Introduction
Shear stress induced by blood flow may play a
pivotal role in the induction or prevention of atherosclerosis by
affecting endothelial functions. Previous studies have demonstrated
that shear stress also inhibits apoptosis of vascular endothelial
cells (ECs) (1,2). In contrast, flow that has a low mean
shear stress and turbulence is markedly correlated with EC
dysfunction, EC apoptosis and atherosclerosis (3–5). In
accordance with our previous studies (6,7), we
identifed that physiological shear stress inhibits EC apoptosis
partly by activating human inhibitor of apoptosis protein-2
(HIAP-2) and X-linked inhibitor of apoptosis protein (XIAP).
Mammalian Omi/HtrA2, a nuclear-encoded mitochondrial protein, is
the antagonist of inhibitor of apoptosis protein (IAP). Following
apoptotic stimulation, the target sequence of Omi/HtrA2 in
mitochondria is cleaved, resulting in a new apoptotic activity of
the N-terminus. When mammalian Omi/HtrA2 is released into the
cytoplasm, it has the ability to bind and antagonize the
baculovirus IAP repeat (BIR) function of IAPs and releases the
caspases binding to the BIR. Omi/HtrA2 also promotes the catalytic
cleavage of IAPs leading to their irreversible inactivation and
promoting the progression of apoptosis (8,9). The
catalytic activity of the Omi/HtrA2 serine protease therefore plays
a major role in induced cell death. It is noteworthy that a number
of studies have demonstrated the importance of the Omi/HtrA2
pathway in different cell types (10–12).
However, its role in the endothelial adaptive response to laminar
shear stress has, to date, not been elucidated.
In the present study, we reveal that Omi/HtrA2
expression and function are markedly inhibited following exposure
of ECs to laminar shear stress. We also analyze the role of
Omi/HtrA2 signaling in the response of ECs to hemodynamic
force.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
harvested from a human umbilical vein using 0.05% trypsin with 0.02
% EDTA and suspended in Dulbecco’s modified Eagle’s medium (DMEM)
containing 20% fetal bovine serum (FBS), 10 μg/l basic fibroblast
growth factor (bFGF), 100 U/ml penicillin and 100 μg/ml
streptomycin sulfate. Cells were plated onto gelatin-coated
polyester sheets (54×89 mm; Costar-Corning, Tewksbury, MA, USA), at
a seeding density of 1–5×106 per sheet. The cells were
cultured until they reached confluence. To avoid phenotypic changes
caused by passaging, only cells experiencing 3–7 passages were
used.
HUVECs were obtained and cultured as previously
described (6). Cells were plated
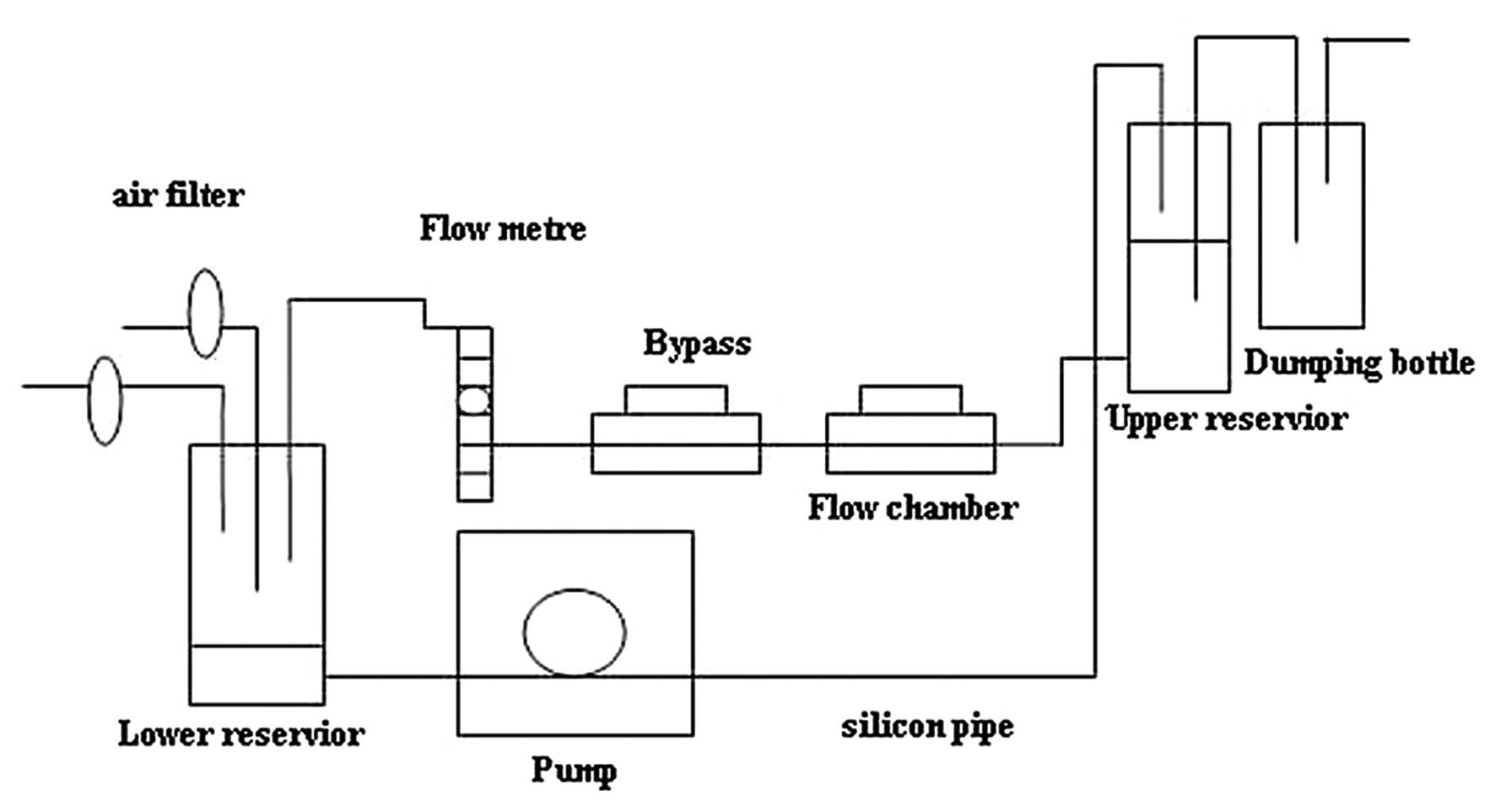
on plastic slides or polyester sheets, and flow experiments using a
parallel-plate flow chamber (Fig.
1) were performed to generate unidirectional laminar shear
stress (6,7). Control ECs were maintained in an
incubator.
DNA fragmentation assay
The isolation of fragmented DNA was conducted as
follows. Briefly, after culturing for 24 h and starving in medium
containing 2% FBS, cells were treated with varying levels of shear
stress for 24 h. HUVECs were lysed and treated with RNAse A and
proteinase K. The DNA fragments were precipitated with ethanol,
resuspended in 50 μl of TE buffer and analyzed by
electrophoresis.
RT-PCR and real-time PCR
The HUVECs were incubated with 2% FBS DMEM without
bFGF for 12 h, then placed in the shear stress flow chamber. ECs
were exposed to 15 dyne/cm2 of shear stress for 1, 2, 4
and 6 h, and harvested for RT-PCR. Total RNA was extracted using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. PCR primers were in accordance
with published sequences as follows: Omi/HtrA2 forward, 5′-GCC GTG
GTC TAT ATC GAG ATC-3′; reverse, 5′-TGA GCC GTT CGA GAT AGG G-3′;
GAPDH forward 5′-GCA CCG TCA AGG CTG AGA AC-3′; reverse, 5′-TGG TGA
AGA CGC CAG TGG A-3′. PCR was performed for one cycle of 2.5 mins
at 95°C, and then run for 33 cycles at 94°C of 30 sec, 58°C for 30
sec and 72°C for 45 sec.
mRNA levels were also quantified by real-time PCR.
The amplification mixtures (20 μl) contained 0.5 μl of cDNA, 0.5 μl
of each primer and 10 μl SYBR-Green PCR Master mix (Applied
Biosystems, Foster City, CA, USA). PCR was performed at 50°C for 2
min, 95°C for 10 min (for AmpliTaq Gold activation) and then run
for 40 cycles at 95°C for 15 sec and 60°C for 1 min. The
fluorescence data for the SYBR-Green dye at each cycle were
collected using an ABI PRISM 7300 Sequence Detection System
(Applied Biosystems). The cycle threshold values were normalized
against the housekeeping gene GAPDH.
Western blot analysis
HUVECs were harvested by scraping, and the cells
were pelleted by centrifugation and resuspended in lysis buffer.
Proteins were separated on a 10% SDS-polyacrylamide gel and
transferred onto a polyvinylidene difluoride (PVDF) membrane.
Anti-Omi/HtrA2 monoclonal antibody was diluted 1:500 (R&D
Systems, Minneapolis, MN, USA). Anti β-actin antibody was diluted
1:1000. Immunoblots were detected by enhanced chemiluminescence
reaction reagents and signals were quantified using the Bio-Rad
system image program (Quantity One; Bio-Rad, Hercules, CA,
USA).
Immunofluorescence microscopy
Cells were exposed to 15 dyne/cm2 shear
stress with 2% FBS in DMEM for 12, 18 and 24 h, and then 100 nM
MitoTracker Red was added for 20 min at 37°C. The cells were washed
three times with PBS, fixed with 4% paraformaldehyde for 20 min,
permeabilized with 0.2% Triton X-100 in PBS for 5 min, and blocked
for 1 h with 3% BSA at 4°C. Cells were then incubated with
anti-Omi/HtrA2 (1:200 in 3% BSA) for 2 h at 37°C, washed three
times with PBS, permeabilized with 0.2% Triton X-100 again, and
then incubated with FITC-goat anti-mouse IgG (1:100 in 3% BSA) for
60 min at 25°C. After three more washes with PBS, cells were
mounted in 1 drop of Dapi-Fluoromount-G. Finally, the fluorescence
was visualized using a laser scanning confocal microscope
(Fluoview, Olympus, Japan).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5 and Bio-Rad Quantity One software. The distribution of data
for each variable was assessed and variables were transformed to
normalize the distribution of data, if necessary. Multiple
comparisons were performed by one-way analysis of variance. P≤0.05
was considered to indicate a statistically significant
difference.
Results
Shear stress inhibits HUVEC
apoptosis
Serum starvation of HUVECs resulted in typical
characteristics of apoptotic cells, including shrinkage, rounding
and membrane blebbing. No such effect was observed with the shear
stress-treated cells. Agarose gel electrophoresis revealed a
typical ladder pattern of internucleosomal DNA fragmentation in the
serum-deprived cells, while the shear stress treated group
demonstrated inhibition of serum starvation-stimulated apoptosis
(Fig. 2).
Omi/HtrA2 expression in HUVECs
To examine whether shear stress induced expression
of Omi/HtrA2 mRNA, RT-PCR and real-time PCR were performed. Before
the shear stress was imposed, the HUVECs were induced to apoptosis
by incubation with 2% FBS DMEM without bFGF for 12 h. The level of
mRNA expression was indicated in terms of the Ct value. The Ct
values reflect the cycle number at which the fluorescence generated
within a reaction crosses the threshold. ΔCt is the difference in
threshold cycles for the target gene and the reference gene. In
this study, GAPDH was used as the reference gene. The Omi/HtrA2
mRNA level decreased from 4 h to 6 h following shear stress
(Fig. 3A and B).
To identify whether shear stress inhibited Omi/HtrA2
expression at the protein level, confluent HUVECs were subjected to
laminar shear stress of 15 dyne/cm2 for varying periods
of time. Reduced levels of Omi/HtrA2 protein were detected at 12,
18 and 24 h, as analyzed by western blot analysis. Densitometry
analysis revealed that exposure to shear stress decreased the
amount of Omi/HtrA2 protein at 12, 18 and 24 h (Fig. 3D), compared with the levels in
HUVECs kept in a static condition. Total protein expression of the
control group, which was not exposed to shear stress, gradually
increased (Fig. 3C). Total protein
expression of the experimental group, which was exposed to 15
dyne/cm2 of shear stress, significantly decreased from
12 h to 24 h and was the lowest at 24 h (Fig. 3E).
Omi/HtrA2 protein migration
To examine whether the Omi/HtrA2 protein migrated
between the cytoplasm and mitochondria following induction of
apoptosis and protection by shear stress, we separated the total
protein into cytoplasmic and mitochondrial fractions. We then used
western blot analysis and immunofluorescence to analyze the
results.
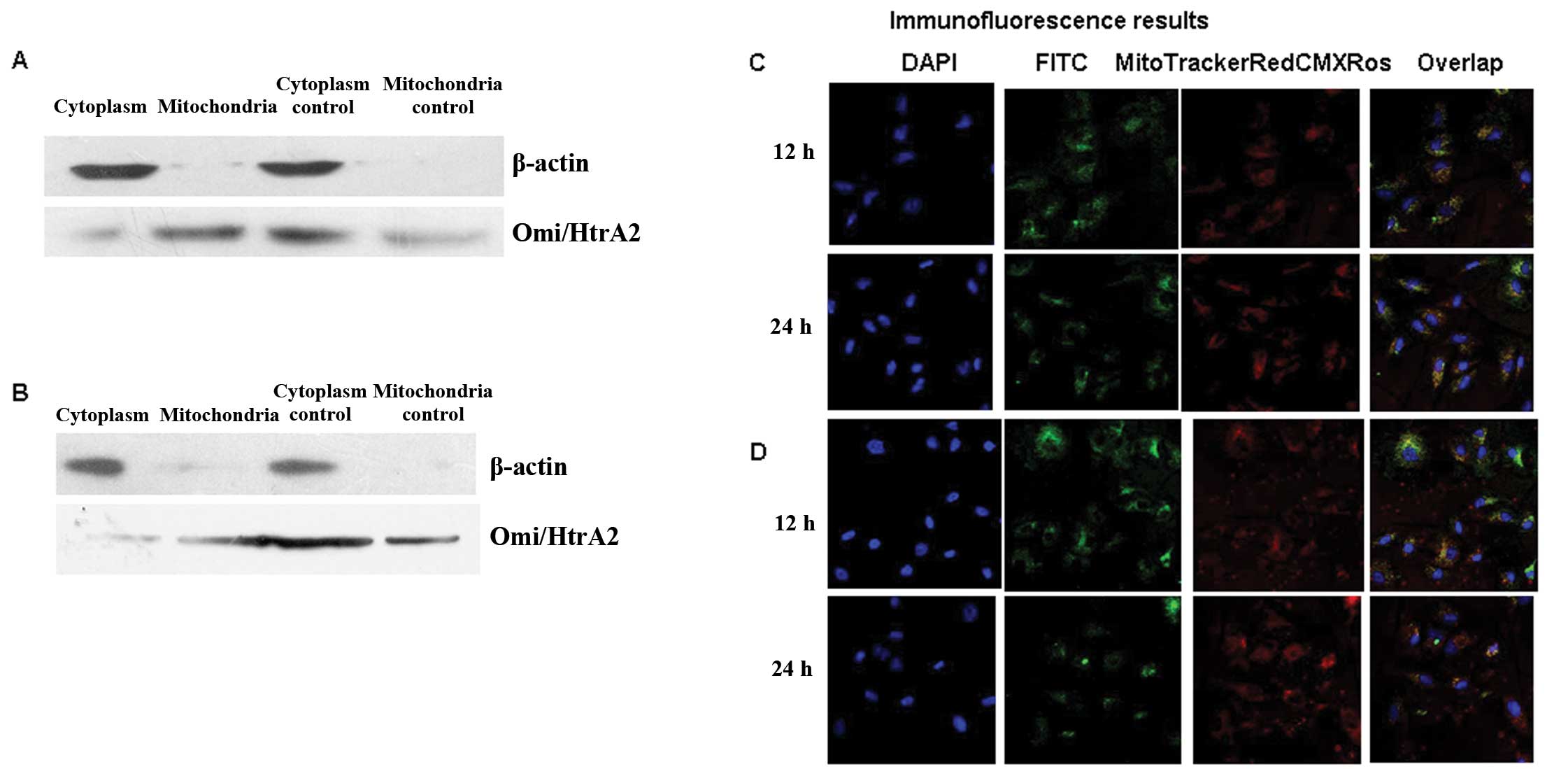
Mitochondrial and cytoplasmic proteins were
separated using the isolation kit (Applygen, Beijing, China), and
the Omi/HtrA2 protein levels were measured by western blot
analysis. As shown in Fig. 4A and
B, the mitochondrial protein was more weakly expressed than the
cytoplasmic protein in the static control group. However, in the
shear stress group, the mitochondrial protein was more strongly
expressed than the cytoplasmic protein; this was more evident at 24
h. In the static control group, mitochondrial protein was expressed
more weakly, but cytoplasmic protein was expressed more strongly;
consistent with the 12 h result. In the shear stress group, the
mitochondrial protein is expressed even more strongly and the
cytoplasmic protein is expressed even more strongly.
For the immunofluorescence analysis, we labeled
nuclei with fluorescent DAPI (blue), Omi/HtrA2 with FITC (green),
and mitochondria with MitoTracker Red CMXRos (red). Based on
analysis of the images detected by fluorescent confocal microscopy
(Fig. 4C and D), Omi/HtrA2 protein
was gradually released into the cytoplasm from the mitochondria in
the static control group following induction due to lack of bFGF
and 2% FBS DMEM medium for 12 and 24 h (Fig 4C). In the shear stress group
(Fig. 4D), the Omi/HtrA2 protein
was expressed mainly in the mitochondria following shear stress for
12 and 24 h, consistent with the western blot analysis result. The
results indicate that the apoptotic signal induced the HUVECs to
express greater Omi/HtrA2 in the absence of the protective effect
of shear stress.
Discussion
The present study demonstrates that Omi/HtrA2 mRNA
and protein expression and release of Omi/HtrA2 from mitochondria
into the cytoplasm in HUVECs was decreased by shear stress.
Atherosclerotic lesions are preferentially found in
areas with low or turbulent shear stress, while areas exposed to
steady laminar shear stress are protected (3,4). A
large body of evidence suggests that endothelial apoptosis
contributes to the development of atherosclerotic lesions in areas
of low or turbulent flow with prevalent occurrence of apoptosis in
the downstream part of the plaque (4,5).
In vitro studies have demonstrated that laminar shear stress
at physiological levels is sufficient to protect HUVECs from
apoptotic cell death (13,14). Several mechanisms have been
proposed to account for the anti-apoptotic effects of laminar shear
stress, including upregulation of anti-apoptotic Bcl-XL (14), IAPs (6,7) and
activation of Akt kinase (15).
According to an earlier study, the intrinsic apoptotic pathway is
involved after ECs receive apoptotic stimuli. In this pathway, the
outer membrane of the mitochondria becomes permeable to cytochrome
C. Once released to the cytosol, cytochrome C binds to Apaf-1 at a
ratio of 2:1 forming an oligomeric Apaf-1/cytochrome C complex in
the presence of dATP or ATP. This oligomerized Apaf-1/cytochrome C
complex then recruits the initiator caspase of this pathway,
pro-caspase-9, and induces its autoactivation and the subsequent
activation of caspase-3 and 7 (16). The first family of endogenous
cellular inhibitors of caspases to be identified in mammals was the
IAP family; these have been characterized over the past few years.
The diversity of triggers against which IAP suppresses apoptosis is
greater than that observed for any family of apoptosis inhibitors,
including the Bcl-2 family. The central mechanisms of IAP apoptotic
suppression are direct caspase and procaspase (caspases 3, 7 and 9)
inhibition. Omi/HtrA2 is a mammalian serine protease that resides
in the mitochondria of healthy cells. Omi/HtrA2 is the antagonist
of IAP and is a nuclear-encoded mitochondrial protein. Following
apoptotic stimulation, the signal sequence of Omi/HtrA2 in the
mitochondria is cleaved, resulting in new apoptotic activity of the
N-terminus. When Omi/HtrA2 is released into the cytoplasm, it binds
and antagonizes the BIR actions of IAPs and releases the caspases
binding to the BIR; thus, the caspases induce apoptosis (17). Omi/HtrA2 also promotes the
catalytic cleavage of IAPs leading to their irreversible
inactivation and the progression of apoptosis (8). In HUVECs, Omi/HtrA2 plays the same
pre-apoptotic role (11).
Inhibition of Omi/HtrA2 by a pharmacological approach provided
significant protection against caspase-3 activation (8–11).
Very little is known about the pathophysiological role of Omi/HtrA2
in ECs during shear stress.
In the present study, we revealed that laminar shear
stress significantly reduced the Omi/HtrA2 mRNA level from 4 to 6
h, and also decreased Omi/HtrA2 protein expression from 12 to 24 h.
Omi/HtrA2 normally resides in the mitochondria. Western blot
analysis and immunofluorescence results indicated that shear stress
inhibited Omi/HtrA2 release from the mitochondria to the cytoplasm.
Our results also demonstrated that in the static control group,
Omi/HtrA2 protein moved from the mitochondria to the cytoplasm due
to lack of bFGF and 2% FBS DMEM in the medium for 12 and 24 h. In
addition, the apoptotic signal led the HUVECs to express even more
Omi/HtrA2 in the absence of protection by shear stress. The
Omi/HtrA2 protein was mainly expressed in the mitochondria,
compared with in the cytoplasm following shear stress for 12 and 24
h. The results suggested that shear stress inhibited Omi/HtrA2
release from the mitochondria to the cytoplasm, and reduced the
expression of Omi/HtrA2.
Our findings suggest that inhibition of Omi/HtrA2
release into the cytoplasm is important for the protection of ECs
from apoptosis. In our previous study, we demonstrated that HIAP-2
and XIAP mRNA and protein are induced by laminar shear stress in
ECs and that IAPs play important roles in protecting ECs from
apoptosis (6,7). Since Omi/HtrA2 promotes apoptosis by
inhibiting IAPs, it is reasonable to assume that the decreased
expression of Omi/HtrA2 may play a role in ECs exposed to laminar
shear stress. Further investigation is required to determine the
direct effects of HtrA2/Omi on ECs exposed to laminar shear
stress.
In conclusion, the present study provides the first
evidence that physiological levels of laminar shear stress reduce
Omi/HtrA2 expression and release into the cytoplasm in HUVECs in
vitro. These data provide an explanation for the inhibitory
effect of shear stress on apoptosis. Further studies to define the
upstream and downstream molecular events involved in shear
stress-induced Omi/HtrA2 expression are required to deepen our
understanding of the mechanisms by which shear stress regulates
endothelial functions. Laminar shear stress-induced inhibition of
Omi/HtrA2 produced by ECs is likely to play an important role in
the formation and development of atherosclerosis.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation (30670512).
References
|
1
|
Dimmeler S, Haendeler J, Rippman V, Nehls
M and Zeiher AM: Shear stress inhibits apoptosis of human
endothelial cells. FEBS Lett. 399:71–74. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haga M, Chen A, Gortler D, Dardik A and
Sumpio BE: Shear stress and cyclic strain may suppress apoptosis in
endothelial cells by different pathways. Endothelium. 10:149–157.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ku DN, Giddens DP, Zarins CK and Glagov S:
Pulsatile flow and atherosclerosis in the human carotid
bifurcation: positive correlation between plaque location and low
oscillating shear stress. Arteriosclerosis. 5:293–302. 1985.
View Article : Google Scholar
|
|
4
|
Asakura T and Karino T: Flow patterns and
spatial distribution of atherosclerotic lesions in human coronary
arteries. Circ Res. 66:1045–1066. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaiser D, Freyberg MA and Friedl P: Lack
of hemodynamic forces triggers apoptosis in vascular endothelial
cells. Biochem Biophys Res Commun. 231:586–590. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin X, Mitsumata M, Yamane T and Yoshida
Y: Induction of human inhibitor of apoptosis protein-2 by shear
stress in endothelial cells. FEBS Letters. 529:286–292. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jin X, Shu Q, Ling GL and Wang HL:
X-linked inhibitor of apoptosis protein is regulated by laminar
shear stress in cultured endothelial cells. Chin J Pharmacol
Toxicol. 17:328–332. 2003.
|
|
8
|
Suzuki Y, Imai Y, Nakayama H, Takahashi K,
Takio K and Takahashi R: A serine protease, HtrA2, is released from
the mitochondria and interacts with XIAP, inducing cell death. Mol
Cell. 8:613–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soustiel JF and Larisch S: Mitochondrial
damage: A target for new therapeutic horizons. Neurotherapeutics.
7:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pruefer FG, Lizarraga F, Maldonado V and
Melendez-Zajgla J: Participation of Omi/Htra2 serine-protease
activity in the apoptosis induced by cisplatin on SW480 colon
cancer cells. J Chemother. 20:348–354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu QB, Liu LL, Lu YM, Tao RR, Huang JY,
Han F and Lou YJ: The induction of reactive oxygen species and loss
of mitochondrial Omi/HtrA2 is associated with
S-nitrosoglutathione-induced apoptosis in human endothelial cells.
Toxicol Appl Pharmacol. 244:374–384. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Kim DS, Park MJ, Cho HJ, Zervos AS,
Bonventre JV and Park KM: Omi/HtrA2 protease is associated with
tubular cell apoptosis and fibrosis induced by unilateral ureteral
obstruction. Am J Physiol Renal Physiol. 298:F1332–F1340. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tricot O, Mallat Z, Heymes C, Belmin J,
Leseche G and Tedgui A: Relation between endothelial cell apoptosis
and blood flow direction in human atherosclerotic plaques.
Circulation. 101:2450–2453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartling B, Tostlebe H, Darmer D, Holtz J,
Silber RE and Morawietz H: Shear stress dependent expression of
apoptosis regulating genes in endothelial cells. Biochem Biophys
Res Commun. 278:740–746. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dimmeler S, Assmus B, Hermann C, Haendeler
J and Zeiher AM: Fluid shear stress stimulates phosphorylation of
Akt in human endothelial cells: involvement in suppression of
apoptosis. Circ Res. 83:334–341. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome C and dATP dependent formation of Apaf-1/ caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hegde R, Srinivasula SM, Zhang Z, et al:
Identification of Omi/ HtrA2 as a mitochondrial apoptotic serine
protease that disrupts inhibitor of apoptosis protein-caspase
interaction. J Biol Chem. 277:432–438. 2002. View Article : Google Scholar : PubMed/NCBI
|