Leuk Res. 34:1506–1516. 2010. View Article : Google Scholar
|
|
22
|
McClung JK, Jupe ER, Liu XT and Dell’Orco
RT: Prohibitin: potential role in senescence, development, and
tumor suppression. Exp Gerontol. 30:99–124. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang S, Nath N, Adlam M and Chellappan S:
Prohibitin, a potential tumor suppressor, interacts with RB and
regulates E2F function. Oncogene. 18:3501–3510. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rastogi S, Joshi B, Dasgupta P, Morris M,
Wright K and Chellappan S: Prohibitin facilitates cellular
senescence by recruiting specific corepressors to inhibit E2F
target genes. Mol Cell Biol. 26:4161–4171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S and Faller DV: Roles of prohibitin
in growth control and tumor suppression in human cancers. Transl
Oncogenomics. 3:23–37. 2008.PubMed/NCBI
|
|
26
|
Singh S, Johnson J and Chellappan S: Small
molecule regulators of Rb-E2F pathway as modulators of
transcription. Biochim Biophys Acta. 1799:788–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ande SR, Xu Z, Gu Y and Mishra S:
Prohibitin has an important role in adipocyte differentiation. Int
J Obes (Lond). 36:1236–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Joshi B, Rastogi S, Morris M, Carastro LM,
DeCook C, Seto E and Chellappan SP: Differential regulation of
human YY1 and caspase 7 promoters by prohibitin through E2F1 and
p53 binding sites. Biochem J. 401:155–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fusaro G, Dasgupta P, Rastogi S, Joshi B
and Chellappan S: Prohibitin induces the transcriptional activity
of p53 and is exported from the nucleus upon apoptotic signaling. J
Biol Chem. 278:47853–47861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He B, Feng Q, Mukherjee A, Lonard DM,
DeMayo FJ, Katzenellenbogen BS, Lydon JP and O’Malley BW: A
repressive role for prohibitin in estrogen signaling. Mol
Endocrinol. 22:344–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davis ME, Zuckerman JE, Choi CH, Seligson
D, Tolcher A, Alabi CA, Yen Y, Heidel JD and Ribas A: Evidence of
RNAi in humans from systemically administered siRNA via targeted
nanoparticles. Nature. 464:1067–1070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moffat J, Grueneberg DA, Yang X, et al: A
lentiviral RNAi library for human and mouse genes applied to an
arrayed viral high-content screen. Cell. 124:1283–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gartel AL and Kandel ES: RNA interference
in cancer. Biomol Eng. 23:17–34. 2006. View Article : Google Scholar
|
|
34
|
Cockrell A and Kafri T: Gene delivery by
lentivirus vectors. Mol Biotechnol. 36:184–204. 2007. View Article : Google Scholar
|
|
35
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|