Introduction
Protein tyrosine phosphatase 1B (PTP1B) is the main
negative regulator of the insulin signaling pathway that has
received significant attention over the last decade. This enzyme is
widely expressed in insulin-sensitive tissues (1). PTP1B binds to the insulin receptor
and efficiently dephosphorylates it in vitro. In vivo and
in vitro studies have shown that changes in the expression
of PTP1B are able to induce or prevent insulin resistance in
muscle, adipose and liver tissues (2–6).
Particularly, obese insulin-resistant and diabetic patients have
demonstrated a higher expression and activity of PTP1B in their
muscle and adipose tissues (7).
The increased expression of PTP1B has also been reported in
insulin-sensitive tissues from various animal models, including
ob/ob mice, animals fed on high fat and fructose diets and
streptozotocin (STZ)-induced diabetic mice or rats (6,8,9).
Furthermore, in vitro studies have also shown that fatty
acids, particularly palmitate, high glucose and cytokines,
including tumor necrosis factor (TNF)-α and interleukin (IL)-6, are
some of the factors that induce the expression of PTP1B in various
tissues (10–13). Further studies using gene-targeting
approaches have provided new insight into the role of PTP1B in the
insulin signaling pathway. The tissue-specific overexpression of
PTP1B in the liver and muscle has been shown to lead to a defect in
insulin signaling, resulting in systemic insulin resistance in mice
(14,15). In addition, genetic variations of
the PTP1B gene have been reported to be associated with
hypertension, insulin resistance and type 2 diabetes in various
populations (16–18).
Given the effect of PTP1B overexpression on the
insulin signaling pathway, a number of attempts have been directed
toward reducing PTP1B expression levels in liver, muscle and
adipose tissues. PTP1B knockout mice indicated an increased
phosphorylation of the insulin receptor in the liver and muscle
tissues following insulin injection (3,4).
Notably, PTP1B-deficient mice are resistant to weight gain, exhibit
increased insulin sensitivity and remain insulin-sensitive when
subjected to a high-fat diet (3).
Also, liver-specific PTP1B knockout mice have demonstrated improved
insulin sensitivity and decreased plasma glucose and insulin levels
(19). In addition,
PTP1B-deficient myocytes have demonstrated increased insulin
sensitivity and protection against TNF-α-induced insulin resistance
(5). The results from these
studies suggest that decreasing the expression and activity of
PTP1B is potentially a therapeutic approach for treating
individuals with metabolic syndrome and type 2 diabetes.
A variety of strategies, including antisense
oligonucleotides, chemical inhibitors and RNA interference (RNAi),
have been used to inhibit PTP1B expression and activity in in
vitro and in vivo studies. The decreased expression of
PTP1B achieved using antisense oligonucleotides has been shown to
lead to increased insulin receptor tyrosine phosphorylation and
insulin sensitivity in monkeys (20). A number of research groups have
also attempted to identify specific inhibitors capable of
decreasing PTP1B activity in vitro(21,22).
However, given the similarities among the phosphatases, these
studies have not been successful in providing a specific PTP1B
inhibitor for in vivo investigation. RNAi has been
introduced as a promising approach for the treatment of certain
metabolic diseases. A certain study revealed that the hydrodynamic
injection of PTP1B-small interfering RNA (siRNA) led to a decrease
in the plasma glucose levels of diabetic mice (23). However, due to the short-term
effects of synthetic siRNA, serious challenges have been
encountered in in vivo studies. Short hairpin RNA (shRNA) is
a member of the RNAi family and, when its sequence is inserted into
bacterial or viral plasmids, it enables the shRNA to be expressed
more potently. In this study, we used PTP1B-shRNA to knockdown
PTP1B expression in the liver of diabetic mice using a hydrodynamic
tail vein injection technique. Although this procedure is not
applicable to humans, it is useful in primary studies where the
liver is the target tissue. To our knowledge, no previous studies
have explored the behavior of shRNA specific for the knockdown of
PTP1B in the liver of diabetic mice. Therefore, the aims of this
study were to generate liver-specific PTP1B knockout mice using
PTP1B-shRNA and to investigate the effects of the PTP1B knockdown
on plasma glucose and lipid levels in STZ-induced diabetic
mice.
Materials and methods
Plasmids
The pGL3-promoter plasmid containing firefly
luciferase and the pRS plasmid containing PTP1B-shRNA were
purchased from OriGene Technologies, Inc. (Rockville, MD, USA). The
PTP1B shRNA sequence (AATTGCACC AGGAAGATAATGACTATATC) was selected
based on our previous study (24).
A scrambled sequence shRNA-vector was also used as the control.
Following transfection of the 2 plasmids into E. coli, they
were cultured in LB overnight and then the plasmids were extracted
using the Maxi kit of Qiagen (Valencia, CA, USA). The purity of the
plasmid preparations was determined by 1% agarose gel
electrophoresis. The assay kit for determining luciferase activity
was purchased from Promega (Madison, WI, USA).
Animal care and treatment
A total of 18 male NMRI mice weighing 15–18 g
(Pasteur Institute, Tehran, Iran) were housed in individual cages
and maintained under controlled conditions of temperature (24±3°C)
and light (12-h light/dark cycle) and fed a standard rodent chow
and water ad libitum. The mice were divided into 2 groups
and received 10 or 20 μg plasmid DNA (pGL3-Luc) via the
hydrodynamic tail vein injection method. Each group was then
separately divided into 3 groups for the measurement of luciferase
activity at different intervals (8, 16 and 24 h). The animals were
sacrificed following ketamine-xylazine anesthesia. After collecting
the whole-body blood, liver, lung, heart, kidney, muscle, intestine
and adipose tissue were dissected and frozen in liquid nitrogen.
The tissues were stored at -80°C for further analysis.
Luciferase studies
The luciferase activity of the pGL3-promoter plasmid
was assessed in protein tissue extracts using a luminometer. Cold
tissue lysis buffer (1 ml; 0.1 M Tris-HCl, 2 mM EDTA, 0.1% Triton
X-100, 0.1 mM phenylmethylsulfonyl fluoride and 1X protease
inhibitor cocktail, pH 7.8) was added to 30 mg of each tissue,
which was then homogenized and centrifuged in a microcentrifuge for
10 min at 13000 × g at 4°C. The protein concentration of the
supernatant was determined using the Bradford method. The
supernatant of the homogenate (10 μl) was mixed with 100 ml
luciferase assay reagent and the luciferase activity was measured
using a luminometer. The firefly luciferase activity was normalized
to the total amount of protein.
Hydrodynamic injection of PTP1B-shRNA
into diabetic mice
A total of 30 male NMRI mice weighing 15–18 g were
divided into 2 groups and allocated to separate cages. The first
group, the non-diabetic control (n=10), received Na-citrate buffer
and the second group (n=20) was injected with 50 mg/kg STZ (Sigma,
St. Louis, MO, USA) for 5 consecutive days based on a low-dose STZ
induction protocol. Body weight and glucose levels were assessed
during the study for 5 weeks prior to the hydrodynamic injection.
Plasma glucose concentrations were measured using a glucometer
(Accu-Check Active; Roche Diagnostic Corporation, Mannheim,
Germany) after fasting for 6 h. After 4–5 weeks, those mice with
blood glucose levels >200 mg/dl were selected and divided into 2
groups, the diabetic control and diabetic groups. The diabetic
control mice were injected with the scrambled shRNA sequence, while
the diabetic group mice were injected with 20 μg plasmid pRS
(PTP1B-shRNA) via hydrodynamic tail vein injection. The plasma
glucose concentration was monitored during the week following the
injection. To investigate the insulin signaling pathway in the
liver of the animals, 1 unit of insulin was injected intravenously
into some animals of all groups. Various tissues of the diabetic
mice, including liver, lung, heart, kidney, muscle and adipose
tissue, were dissected after collecting the whole-body blood.
Non-diabetic control mice (n=10) also received an injection of the
PTP1B-shRNA and the scrambled shRNA sequence. All procedures and
protocols were performed in accordance with the institutional
guidelines for animal care which were approved by the ethics
committee of Tehran University of Medical Sciences.
Gene expression analysis
Total RNA was extracted from certain tissues,
including liver, lung, heart, kidney, muscle and adipose tissues,
from all groups (non-diabetic, diabetic, diabetic receiving the
scrambled shRNA and diabetic receiving PTP1B-shRNA) using QIAzol.
Total RNA (1 μg) was reverse transcribed using Qiagen reverse
transcriptase and random hexamer primer. Real-time PCR was
performed on a RotorGene 3000 instrument (Corbett Research, Sydney,
Australia). PTP1B expression levels were assessed by a QuantiTect
primer assay for PTP1B using QuantiFast SYBR-Green PCR master mix.
The data were normalized against the β-actin transcript level. The
amplification protocol for 40 cycles was as follows: 5 min at 95°C
for initial activation, 10 sec at 95°C for denaturation and 30 sec
at 60°C for annealing/extension.
Western blot analysis
A tissue lysate was prepared by homogenization in
modified RIPA buffer (50 mM Tris-HCl pH 7.4, 1% Triton X-100, 0.2%
sodium deoxycholate, 0.2% SDS, 1 mM Na-EDTA and 1 mM PMSF)
supplemented with protease inhibitor cocktail (Roche, Mannheim,
Germany). For detection of phosphoprotein, a buffer consisting of
50 mM HEPES pH 7.5, 150 mM NaCl, 100 mM NaF, 10 mM EDTA, 10 mM
Na4P2O7, 2 mM NaVO4 and
protease inhibitor cocktail was used. The protein concentration was
determined using the Bradford method. The total protein (20–30 mg)
was fractionated by SDS-PAGE and the gel was transferred onto a
PVDF membrane (Millipore, Schwalbach, Germany). The membrane was
blocked overnight in blocking buffer (5% skimmed milk in TBST
buffer) and then incubated for 1 h with primary antibodies diluted
in TBST containing 1% BSA. The primary antibodies used were as
follows: Akt and phospho-Akt (Ser473) (Cell Signaling Technology,
Beverly, MA, USA) and β-actin (Abcam, Cambridge, MA, USA). The
membrane was then incubated with secondary antibody conjugated to
HRP (Santa Cruz Biotechnology, Santa Cruz, USA) for 1 h and
detection was performed using ECL reagents (Amersham Pharmacia
Corp., Piscataway, NJ, USA). The films were scanned and protein
bands were quantified using Scion Image software. Each experiment
was performed at least 3 times.
Measurements of clinical biochemistry
parameters
The concentrations of aspartate transaminase (AST),
alanine transaminase (ALT), lactate dehydrogenase (LDH), uric acid,
albumin and total protein were measured using commercial kits to
analyze the serum of diabetic mice receiving either the scrambled
or PTP1B-shRNA, and in that of the normal mice that did not receive
any injection, 24 h post-injection. The levels of triglyceride,
LDL, cholesterol and HDL were measured 1 week after hydrodynamic
injection.
Statistical analysis
Data are expressed as the means ± SE and were
analyzed using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA). One-way
ANOVA was used for statistical analysis to determine differences
between groups. The independent t-test was performed when a
statistical significance was found. A p-value <0.05 was
considered to indicate a statistically significant result.
Results
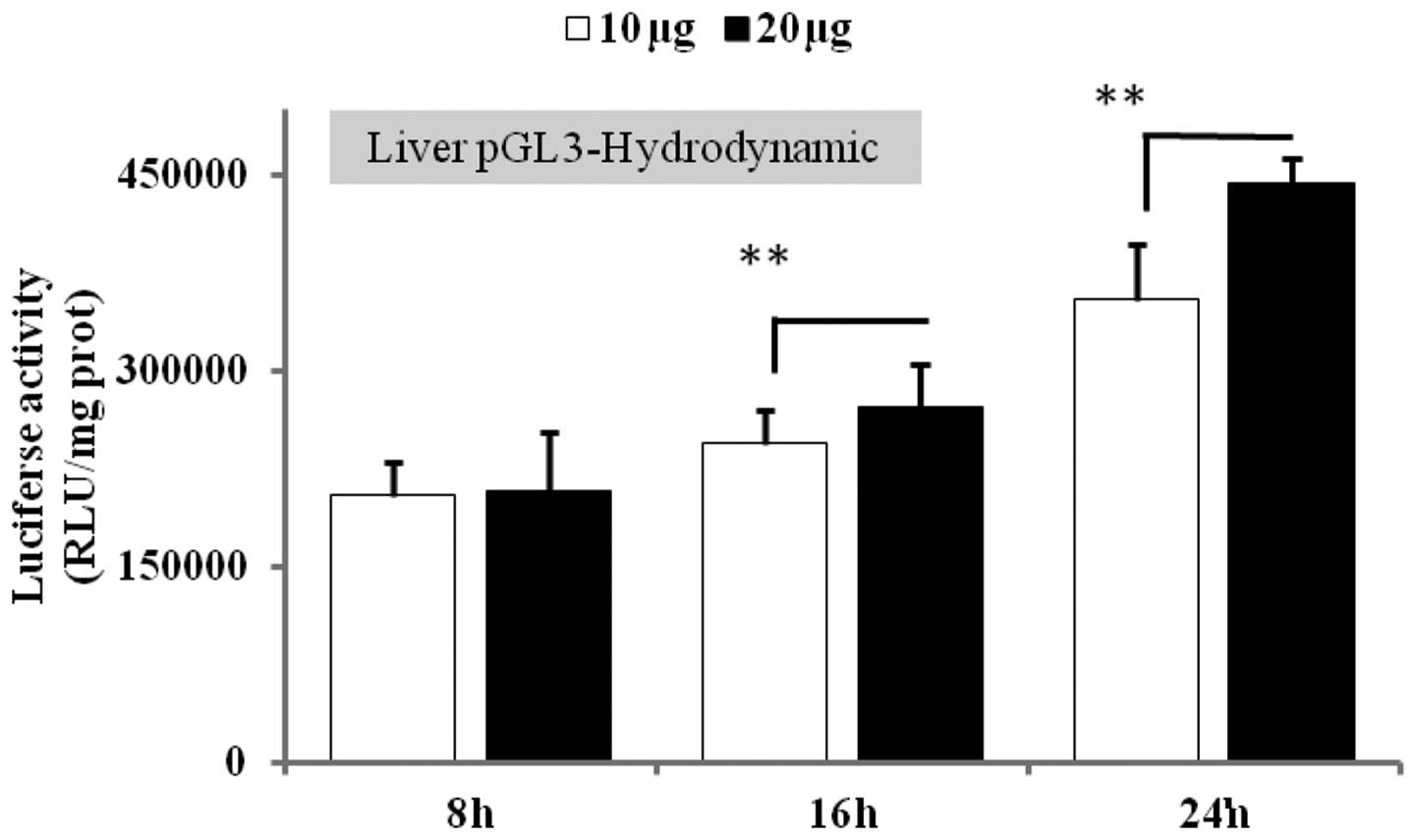
Optimization of the dose and time of in
vivo gene expression following hydrodynamic injection
To validate the hydrodynamic-based procedure as an
appropriate method for the specific delivery of the gene of
interest into the liver, we first set up experiments to determine a
suitable dose and time of luciferase gene expression in the liver.
Luciferase gene activity was measured in various tissues 8, 16 and
24 h post-injection of the plasmid at doses of 10 and 20 μg. The
results revealed no significant difference between the 2 doses 8 h
after injection, but, at 16 and 24 h post-injection, an efficient
and specific luciferase activity was observed in the liver (data
not shown). However, 20 μg plasmid after 24 h had greater
luciferase activity (19.5%, p<0.001) than 10 μg at 24 h
(Fig. 1). According to these data,
we selected 20 μg plasmid at 24 h to study the effect of PTP1B
knockdown on gene expression analysis.
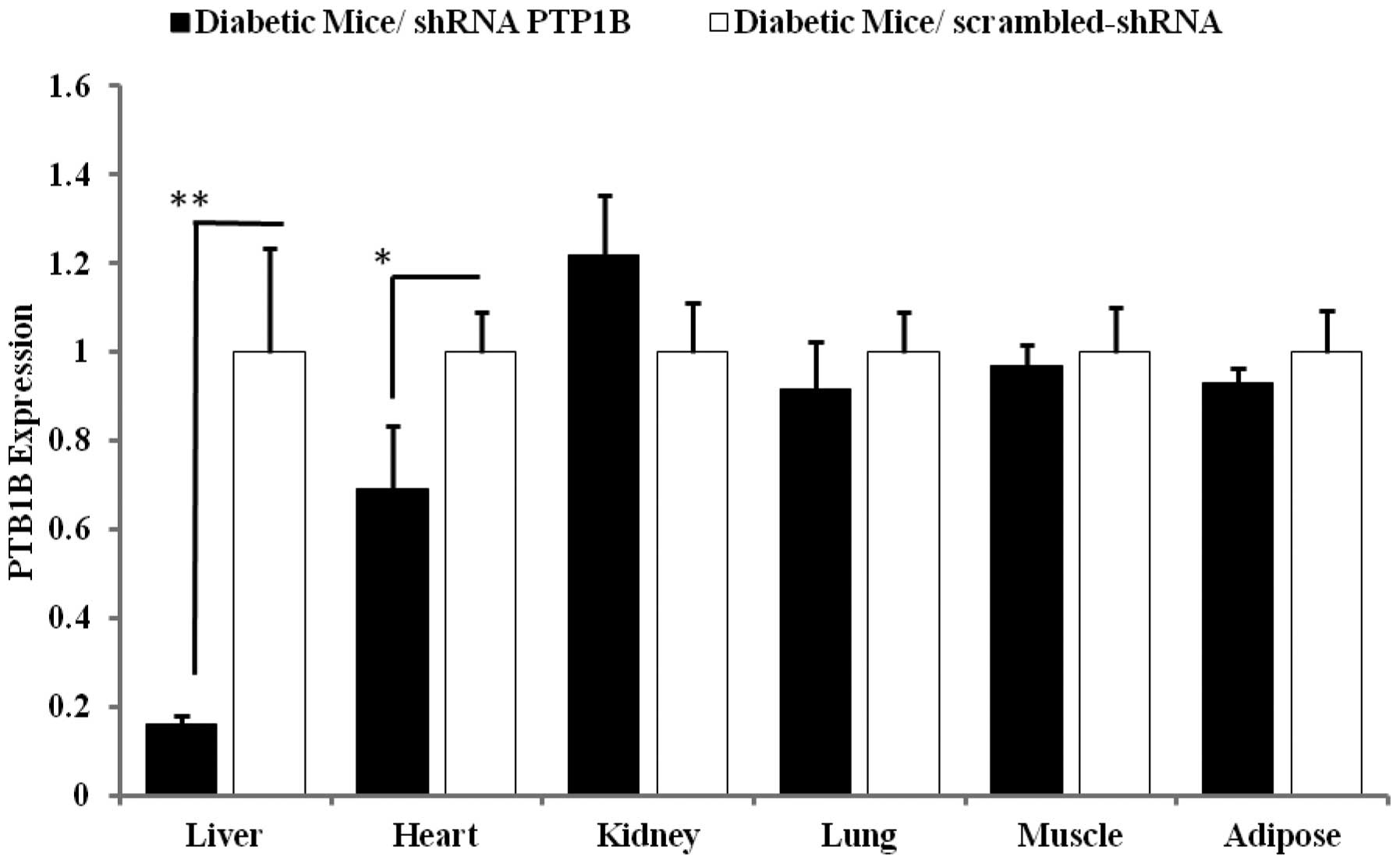
Liver-specific knockdown of PTP1B by
shRNA in diabetic mice
To confirm that the hydrodynamic tail vein injection
of 20 μg PTP1B-shRNA led to a specific decrease of PTP1B expression
in the liver, the mRNA levels of PTP1B were quantified in various
tissues. As shown in Fig. 2,
PTP1B-shRNA injection significantly reduced PTP1B expression in the
liver of the mice by up to 84% (p<0.0001) compared with the
control group receiving the scrambled shRNA. No significant
differences were identified in the levels of PTP1B expressed in the
muscle, adipose, lung and kidney tissues of the mice compared with
the controls, whereas the heart exhibited a 30% reduction of PTP1B
expression following PTP1B-shRNA injection. These findings
demonstrate that PTP1B-shRNA is able to decrease PTP1B expression
somewhat specifically in the liver.
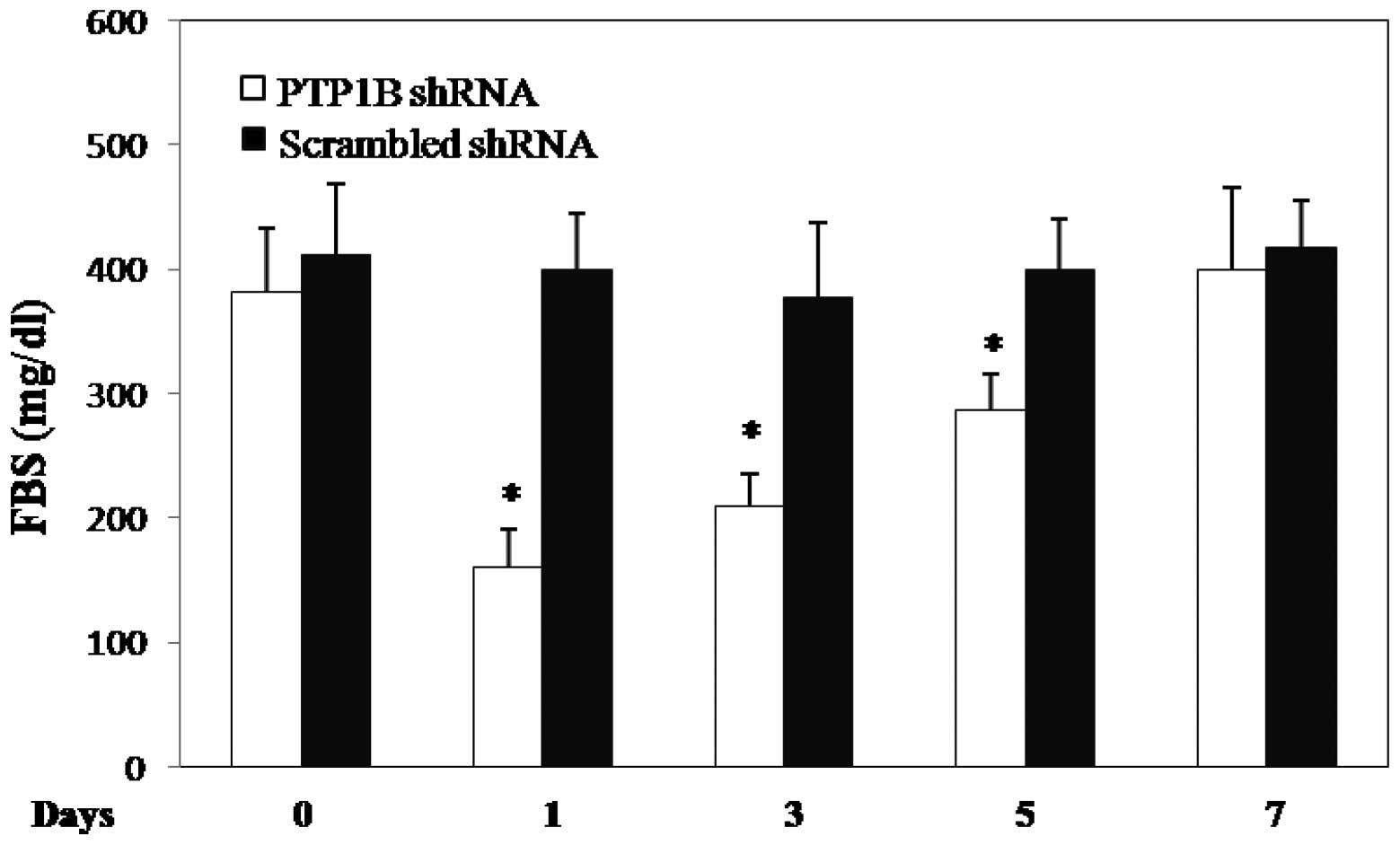
Effects of liver-specific knockdown on
glucose and lipid parameters
We then aimed to evaluate whether hepatic PTP1B
inhibition could be an efficient and specific strategy to reduce
hyperglycemia in diabetic mice. All diabetic mice with plasma
glucose levels >200 mg/dl received either 20 μg pRS
(PTP1B-shRNA) plasmid or the scrambled shRNA vector. At 1 day
post-injection, the plasma glucose concentration markedly decreased
by up to 58% (382±51 vs. 160.8±31 mg/dl, n=6, p<0.0001; Fig. 3) in diabetic mice, while no
significant changes were observed in the mice receiving the
scrambled shRNA vector. On days 3 (45%) and 5 (25%) the plasma
glucose levels remained significantly lower than on the day of
injection of PTP1B-shRNA; however, there was no significant
difference in the plasma glucose levels 1 week after PTP1B-shRNA
injection. These findings demonstrate a significant effect of PTP1B
inhibition on the plasma glucose levels for a few days in diabetic
mice. No significant difference in the lipid profile (triglyceride,
cholesterol, HDL and LDL) was observed between the diabetic and
non-diabetic mice (data not shown). An injection of PTP1B-shRNA or
the scrambled shRNA did not change the lipid levels of the diabetic
mice.
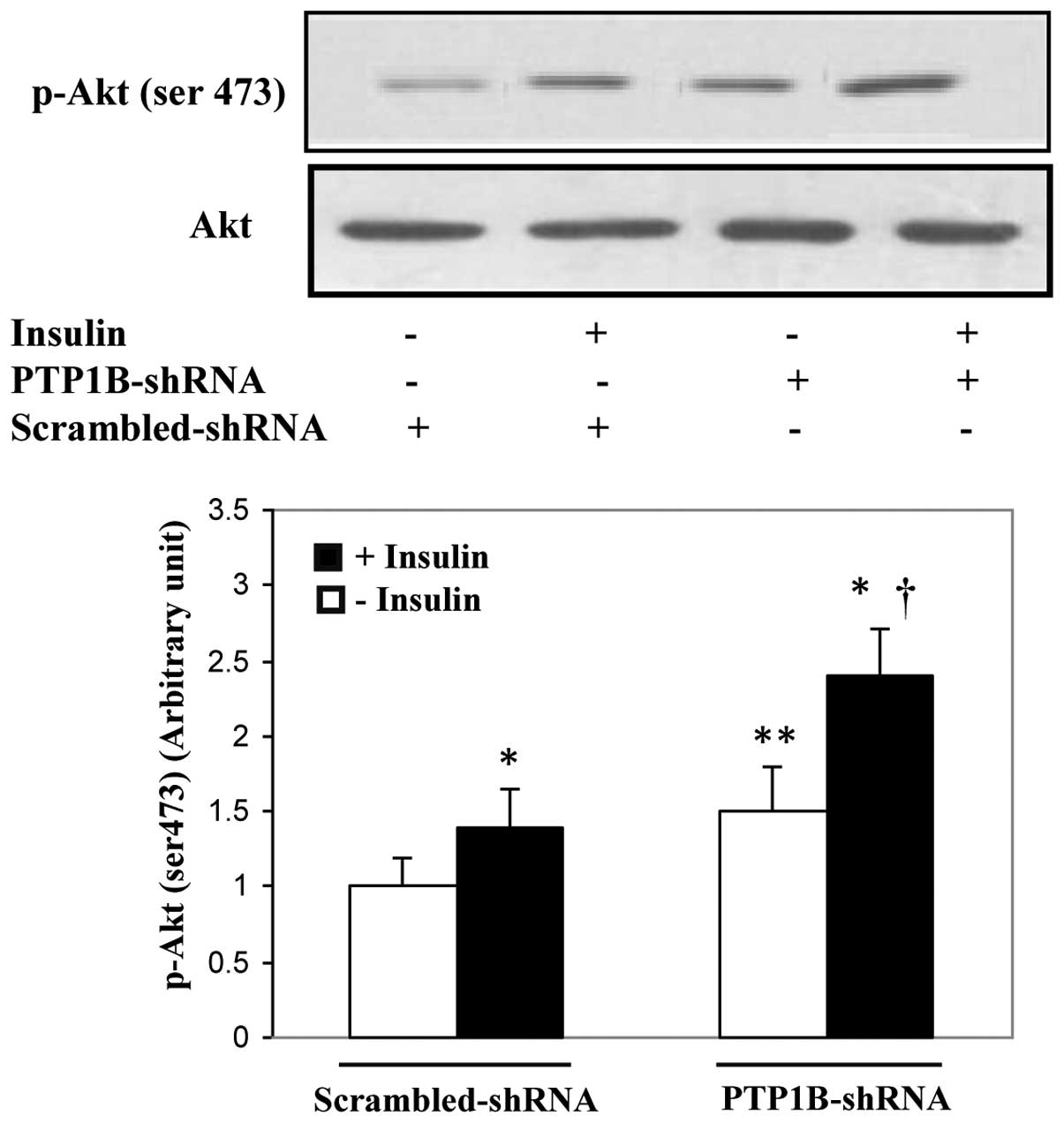
Enhancement of hepatic insulin signaling
in mice receiving PTP1B-shRNA
To investigate whether the decreased plasma glucose
levels in diabetic mice receiving PTP1B-shRNA were at least partly
due to the enhancement of insulin signaling activity, the
phosphorylation of the key element of this pathway (Akt, Ser473)
was studied in the liver. As shown in Fig. 4, the results demonstrated that
insulin induced the phosphorylation of Akt in the PTP1B-shRNA- and
scrambled-shRNA-treated mice by 70 and 50%, respectively
(p<0.001). In addition, mice receiving PTP1B-shRNA in the basal
and insulin-stimulated states had higher Akt phosphorylation levels
in the liver cells than the corresponding mice that were injected
with the scrambled sequence (35 and 60%, respectively;
p<0.01).These findings suggest that PTP1B inhibition in the
liver results in insulin sensitivity leading to decreased plasma
glucose levels in diabetic mice.
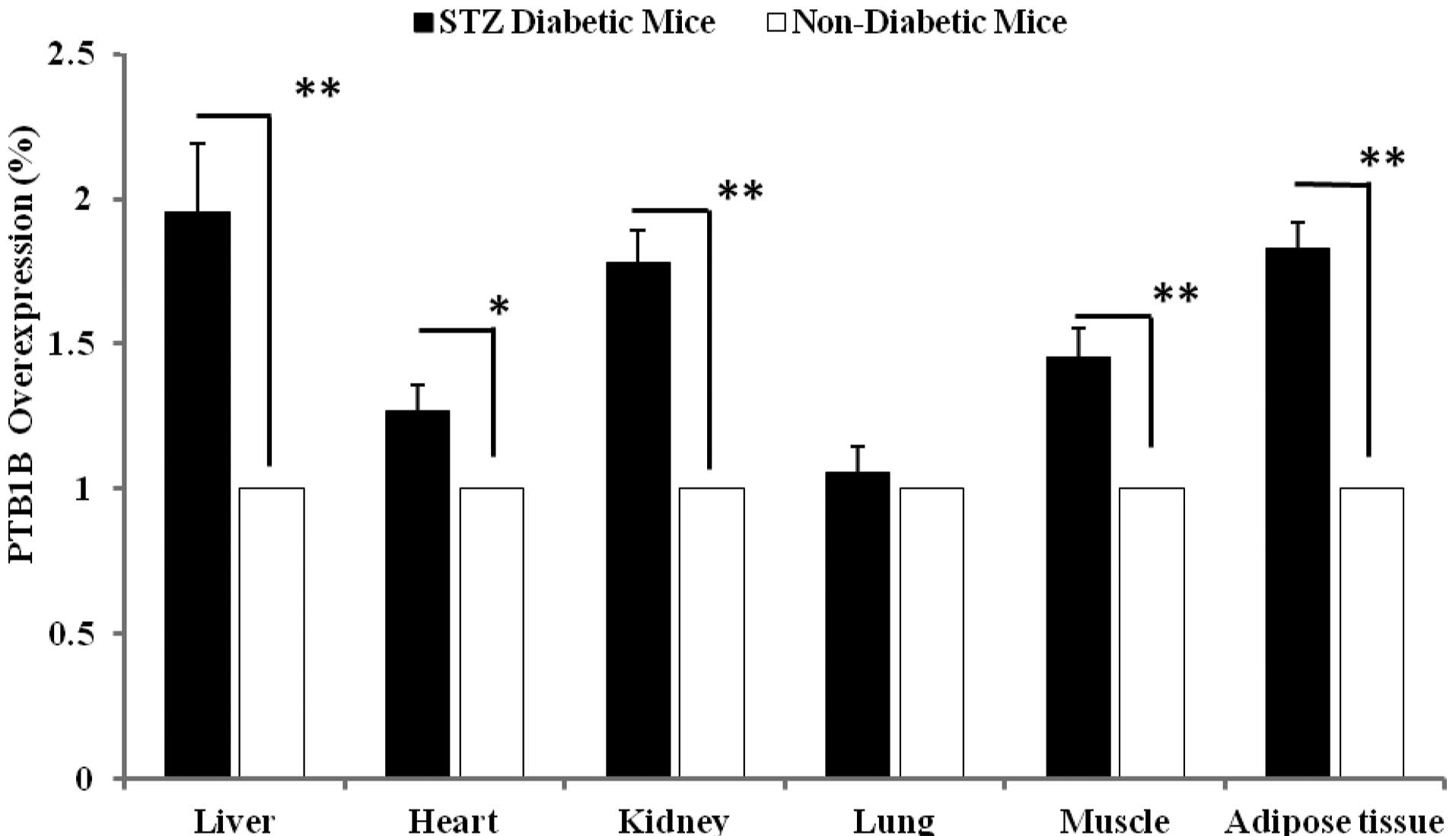
Effect of diabetes on PTP1B expression in
various tissues
We assessed the PTP1B expression levels in various
tissues of the healthy and diabetic mice. No injection was
administered to these animals. As shown in Fig. 5, 3
insulin-sensitive tissues (liver, muscle and adipose) significantly
demonstrated the elevated levels of PTP1B expression in the
diabetic mice compared with those of the healthy group. The PTP1B
gene demonstrated a 95% (p<0.001), 45% (p<0.001) and 82.0%
(p<0.001) overexpression in liver, muscle and adipose,
respectively. These data also demonstrate the overexpression of
PTP1B in the kidney (78%, p<0.001) and heart (26.6%, p<0.01)
in the diabetic mice compared with the controls.
Metabolic profile in diabetic and healthy
mice
To investigate the effect of the hydrodynamic tail
vein injection on liver function, several parameters were assessed.
The concentrations of hepatic enzymes, albumin, uric acid and total
protein were measured in the serum of mice receiving either the
scrambled or PTP1B-shRNA and in that of the normal mice that did
not receive any injection (data not shown). The levels of ALT and
AST were ~10-fold (p<0.001) higher than those in normal mice
following hydrodynamic injection. In the case of LDH, a marked
increase of 172-fold (p<0.001) was observed at 24 h
post-injection compared with the normal mice. The concentrations of
albumin and total protein were reduced by 1.2- and 1.5-fold
(p<0.001), respectively, compared with those in normal animals.
These altered parameters tended to return to normal levels after 1
week (25).
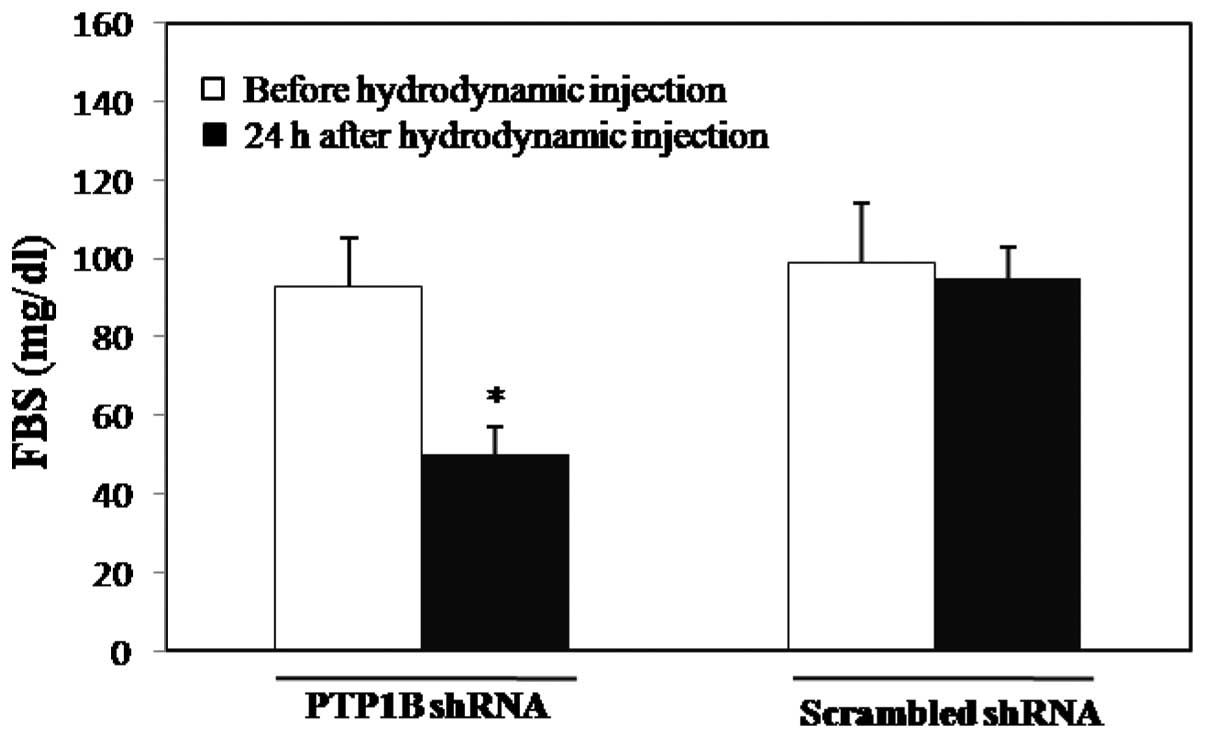
Liver-specific knockdown of PTP1B by
shRNA in healthy mice
We revealed that the PTP1B gene was inhibited by
PTP1B-shRNA, even in healthy animals, 24 h post-hydrodynamic tail
vein injection. In particular, the plasma glucose level was
decreased by 46% in the 6-h fasted mice (93.5±8 vs. 50.5± 9 mg/dl,
n=6, p<0.001; Fig. 6). These
animals remained healthy and no hypoglycemic symptoms were observed
until 24 h post-injection.
Discussion
There is increasing evidence supporting the fact
that type 2 diabetes is now a serious health problem all over the
world. During the last decade, a number of cellular and molecular
aspects of the diseases have been recognized, and these have aided
researchers in the search for therapeutic targets for the treatment
of diabetes. Evidence from human and animal studies support PTP1B
as a new candidate in the field of diabetes. The increased
expression of PTP1B in various tissues of diabetic patients is a
major concern, and strategies based on the inhibition of PTP1B may
have a beneficial effect on diabetes-associated complications.
Numerous efforts have been made to identify specific chemical
inhibitors of PTP1B for use in diabetes treatment. However, they
face many difficulties as PTP1B resembles other phosphatases,
including leukocyte antigen-related (LAR), SH2-containing inositol
5-phosphatase 2 (SHIP2) and T-cell protein tyrosine phosphatase or
PTN2 (TC-PTP). The use of oligonucleotides is another strategy
under investigation and a few studies have used antisense
oligonucleotides and siRNA against PTP1B to investigate different
aspects of the disease in animal models (20, 23). However, none of the studies have
used shRNA against PTP1B and the present study is the first to
apply PTP1B-shRNA in the treatment of diabetes. shRNA is superior
to siRNA since it may be expressed more stably.
The liver is a vital organ in vertebrates that plays
a central role in coordinating the whole body metabolism, including
carbohydrate, lipid and protein metabolism, xenobiotic metabolism
and detoxification (26). PTP1B
overexpression in liver has been reported to be significant in the
development of whole body insulin resistance in various animal
models (14). Therefore,
inhibition of PTP1B expression appears to have a beneficial effect
on insulin resistance and diabetes. In this study we generated
liver-specific PTP1B knockdown mice using shRNA by a hydrodynamic
tail vein injection procedure. This method has been previously
shown to deliver transgenes (pCMV-Luc, pGL3 and pSHAG-664)
specifically into the liver (23,27).
We first attempted to validate this procedure in our study by
luciferase gene delivery. Our results confirm that the hydrodynamic
tail vein injection is a suitable method for delivering the gene of
interest specifically into the liver. According to the results of
our validation assay, we selected 20 μg plasmid (pRS-PTP1B-shRNA)
for the following steps of the study. The injection of 20 μg
plasmid (pRS-PTP1B-shRNA) via the tail vein led to a marked
decrease in plasma glucose levels in the diabetic and non-diabetic
mice, reduced the expression of PTP1B specifically in the liver and
increased the sensitivity of the insulin signaling pathway in the
liver.
The glucose lowering effect of PTP1B inhibition in
the liver is the main finding of this study. Notably, a single
injection of 20 μg PTP1B-shRNA plasmid resulted in a 58% decrease
in plasma glucose levels after 24 h. Although the plasma glucose
levels tended to gradually increase on days 3 and 5, the animals
that received PTP1B-shRNA had significantly lower glucose levels
than those that received scrambled-shRNA on those days. The same
results were also observed in non-diabetic mice. These results are
in the line with previous reports that PTP1B inhibition leads to a
reduction in plasma glucose levels in animal models (3,5,20,23).
For example, PTP1B knockout mice are not only insulin-sensitive but
also maintain euglycemia in the fed state (3). In another study, the hydrodynamic
injection of PTP1B-siRNA led to a decrease in the plasma glucose
levels of diabetic mice only for 24 h, whereas, in this study,
PTP1B-shRNA reduced glucose levels for 5 days (23). The short-term effect of PTP1B-siRNA
on the glucose levels of diabetic mice may be attributed to the
nature of the siRNA. Although siRNA delivery to the cytosol should
be easier to achieve compared with the delivery of shRNA, which
must enter the nucleus and undergo transcription, the shRNA has
been established as an improved substrate for dicer and exhibits
improved RISC loading. It has been previously demonstrated that
plasmid-based shRNA is much more potent than siRNA (28). It has also been suggested that a
significant amount of siRNA is lost to premature metabolism
following hydrodynamic administration (29). It is noteworthy that PTP1B
antisense oligonucleotides have been investigated for ability to
normalize blood glucose levels in phase II clinical trials
(30).
To confirm that the decreased plasma glucose levels
in diabetic mice are due to the inhibition of PTP1B expression in
the liver, expression analysis was performed. The data demonstrated
that among the insulin-sensitive tissues (liver, muscle and
adipose), the liver was the only one that had a reduction in the
expression of PTP1B. An investigation of the phosphorylation of Akt
also provided further evidence on the role of PTP1B in the insulin
signaling pathway. The results demonstrated that in the liver,
PTP1B has a major effect on glucose homeostasis and its reduced
expression led to insulin sensitivity in liver resulting in
decreased plasma glucose levels in the mice. However, in the
present study, we did not observe a difference in the lipid profile
(triglyceride, cholesterol, LDL and HDL cholesterol) between the
diabetic and healthy mice. The lack of a change in the lipid
profile may be due to the method of diabetes induction (low-dose
STZ) in this study. Corresponding results were also reported in
another study in which low-dose STZ injection was used to induce
diabetes in mice (31).
In this study, we also investigated the expression
of PTP1B in various tissues of the diabetic mice. In addition to
increased expression levels of PTP1B in the liver, muscle and
adipose tissues, the observation of enhanced expression of PTP1B in
the heart and kidney is unique and it is the first time that such
results have been reported. In addition, with respect to the
hydrodynamic procedure (1) we
assessed the levels of hepatic enzymes and certain other proteins
in the diabetic animals. According to the data, the liver showed
symptoms of primary damage due to the high pressure of the
hydrodynamic injection which is understandable from the high levels
of ALT, AST and LDH. However, these abnormal factors appeared to
reverse with time and the levels of the parameters returned to
normal values after a few days.
In conclusion, the data from this study provide
evidence that PTP1B inhibition by PTP1B-shRNA administration may be
a promising approach for lowering plasma glucose levels in diabetic
patients. The study used hydrodynamic tail vein injection but the
application of this method to humans is not possible. Therefore,
the targeting of the PTP1B-shRNA to the liver using non-viral
carriers may be a subject for investigation in further studies.
Abbreviations:
|
PTP1B
|
protein tyrosine phosphatase-1B
|
|
RNAi
|
RNA interference
|
|
shRNA
|
short hairpin RNA
|
|
STZ
|
streptozotocin
|
References
|
1
|
Goldstein BJ: Regulation of insulin
receptor signaling by protein-tyrosine dephosphorylation. Receptor.
3:1–15. 1993.PubMed/NCBI
|
|
2
|
Zabolotny JM, Haj FG, Kim YB, Kim HJ, et
al: Transgenic overexpression of protein-tyrosine phosphatase 1B in
muscle causes insulin resistance, but overexpression with leukocyte
antigen-related phosphatase does not additively impair insulin
action. J Biol Chem. 279:24844–24851. 2004. View Article : Google Scholar
|
|
3
|
Elchebly M, Payette P, Michaliszyn E, et
al: Increased insulin sensitivity and obesity resistance in mice
lacking the protein tyrosine phosphatase-1B gene. Science.
283:1544–1548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klaman LD, Boss O, Peroni OD, et al:
Increased energy expenditure, decreased adiposity, and
tissue-specific insulin sensitivity in protein-tyrosine phosphatase
1B-deficient mice. Mol Cell Biol. 20:5479–5489. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nieto-Vazquez I, Fernández-Veledo S, de
Alvaro C, Rondinone CM, Valverde AM and Lorenzo M: Protein-tyrosine
phosphatase 1B-deficient myocytes show increased insulin
sensitivity and protection against tumor necrosis
factor-alpha-induced insulin resistance. Diabetes. 56:404–413.
2007. View Article : Google Scholar
|
|
6
|
Dadke SS, Li HC, Kusari AB, Begum N and
Kusari J: Elevated expression and activity of protein-tyrosine
phosphatase 1B in skeletal muscle of insulin-resistant type II
diabetic Goto-Kakizaki rats. Biochem Biophys Res Commun.
274:583–589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmad F, Azevedo JL, Cortright R, Dohm GL
and Goldstein BJ: Alterations in skeletal muscle protein-tyrosine
phosphatase activity and expression in insulin-resistant human
obesity and diabetes. J Clin Invest. 100:449–458. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y, Ouyang JP, Wu K, Wang SS, Wen CY and
Xia ZY: Rosiglitazone ameliorates abnormal expression and activity
of protein tyrosine phosphatase 1B in the skeletal muscle of
fat-fed, streptozotocin-treated diabetic rats. Br J Pharmacol.
146:234–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adeli K, Macri J, Mohammadi A, Kito M,
Urade R and Cavallo D: Apolipoprotein B is intracellularly
associated with an ER-60 protease homologue in HepG2 cells. J Biol
Chem. 272:22489–22494. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zabolotny JM, Kim YB, Welsh LA, Kershaw
EE, Neel BG and Kahn BB: Protein-tyrosine phosphatase 1B expression
is induced by inflammation in vivo. J Biol Chem. 283:14230–14241.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Parvaneh L, Meshkani R, Bakhtiyari S, et
al: Palmitate and inflammatory state additively induce the
expression of PTP1B in muscle cells. Biochem Biophys Res Commun.
396:467–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
MohammadTaghvaei N, Meshkani R, Taghikhani
M, Larijani B and Adeli K: Palmitate enhances protein tyrosine
phosphatase 1B (PTP1B) gene expression at transcriptional level in
C2C12 skeletal muscle cells. Inflammation. 34:43–48. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Yang LJ and Zou DJ: Rosiglitazone
attenuates tumor necrosis factor-α-induced protein-tyrosine
phosphatase-1B production in HepG2 cells. J Endocrinol Invest.
35:28–34. 2012.
|
|
14
|
Haj FG, Zabolotny JM, Kim YB, Kahn BB and
Neel BG: Liver-specific protein-tyrosine phosphatase 1B (PTP1B)
re-expression alters glucose homeostasis of PTP1B−/−
mice. J Biol Chem. 280:15038–15046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delibegovic M, Bence KK, Mody N, et al:
Improved glucose homeostasis in mice with muscle-specific deletion
of protein-tyrosine phosphatase 1B. Mol Cell Biol. 27:7727–7734.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu P, Jiang W, Du H, Shao J, Lu B, Wang J
and Zou D: Protein tyrosine phosphatase 1B gene polymorphisms and
essential hypertension: a case-control study in Chinese population.
J Endocrinol Invest. 33:483–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meshkani R, Taghikhani M, Al-Kateb H,
Larijani B, et al: Polymorphisms within the protein tyrosine
phosphatase 1B (PTPN1) gene promoter: functional characterization
and association with type 2 diabetes and related metabolic traits.
Clin Chem. 53:1585–1592. 2007. View Article : Google Scholar
|
|
18
|
Meshkani R, Taghikhani M, Mosapour A, et
al: 1484insG polymorphism of the PTPN1 gene is associated with
insulin resistance in an Iranian population. Arch Med Res.
38:556–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Delibegovic M, Zimmer D, Kauffman C, et
al: Liver-specific deletion of protein-tyrosine phosphatase 1B
(PTP1B) improves metabolic syndrome and attenuates diet-induced
endoplasmic reticulum stress. Diabetes. 58:590–599. 2009.
View Article : Google Scholar
|
|
20
|
Swarbrick MM, Havel PJ, Levin AA, et al:
Inhibition of protein tyrosine phosphatase-1B with antisense
oligonucleotides improves insulin sensitivity and increases
adiponectin concentrations in monkeys. Endocrinology.
150:1670–1679. 2009. View Article : Google Scholar
|
|
21
|
Koren S and Fantus IG: Inhibition of the
protein tyrosine phosphatase PTP1B: potential therapy for obesity,
insulin resistance and type-2 diabetes mellitus. Best Pract Res
Clin Endocrinol Metab. 21:621–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang S and Zhang ZY: PTP1B as a drug
target: recent developments in PTP1B inhibitor discovery. Drug
Discov Today. 12:373–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Li L, Hong J and Huang W: Effects of
small interference RNA against PTP1B and TCPTP on insulin signaling
pathway in mouse liver: evidence for non-synergetic cooperation.
Cell Biol Int. 31:88–91. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bakhtiyari S, Meshkani R, Taghikhani M,
Larijani B and Adeli K: Protein tyrosine phosphatase-1B (PTP-1B)
knockdown improves palmitate-induced insulin resistance in C2C12
skeletal muscle cells. Lipids. 45:237–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maruyama H, Higuchi N, Nishikawa Y, et al:
High level expression of naked DNA delivered to rat liver via tail
vein injection. J Gene Med. 4:333–341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meshkani R and Adeli K: Hepatic insulin
resistance, metabolic syndrome and cardiovascular disease. Clin
Biochem. 42:1331–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu F, Song YK and Liu D:
Hydrodynamics-based transfection in animals by systemic
administration of plasmid DNA. Gene Ther. 6:1258–1266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
McAnuff MA, Rettig GR and Rice KG: Potency
of siRNA versus shRNA mediated knockdown in vivo. J Pharm Sci.
96:2922–2930. 2007. View Article : Google Scholar
|
|
29
|
Ventura-Sobrevilla J, Boone-Villa VD,
Aguilar CN, et al: Effect of varying dose and administration of
streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol
Soc. 54:5–9. 2011.PubMed/NCBI
|
|
30
|
Lewis DL and Wolff JA: Systemic siRNA
delivery via hydrodynamic intravascular injection. Adv Drug Deliv
Rev. 59:115–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galic S, Hauser C, Kahn BB, et al:
Coordinated regulation of insulin signaling by the protein tyrosine
phosphatases PTP1B and TCPTP. Mol Cell Biol. 25:819–829. 2005.
View Article : Google Scholar : PubMed/NCBI
|