Introduction
Bronchial asthma (referred to as asthma) is a common
chronic inflammatory disease of the airways characterized by
variable and recurring bronchospasm that causes periodic attacks of
coughing, wheezing, shortness of breath and chest tightness
(1). A small number of patients
also present with chest pain as a main symptom. These symptoms may
be initiated when patients are exposed to irritant gases or
allergens and they are often exacerbated at night and/or in the
morning (2). The principal
pathological features of asthma include reversible
bronchoconstriction, increased airway reactivity and increased
inflammatory response (3). Studies
have shown that the imbalance of different types of helper T cells
(Th), particularly the imbalance of Th1/Th2 cells, may be the main
cause of asthma. Specifically, increased numbers of Th2 cells and
the secretion of inflammatory factors may be key factors in airway
inflammation (4,5). Interleukin-18 (IL-18) regulates the
immune response of Th1/Th2 cells, and polymorphisms of the IL-18
gene promoter are associated with diseases such as rheumatoid
arthritis (6). This study
investigated the association of asthma with polymorphisms of the
IL-18 promoter at sites -607C/A and-137G/C by measuring these
polymorphisms in asthma patients and in healthy individuals.
Materials and methods
Case records
The experimental group samples were collected from
asthma patients (n=120) diagnosed in The People’s Hospital of
Lishui (Zhejiang, China) from March 2007 to June 2011. The subjects
comprised 59 men and 61 women and their median age was 25.58 years
(±7.78, range 15–37 years). Among the asthma patients, 49 were
cases of allergic rhinitis in asthma (experimental group 1) and 71
were without allergic rhinitis (experimental group 2). Healthy
individuals (n=120) who had received a health examination in the
Physical Examination Center in the hospital were selected as a
control group during the same time period. These individuals
comprised 61 men and 59 women of median age, 36.89 years (±7.67,
range 15–37 years). No statistically significant differences in age
or gender existed between the experimental and control groups. The
Ethics Committee of Lishui University approved the study.
Genotype identification of IL-18 promoter
at -607C/A and -137G/C
A polymerase chain reaction sequence-specific primer
(PCR-SSP) (7) was used to identify
the genotypes of the IL-18 promoter at -607C/A and-137G/C. Primer 5
primer design software was used to design specific F1, F2, common
reverse and control forward primers of the promoter at -607C/A
and-137G/C, respectively. Samples of venous blood (5 ml) were
obtained from all patients for extraction of genomic DNA by the
phenolic-chloroform-isoamyl alcohol method (8). Polymerase chain reaction (PCR) was
adapted to amplify the specific segment. The primer sequences for
the promoter at the -607C/A site were: specific forward F1:
5′-GTTGCA GAAAGTGTAAAAATTATTAC-3′; specific forward F2:
5′-GTTGCAGAAAGTGTAAAAATTATTAA-3′; common reverse:
5′-CTTTGCTATCATTCCAGGAA-3′; and internal control forward:
5′-TAACCTCATTCAGGACTTCC-3′. The primer sequences for the promoter
at the -137G/C site were: specific F1 forward:
5′-CCCCAACTTTTACGGAAGAAAAG-3′; specific F2 forward:
5′-CCCCAACTTTTACGGAAGAAAAC-3′; common reverse:
5′-CCAATAGGACTGATTATTCCGCA-3′; and internal control forward:
5′-AGGAGGGCAAAATGCACTGG-3′. The primers were synthesized by Takara
Biotech Co., Ltd. (Dalian, China). The specific forward primers F1
and F2 were used for all PCR reactions of each sample. The PCR
reaction system included: 21 μl genotype DNA; 2.5 μl
10X PCR buffer (Promega Corp., Madison, WI, USA); 1.5 μl
dNTP (25 mmol/l, Promega Corp.); 0.25 μl Taq DNA polymerase
(2.5 U/ml, Promega Corp.); 1.5 μl MgCl2 (25
mmol/l, Promega Corp.); 0.5 μl specific forward primer F1 or
F2; 0.5 μl common reverse primer; 0.5 μl internal
control forward primer; and water to a volume of 25 μl. PCR
reaction conditions at -607C/A were as follows: 7 cycles of
denaturation for 30 sec at 94°C; annealing for 40 sec at 64°C;
extension for 45 sec at 72°C; then 30 cycles of denaturation for 60
sec at 94°C; annealing for 60 sec at 57°C; extension for 60 sec at
72°C; and a final extension for 5 min at 72°C. PCR reaction
conditions at -137G/C were as follows: 5 cycles of denaturation for
5 min; denaturation for 30 sec at 94°C, annealing for 40 sec at
68°C; and extension for 60 sec at 72°C; then 30 cycles of
denaturation for 60 sec at 94°C; annealing for 60 sec at 62°C; and
extension for 60 sec at 72°C; and a final extension for 5 min at
72°C.
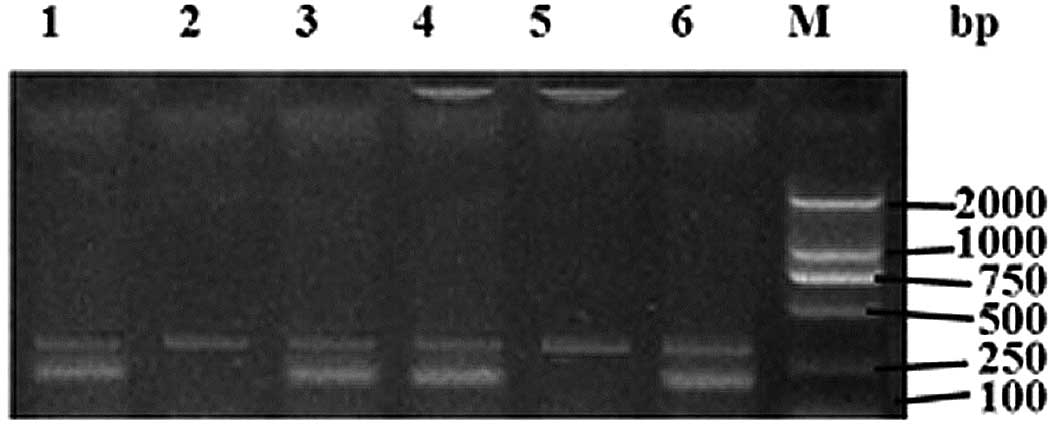
Genotypes were determined from the bands obtained by
agarose gel electrophoresis. The genotype CC at -607C/A gave a
primer F1 product with two bands (301 and 196 bp) and a primer F2
product with one band (301 bp). The genotype CG yielded primer F1
and F2 products with two bands (301 and 196 bp). The genotype GG
yielded a primer F1 product of one band (301 bp) and a primer F2
product of two bands (301 and 196 bp) (Fig. 1). For the genotype CC at -137G/C,
the primer F1 product produced two bands (261 and 446 bp) and the
primer F2 product yielded one band (446 bp). For genotype GC, the
products of primers F1 and F2 each produced two bands (261 and 446
bp). For genotype CC, the primer F1 product produced one band (446
bp) and the primer F2 product yielded two bands (261 and 446 bp)
(Fig. 2).
Statistical analysis
SPSS 13.0 for Windows (IBM, Armonk, NY, USA)
statistical software was used for statistical analysis. The
χ2 test was adopted to analyze the data and P<0.05
was considered to indicate a statistically significant result.
Results
Polymorphism analysis of the IL-18 gene
promoter at -607C/A
The genotype distributions and allele frequencies of
the IL-18 gene promoter at -607C/A between the experimental and
control groups conformed to the Hardy-Weinberg equilibrium. No
statistically significant differences in the genotype distributions
and allele frequencies between the experimental and control groups
were identified (P>0.05). The frequencies of genotypes CC, CA
and AA in experimental group 1 were 18.37, 40.82 and 40.82%,
respectively, and in experimental group 2 were 32.39, 54.92 and
12.68%, respectively. Therefore, there was a statistically
significant difference in the genotype distribution at -607C/A
between experimental groups 1 and 2 (χ2=12.81;
P<0.05; Table I). The allele
frequencies of C and A in experimental group 1 were 38.78 and
61.22%, respectively, and in experimental group 2 were 59.86 and
40.14%, respectively. There was a significant difference in the
allele frequency at -607C/A between experimental groups 1 and 2
(χ2=10.32; P<0.05).
 | Table IGenotype and allele frequencies of
IL-18 gene -607C/A site polymorphism. |
Table I
Genotype and allele frequencies of
IL-18 gene -607C/A site polymorphism.
| | Genotype
frequency | Allele frequency |
|---|
| |
|
|
|---|
| Groups | n | CC | CA | AA | C | A |
|---|
| Control group | 120 | 30 | 49 | 41 | 109 | 131 |
| Experimental
group | 120 | 32 | 59 | 29 | 123 | 117 |
| Experimental group
1 | 49 | 9 | 20 | 20 | 38 | 60 |
| Experimental group
2 | 71 | 23 | 39 | 9a | 85 | 57a |
Polymorphism analysis of the IL-18 gene
promoter at -137G/C
The genotype distributions and allele frequencies of
the IL-18 gene promoter at -137G/C between the experimental and
control groups conformed to the Hardy-Weinberg equilibrium. No
statistically significant differences were identified in the
genotype distributions and allele frequencies between the
experimental and control groups (χ2=15.73; P>0.05;
Table II). The frequencies of
genotypes GG, GC and CC in experimental group 1 were 48.98, 46.94
and 4.08%, respectively, and in experimental group 2 were 80.28,
14.08 and 4.08%, respectively. Therefore, there was a significant
difference in the genotype distribution at -137G/C between
experimental groups 1 and 2 (χ2=15.73; P<0.05). The
allele frequencies of G and C in experimental group 1 were 72.45
and 27.55%, respectively, and in experimental group 2 were 87.32
and 12.68%, respectively. Therefore, there was a statistically
significant difference in the allele frequency at -137G/C between
experimental groups 1 and 2 (χ2=8.42; P<0.05).
 | Table IIGenotype and allele frequencies of
IL-18 gene -137G/C site polymorphism. |
Table II
Genotype and allele frequencies of
IL-18 gene -137G/C site polymorphism.
| | Genotype
frequency | Allele frequency |
|---|
| |
|
|
|---|
| Groups | n | GG | GC | CC | G | C |
|---|
| Control group | 120 | 83 | 32 | 5 | 198 | 42 |
| Experimental
group | 120 | 81 | 33 | 6 | 195 | 45 |
| Experimental group
1 | 49 | 24 | 23 | 2 | 71 | 27 |
| Experimental group
2 | 71 | 57 | 10 | 4a | 124 | 18a |
Discussion
Studies have shown that an imbalance of Th1/Th2 cell
function may cause asthma (9).
Apter and Szefler (10) indicated
that Th1 and Th2 cells had similar in vivo effects; for
example, both were able to induce bronchial asthma, and cytokines
from Th1 and Th2 cells may cooperate to aggravate bronchial asthma
symptoms. This contrasts with the earlier theory that Th1 and Th2
cytokines have contrasting effects on airway hyper- responsiveness
(10). IL-18 is a member of the
IL-1 family and is synthesized and secreted by a variety of immune
effector cells. As a Th1-type cytokine, IL-18 is able to induce the
occurrence of Th1-type cytokines, including IFN-γ and TNF-α, and
contribute to the damage of tissues. However, IL-18 is also able to
stimulate the expression of Th2 cytokines (11). IL-18 improves IgE and IgG1
expression in bronchioloalveolar eosinophils and increases cytokine
release through the promotion of eosinophil activation. Therefore,
IL-18 may trigger allergic inflammation by causing the accumulation
of eosinophils and stimulating IgE (12). The plasma IL-18 levels of children
with asthma are significantly higher than those of healthy children
(13,14), thus IL-18 may be associated with
the occurrence of bronchial asthma.
The current study analyzed the genotypes of the
IL-18 gene promoter at -607C/A and -137G/C in patients with
bronchial asthma and healthy individuals. The results indicated
that there were no statistically significant differences in the
genotype distributions and allele frequencies at -607C/A and
-137G/C between subjects with bronchial asthma and healthy
subjects. However, there were significance differences in genotype
distributions and allele frequencies at -607C/A and -137G/C in
patients with bronchial asthma and allergic rhinitis
(P<0.05).
IL-18 is located at chromosome 11q22.2–22.3, which
has two promoters upstream of exons 1 and 2. The analysis of the
IL-18 sequence revealed that the -137G/C site, located at the GATA3
binding site, is able to induce the expression of Th2 cytokines,
while inactivation of GATA3 may reduce the airway inflammation in
asthmatic mice. Furthermore, the presence of allele G is required
for increased IL-18 expression following cell stimulation by PMA,
but substitution with allele C attenuates this effect (15).
Pawlik et al(6) also found an association between
polymorphisms of the IL-18 promoter at site -607C/A and severe
bronchial asthma. The results of our study revealed that
polymorphisms of the IL-18 gene promoter at -137G/C and -607C/A are
not correlated with bronchial asthma without allergic rhinitis, but
correlate with bronchial asthma with allergic rhinitis. As such,
our results are not entirely consistent with the previous studies
possibly to ethnic/geographic differences in the study populations.
Further studies are required in diverse ethnic regions to elucidate
the correlation of IL-18 promoter polymorphisms with asthma.
In brief, our results have shown that polymorphisms
of the IL-18 gene promoter at -137G/C and -607C/A do not correlate
with bronchial asthma, but correlate with allergic rhinitis in
bronchial asthma. Polymorphisms of the IL-18 promoter at -137G/C
and -607C/A may have unique effects on the pathogenesis of asthma
and allergic rhinitis.
References
|
1
|
Krishnan JA, Lemanske RF Jr, Canino GJ,
Elward KS, Kattan M, Matsui EC, et al: Asthma outcomes: symptoms. J
Allergy Clin Immunol. 129(Suppl 3): S124–S135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilson SR, Rand CS, Cabana MD, Foggs MB,
Halterman JS, Olson L, et al: Asthma outcomes: quality of life. J
Allergy Clin Immunol. 129(Suppl 3): S88–S123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehring M, Gillissen A and Schneider A:
Asthma - what is to do in general practice? MMW Fortschr Med.
153:47–51. 2011.(In German).
|
|
4
|
Tattersfield AE, Knox AJ, Britton JR and
Hall IP: Asthma. Lancet. 360:1313–1322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang XZ, Zhuang JH, Ren YG, Zhou LJ and
Zhou Q: Association of interleukin-6 and interleukin-18 gene
polymorphism with rheumatoid arthritis in Guangdong Han population.
Nan Fang Yi Ke Da Xue Xue Bao. 27:1661–1664. 2007.(In Chinese).
|
|
6
|
Pawlik A, Kaminski M, Kuśnierczyk P, et
al: Interleukin-18 promoter polymorphism in patients with atopic
asthma. Tissue Antigens. 70:314–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giedraitis V, He B, Huang WX and Hillert
J: Cloning and mutation analysis of the human IL-18 promoter: a
possible role of polymorphisms in expression regulation. J
Neuroimmunol. 112:146–152. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panda DK, Miao D, Bolivar I, Li J, Huo R,
Hendy GN and Goltzman D: Inactivation of the 25-hydroxyvitamin D
1alpha-hydroxylase and vitamin D receptor demonstrates independent
and interdependent effects of calcium and vitamin D on skeletal and
mineral homeostasis. J Biol Chem. 279:16754–16766. 2004. View Article : Google Scholar
|
|
9
|
Liu L, Jarjour NN, Busse WW and Kelly EA:
Enhanced generation of helper T type 1 and 2 chemokines in
allergen-induced asthma. Am J Respir Crit Care Med. 169:1118–1124.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Apter AJ and Szefler SJ: Advances in adult
and pediatric asthma. J Allergy Clin Immunol. 113:407–414. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Volin MV and Koch AE: Interleukin-18: a
mediator of inflammation and angiogenesis in rheumatoid arthritis.
J Interferon Cytokine Res. 31:745–751. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wild JS, Sigounas A, Sur N, et al:
IFN-gamma-inducing factor (IL-18) increases allergic sensitization,
serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model
of allergic asthma. J Immunol. 164:2701–2710. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rachmiel M, Bloch O, Shaul AA, et al:
Young patients with both type 1 diabetes mellitus and asthma have a
unique IL-12 and IL-18 secretory pattern. Pediatr Diabetes.
12:596–603. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melén E, Himes BE, Brehm JM, et al:
Analyses of shared genetic factors between asthma and obesity in
children. J Allergy Clin Immunol. 126:631–637. e1–8.
2010.PubMed/NCBI
|
|
15
|
Imboden M, Nicod L, Nieters A, et al: The
common G-allele of interleukin-18 single-nucleotide polymorphism is
a genetic risk factor for atopic asthma. The SAPALDIA Cohort Study.
Clin Exp Allergy. 36:211–218. 2006. View Article : Google Scholar : PubMed/NCBI
|