Introduction
Heart failure (HF) is a clinical syndrome
characterized by impaired contractile function of the heart, and
represents the final pathway of a variety of diseases.
The sympathetic nervous system and its
neurotransmitters, noradrenaline and adrenaline, play a key role in
the development and prognosis of heart failure. Chronic
neurohumoral activation with high catecholamine levels increases
afterload, as well as myocardial consumption of energy, hypertrophy
and apoptosis of myocytes, which leads to deterioration of cardiac
function, thus triggering a vicious cycle (1). Sympathetic activation is observed
from the early stages of HF, with rates of catecholamine associated
with functional class and prognosis of this disease (2).
The persistent sympathetic activation in HF will
also cause increased heart rate, arteriolar vasoconstriction with
increased peripheral vascular resistance and renal function,
reduced blood flow and sodium excretion with a consequent increase
in blood volume, venous return and ventricular pressures and
volumes (3).
The beneficial effects of β-blocking agents appear
to be due to their ability to prevent the deleterious effects of
catecholamine on the heart and circulation. However, several
studies have shown that the response to β-blockers is variable
among patients and this is partly explained by genetic changes,
mainly related to the polymorphisms of the β-adrenergic receptor
gene (ADRB).
The most commonly studied polymorphism in HF is the
ADRB1 polymorphism Arg389Gly, which is related to a poorer response
to bucindolol in patients with the Gly389 variant (4). Recently, several other functionally
relevant polymorphisms of adrenergic receptors α2, β1 and β2 and
their specific genotypes were found to be associated with incidence
and clinical severity of HF (5).
Additionally, there has been debate concerning the
impact of ethnicity on the efficacy of β-blockers. Certain studies
suggest that many of the perceived differences in the efficacy of
β-blockers, as well as the variability of responses to β-blockers
among African-Americans, may be attributed to genetic variations
that affect β-receptors and their signaling pathways.
The aim of this study was to evaluate the
polymorphisms of β-adrenergic receptors in the risk of development
of HF and response to therapy and prognosis in patients with left
ventricular systolic dysfunction using carvedilol.
Materials and methods
Study population
Patients
A total of 146 patients with heart failure caused by
systolic dysfunction from the Heart Failure Clinic at the
Fluminense Federal University were enrolled in this study between
December 2005 and March 2009.
In this prospective cohort, the inclusion criteria
were: age ≥18 years; patients with a history and physical
examination compatible with HF; left ventricular ejection fraction
(LVEF) ≤50% by Simpson's method echocardiography. The exclusion
criteria were situations that could affect prognosis or drug
response, regardless of β-blocker use: implantation of a cardiac
resynchronization therapy device (CRTD) within 3 months prior to
admission in this study or intent to implant a CRTD; active
myocarditis; episode of aborted sudden death or presence of
defibrillators; indication of percutaneous or surgical coronary
intervention within the 3 months prior or 6 months following
admission; intolerance to carvedilol therapy on admission.
Peripheral blood was obtained for DNA isolation and
genotyping. The demographic data recorded were gender, age and
self-identified ethnicity. Clinical data assessed were smoking,
body mass index, presence of hypertension, coronary artery disease,
diabetes mellitus, chronic renal failure, anemia, atrial
fibrillation and use of cardiovascular medications. Patients with a
history of acute myocardial infarction, percutaneous or surgical
coronary intervention were considered as having coronary artery
disease. All patients underwent standard treatment for congestive
heart failure. Carvedilol was the β-blocker used and a target dose
of 25 mg twice daily was considered or the maximum tolerable
dose.
Control group
The control group consisted of 180 healthy
individuals, without structural heart disease according to clinical
evaluation guided by history, physical examination and
electrocardiogram. The exclusion criteria in this group were age
<18 years and presence of cardiovascular risk factors
(dyslipidemia, hypertension, diabetes mellitus, atherosclerotic
disease, family history of cardiomyopathy).
Ethics considerations
This investigation conformed to the principles
outlined in the Declaration of Helsinki, the Institutional Ethics
Committee of Faculty of Medicine/Antonio Pedro University Hospital
approved the protocol and all patients signed an informed consent
form prior to entering the study.
Echocardiographic assessment
Echocardiographic examinations were performed by two
experienced echocardiographers, both on admission and following six
months of monitoring, in accordance with the guidelines of the
European Society of Cardiology (6). The analyses made in this study were:
left ventricular cavity diameters in systole and diastole and left
atrium and LVEF by volume calculation (Simpson) (Vivid 3, GE
Medical Systems Ultrasound, WI, USA).
Follow-up
Patients were followed up for a minimum period of 12
months and a maximum of 40 months to evaluate the outcomes of
hospitalization as well as death. The information was registered
through the subsequent visits (in case of hospitalization) or phone
or medical records (in the case of death).
The patients who met the inclusion criteria and
consented to participate in the study were submitted to laboratory
exams and genetic evaluation, and drug therapy was optimized or
initiated.
Six months after the study onset, patients were
reassessed with clinical, laboratory and echocardiographic exams.
We considered echocardiographic improvement compared to the
admission exam as an increase in LVEF ≥20% and reduction of left
cavity diameters ≤5%.
Genotyping of the β-adrenergic
polymorphism
The samples were subjected to cell lysis with 1000
μl of Tris-1 (Tris-HCl 10 mM pH 8.0, KCl 10 mM, MgCl2 10
mM, EDTA 2 mM pH 8.0) containing Triton X-100 2.5%. Following
centrifugation at 5,000 rpm for 5 min in a Beckman centrifuge, the
cell nuclei were lysed with 200 μl of Tris-2 containing SDS 1%. The
proteins were removed by saline precipitation with 100 μl NaCl 5M.
The DNA present in the supernatant was isolated by ethanol
precipitation and finally resuspended in 100 μl TE (Tris-HCl 10 mM
and 1 mM EDTA, pH 8.0) and stored at −20°C until use.
Following extraction, the integrity of DNA samples
was analyzed by an electrophoresis system (Bio-Rad electrophoresis)
in 0.8% agarose gel in TBE 1× (Tris-HCl 90 mM, 90 mM boric acid and
2 mM EDTA) stained with ethidium bromide.
The ADRB1 polymorphisms [Arg389Gly (rs1801253);
Ser49Gly (rs1801252)] and ADRB2 polymorphisms [Gln27Glu
(rs1042713); Arg16Gly (rs1042713)] were analyzed by polymerase
chain reaction and restriction fragment length polymorphism
(PCR-RFLP) (7,8). The PCR was performed in a total
volume of 25 μl, utilizing 50–100 ng genomic DNA, after adjusting
the concentration, 1 unit of Fermentas Taq DNA polymerase, reaction
buffer (50 mM KCl, 1.5 mM MgCl2 and 10 mM Tris-HCl), 200
μM of each deoxynucleotide (dATP, dCTP, dGTP and dTTP) and 15 pmol
of each oligonucleotide.
The conditions of amplification and digestion for
the identification of the polymorphisms are detailed in Table I. The wild allele was defined
according to the allele frequency of the study population.
 | Table IConditions of amplification and
digestion for the identification of the β-adrenergic receptor
polymorphisms. |
Table I
Conditions of amplification and
digestion for the identification of the β-adrenergic receptor
polymorphisms.
| ADBR1 | ADBR2 |
|---|
|
|
|
|---|
| Arg389Gly | Ser49Gly | Glu27Gln | Arg16Gly |
|---|
| Technique | PCR-RFLP | PCR-RFLP | PCR-RFLP | PCR-RFLP |
| Amplified product
(bp) | 530 | 564 | 353 | 201 |
| Cycling |
| Initial
denaturation | 94°C-5 min | 94°C-5 min | 94°C-5 min | 94°C-5 min |
| Denaturationa | 94°C-30 sec | 94°C-30 sec | 94°C-1min | 94°C-1 min |
| Annealinga | 58°C-30 sec | 61°C-30 sec | 56°C-60 sec | 56°C-60 sec |
| Extensiona | 72°C-1 min | 72°C-1 min | 72°C-1 min | 72°C-1 min |
| Final extension | 72°C-7 min | 72°C-7 min | 72°C-7 min | 72°C-7 min |
| Restriction
enzyme | BCG1 | Eco0109I | Fnu4HI | BsrDI |
| SNP effect in the DNA
sequence | Abolishes binding
site | Abolishes binding
site | Abolishes binding
site | Creates binding
site |
| Fragments of the wild
allele (bp) | 342, 154, 34 | 345, 219 | 27, 55, 97, 174 | 14, 56, 131 |
| Fragments of the
mutante allele (bp) | 530 | 564 | 27, 97, 229 | 14, 23, 56,
108 |
| Digestion
temperature (°C) | 37 | 37 | 37 | 37 |
| Digestion time
(h) | 20 | 20 | 2h | 20 |
Statistical analysis
The correlation between clinical variables and
genotype with the outcomes and therapeutic responses (univariate
and multivariate) was assessed by the following methods: i) for
comparison with categorical data the χ2 test or Fisher's
exact test were applied; ii) for comparison with numerical data we
used the Student's t-test for independent samples, and iii)
logistic regression analysis was used to identify independent
variables that predict (or explain) the outcomes for therapeutic
response.
Kaplan-Meier survival curve and log-rank tests were
used for survival analysis and to estimate the difference between
the survival probabilities of ADRB1 polymorphisms and
self-identified ethnicity.
The χ2 test showed that the genotype
frequencies of polymorphisms of genes ADRB1 and ADRB2 were in
agreement with the assumptions of Hardy-Weinberg.
The criterion for determining significance was the
level of 5%. Statistical analysis was conducted using the
statistical software SAS® System.
Results
Baseline demographics
Of the 146 patients included in the study, 140
remained until the completion, with 6 losses occurring due to loss
of contact or waiver monitoring. The mean age of the cohort was
59±13 years; 72.9% were male, 46% were ischemic (no patient had
Chagas disease) and 49.3% were non-black (self-identified). In
total, 94% of patients were treated with either an ACE inhibitor or
an angiotensin receptor blocker, and all the patients were taking
β-blockers. All patients had the maximum tolerated dose. Clinical,
laboratory and echocardiographic variables are shown in Table II.
 | Table IIClinical, laboratory and
echocardiographic variables of the patient group. |
Table II
Clinical, laboratory and
echocardiographic variables of the patient group.
| Variable | Mean ± SD |
|---|
| Age (years) | 58.5±13.0 |
| BMI
(kg/m2) | 25.9±5.2 |
| SBP (mmHg) | 129.7±26.1 |
| DBP (mmHg) | 80.2±15.3 |
| HR (bpm) | 75.4±11.6 |
| Uric acid
(mg/dl) |
| Female | 6.3±2.3 |
| Male | 7.0±2.1 |
| Creatinine
(mg/dl) | 1.3±1.0 |
| Sodium (mEq/l) | 139.2±3.7 |
| Hemoglobin
(g/dl) |
| Female | 12.7±1.5 |
| Male | 13.9±1.8 |
| Admission
echocardiography |
| Left atria
(mm) | 4.6±0.8 |
| LVEDD (mm) | 6.8±1.0 |
| LVESD (mm) | 5.5±1.1 |
| LVEF (%) | 35.3±9.3 |
Clinical characteristics
With regard to comorbidities associated with HF,
there was a predominance of hypertension (73.6%), followed by
coronary artery disease (42.1%), diabetes mellitus type II (34.3%),
anemia (25.7 %), chronic renal failure (16.4%) and atrial
fibrillation (15.7%).
In assessing the functional class, it was observed
that the majority of patients included were in functional class I
or II (75%) according to the New York Heart Association (NYHA)
classification. On the laboratory tests, only the mean serum uric
acid for both genders was higher than the normal range.
Echocardiogram performed at inclusion of the study demonstrated the
prevalence of patients with severe LV systolic dysfunction (mean
LVEF, 35.3%). Only advanced functional class [RR=3.90 (1.73–8.80),
P=0.001] was significant for predicting hospitalization. The other
variables showed no independent contribution.
Analysis of genotype prevalence in
control and patient groups
In order to establish the prevalence of genotypes
for the ADRB1 and ADRB2 polymorphisms between cases and controls,
180 healthy subjects were analyzed. The control group showed that
the Gln27Gln genotype (52.8%) was significantly higher compared
with the case group (32.9%) (P=0.0004). Moreover, the number of
cases presenting the Glu27Glu genotype (24.7%) was significantly
higher than the control group (6.1%). In the same way, the patient
group had a higher prevalence of Gly16Gly and Gly49Gly genotypes
(P<0.0001) when compared with the control group. No association
was found for Arg389Gly genotypes (Table III).
 | Table IIIADBR1 and ADBR2 genotypes in control
and patient groups. |
Table III
ADBR1 and ADBR2 genotypes in control
and patient groups.
| | | Controls | Patients | |
|---|
| | |
|
| |
|---|
| Gen | Polymorphisms | Genotype | n (%) | n (%) | P-value |
|---|
| ADBR1 | Arg389Gly | ArgArg | 82 (45.6) | 71 (48.6) | 0.84 |
| | ArgGly | 77 (42.80 | 58 (39.8) | |
| | GlyGly | 21 (11.7) | 17 (11.6) | |
| Ser49Gly | SerSer | 129 (93.5) | 33 (22.60 | <0.0001 |
| | SerGly | 43 (31.2) | 64 (43.8) | |
| | GlyGly | 6 (4.3) | 49 (33.6 | |
| ADBR2 | Gln27Glu | GlnGln | 95 (52.8) | 48 (32.9) | 0.0004 |
| | GlnGlu | 74 (41.1) | 62 (42.4) | |
| | GluGlu | 11 (6.1) | 36 (24.7 | |
| Arg16Gly | ArgArg | 41 (22.8) | 106 (72.6) | <0.0001 |
| | ArgGly | 76 (42.2) | 37 (25.3) | |
| | GlyGly | 63 (35.0) | 3 (2.1) | |
Outcomes
Event-free survival
During follow-up, 15 patients succumbed to the
disease (10.7%) and 46 patients required hospitalization
(32.9%).
Analysis of outcomes in relation to
allele frequency
There was no statistical significance in the
analysis of the correlation between >20% LVEF improvement and
allele frequencies of the different β-adrenergic receptors
polymorphisms.
In the analysis of clinical outcomes and
polymorphisms for ADRB1 Arg389Gly, no significant association was
observed with the χ2 test between mortality after 12
months and allele frequency of Arg/Gly, at 5% (P=0.065). However,
according to Fisher's exact test, it was found that patients who
succumbed to the disease had a significantly higher Gly389 allele
frequency (61.1%) than the group that did not (44.7%), P=0.047.
Additionally, and reinforcing the first finding, it
was observed that patients admitted to hospital after 12 months
showed a Gly389 allele frequency that was significantly higher
(54.9%) than the group without hospitalization (42.1%), P=0.039. In
the evaluation of ADRB2 and ADRB1 Ser49Gly polymorphisms, the
difference was not statistically significant for the analysis of
clinical outcomes and allele frequency.
Evaluation of survival or event-free
survival in relation to genotypes
It was observed that there were no significant
differences in survival between the two subgroups of ADRB1 Ser49Gly
(Ser49Ser vs. Ser49Gly + Gly49Gly). The subgroup with the Ser49Ser
genotype presented a reduced survival rate compared with the
subgroup with a non-Ser49Ser genotype; however, this showed no
statistical significance (P=0.085).
The subgroup with the presence of the Gly389 allele
had lower survival than the subgroup without the Gly389 allele
(Arg389Arg), but there was no significant difference in survival
between the two subgroups of genotype ADRB1 Arg389Gly (Gly389Gly +
Arg389Gly vs. Arg389Arg) (P=0.054).
There was no significant difference in the
evaluation of the polymorphisms studied for the ADRB2 gene and
event-free survival.
Assessment of survival in relation to
polymorphisms of the β-adrenergic receptors and self-identified
ethnicity
In the analysis of hospital outcomes in relation to
polymorphisms of the β-adrenergic receptors, we found no
significant difference between the studied groups; however, the
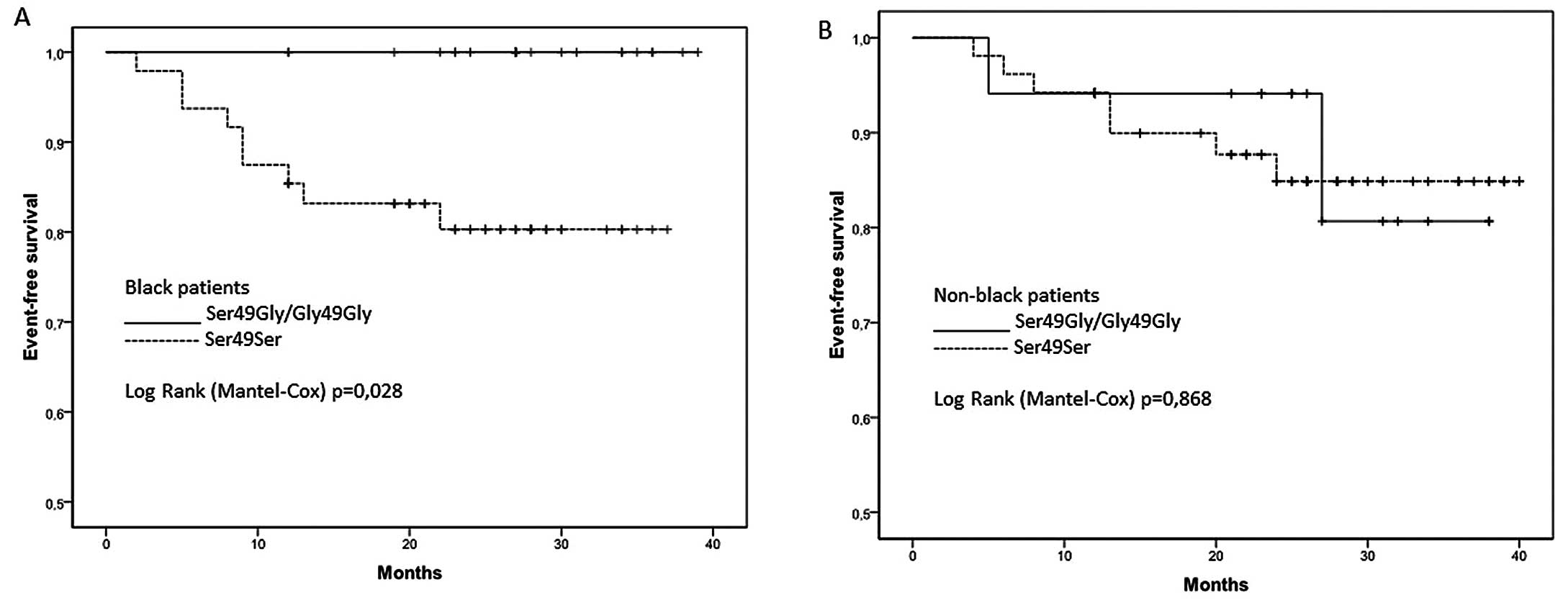
survival analysis, shown in Fig.
1, revealed that homozygous ADRB1 Ser49Ser patients who
described themselves as black had lower survival compared to
homozygous Gly49Gly and heterozygous Ser49Gly patients (P=0.028).
There was no such difference between the patients who described
themselves as non-black (P=0.868).
In the analysis for polymorphism Arg389Gly, we found
a higher mortality rate among patients who described themselves as
black and who had the presence of 1 or 2 Gly389 alleles (P=0.06),
although this was not statistically significant, while the same was
not observed in the patients who described themselves as non-black
(P=0.33).
Discussion
For a considerable number of cardiovascular
diseases, it has been proposed that both susceptibility to disease
and individual variability in relation to the treatment were
associated in part to genetic polymorphisms, particularly those
polymorphisms that are related to neurotransmitter receptors.
The central role of the sympathetic nervous system
and its receptors in HF makes the genetic polymorphisms of these
receptors strong candidates for risk factors and predictors of
response to treatment of this disease.
The allelic prevalence studies in different
populations have shown that for ADRB1 Arg389Gly polymorphism, the
allele frequency of Arg389 is greater than of Gly389, at 70 and 30%
respectively, and there was a higher frequency for the Gly389
allele in African-Americans (42%) compared to Caucasians (27%). In
the control group of this study, we found a similar allele
frequency distribution to Hispanic-Americans (67% for Arg389 and
33% for Gly389) (9). With regard
to the allelic distribution of the gene polymorphism ADRB1
Ser49Gly, Moore et al(10)
demonstrated no difference in the prevalence of the Gly49 allele
among Caucasians and Asians (15%). In a multiethnic population,
such as in Brazil, the Gly49 allelic frequency is equal, whereas,
among African-Americans the frequency for the same allele is
13%.
The estimated frequency of the Arg16 variant is
39.3% in Caucasians, 49.2% in African Americans and 51.0% among
Chinese patients. The estimated frequency of the Glu allele is
24.6% among Caucasian, 18.7% among black and 9% among Chinese
patients. In this study the estimated frequency of the Arg16
variant is 44% and 27% for the Glu allele in a Brazilian population
(11).
In the investigation of gene polymorphisms of ADRB1
and risk for development of HF, no association between polymorphism
Arg389Gly and susceptibility to HF was found. The same finding had
been shown by Small et al(12) in an evaluation of 159 patients with
HF (ischemic and idiopathic) and 189 controls; and Tesson et
al(13) in 426 patients with
idiopathic dilated cardiomyopathy. For the Ser49Gly polymorphism,
Borjesson et al(14)
concluded that the prevalence of Ser49Gly was similar in 184
patients with idiopathic HF and 77 controls. However, Podlowski
et al(15) observed that
the presence of the Gly49 allele was more prevalent in patients
with idiopathic cardiomyopathy, as was found in this study.
Regarding susceptibility to HF and gene
polymorphisms of ADRB2, Liggett et al(16) studied the effect of these in 259
patients with idiopathic or ischemic HF and concluded that Arg16Gly
and Gln27Glu polymorphisms were not associated with risk of
developing HF or with prognosis, fitting with the conclusion of
other studies that have made this analysis. Forleo et
al(17), after analyzing
haplotypes of Gly16Glu27, concluded that patients with dilated
cardiomyopathy had a higher risk of developing HF.
In this population, a higher prevalence of the
genotypes Glu27Glu and Gly16Gly was found among patients with HF
compared to healthy volunteers. The same fact was found by Mogara
et al(18) in a study
conducted in the Latin American population, where the presence of
Glu allele was considered a possible predictor of risk for HF.
Studies in other populations indicate a higher
susceptibility to develop hypertension in carriers of the Glu27 and
Gly16 alleles. Bray et al(19) showed higher frequencies for the
Gly16 and Glu27 alleles in hypertensive compared with normotensive
patients, and the odds ratio for the occurrence of hypertension was
1.80 (P=0.023) for the Glu27 allele. Therefore, as the prevalence
of hypertension was 74% among individuals with HF in our study,
this comorbidity could be attributed to the higher prevalence of
Glu27Glu and Gly16Gly genotypes and not directly related to HF.
Studies are inconclusive on reverse remodeling in
patients with HF and polymorphisms of the β-adrenergic receptors.
While some have shown that improvement in LVEF and reduction of
cavity diameters with the use of β-blockers is associated with the
presence of the allele Arg389, some trials such as MERITH found no
association of these polymorphisms with reverse remodeling. Other
studies showed improvement in LVEF in the presence of the Gly49 and
Glu27 alleles, but this was not confirmed by larger studies
(20,21).
In this population no association was found for any
of the polymorphisms studied with improvement in LVEF following six
months of treatment with carvedilol, both for assessing genotypes
and allele frequency.
For the assessment of prognosis and ADRB1
polymorphisms, one sub-study of BEST analyzed 1,040 patients for
the polymorphism Arg389Gly and showed that those patients
homozygous for Arg389 and treated with bucindolol had a significant
reduction in mortality compared with placebo, while there was no
difference in same evaluation for the homozygous Gly389 (4). As a result of this study, the FDA
approved the use of this genetic test for patients with HF.
In a retrospective analysis of 375 patients with
dilated cardiomyopathy receiving β-blockers and 492 controls, a
significant association was observed between survival and the
polymorphism Ser49Gly, and the Ser49 variant was associated with
reduced 5-year survival. However, this study was aimed at patients
with HF with preserved LVEF and did not specify the use of
β-blockers in the study (22). In
the present study, the assessment of prognosis in relation to
outcomes of death and hospitalization over an average of 23 months
had a decreased survival in patients with the Gly389 allele and
tendency to mortality for those with the genotype.
Ethnic differences and indicators of socioeconomic
status cannot fully explain the excess mortality in the
self-identified black population compared to the white population,
particularly mortalities from cardiovascular disease and
complications such as heart failure. This finding has reinforced
the theory that the racial differences in cardiovascular events are
mediated by genetic factors that determine disease severity and
response to medications (23).
In the analysis of survival, Ser49Gly polymorphism
and self-identified ethnicity, it was observed that black patients
with the Ser49Ser homozygote had a poorer survival compared to
non-black patients with the same genotype. This corroborates the
interaction of gene-ethnicity-response to drugs that had already
been described in relation to the polymorphism of nitric oxide
synthase and favorable response to the hydralazine-nitrate
combination in African-American HF patients. The results of Genetic
Risk Assessment of Heart Failure (GRAHF) study suggested that
genetic variation for the enzyme nitric oxide synthase 3 affects
the progression to HF and may impact the therapeutic efficacy of
the nitrate-hydralazine combination (24).
The studies are controversial regarding the response
to β-blockers according to ethnicity. The BEST study showed an 18%
increase in survival of non-African-Americans (P=0.01) vs. a 17%
reduction in survival of African-Americans (P=0.27) with the use of
bucindolol compared with placebo (25). The U.S. Carvedilol and COPERNICUS
studies showed no difference in drug response in favor of
β-blockers in relation to whether or not patients were
African-American (26,27).
It is worth noting that the studies conducted to
evaluate the effectiveness of β-blockers in HF include a
significantly smaller number of African-Americans, which may
compromise the analysis of drug response according to ethnicity
(28). Moreover, none of the
studies has been developed specifically to test whether there was
any difference in therapeutic response between ethnic groups. The
comparisons made by analysis of subgroups are not involved in the
original design of the study.
Another fact that should be taken into account is
the different pharmacological actions of β-blockers. Carvedilol is
a nonselective β-adrenergic blocking agent with α1-blocking
activity and is a multiple-action neurohormonal antagonist. It has
sympathomimetic activity besides membrane stabilizing properties,
and is a potent antioxidant and neutralizer of oxygen radicals,
which may explain the better results in African-Americans; whereas,
bucindolol is a phenoxypropanolamine with potent non-selective
β-antagonist activity but mild vasodilatory properties (29).
Finally, ethnic differences in drug response may be
due to genetic differences and the environmental effects on
individuals, as well as the pathogenesis of the disease itself. The
genetic differences between ethnic groups, in turn, result from
differences in the distribution of polymorphisms that are related
to both the enzymes responsible for drug metabolism as well as the
recipients of these medications. For example, African-Americans and
Africans have a high frequency of the CYP2D6 allele, which encodes
an enzyme deficiency. This allele is virtually absent in Caucasians
and Asians. This is particularly notable as regards the response to
carvedilol in black patients, and since CYP2D6 is responsible for
the metabolism of one isomer of this product, it may contribute to
the efficacy and toxicity, according to the genotype (30).
Prospective validation of predictive impact of
genetic variations is necessary prior to implementation of routine
treatments that are genetically individualized.
HF patients with β-blocker therapy and the Gly389
allele have worse outcome, with reduced event-free survival
compared to those carrying the Arg389 allele. HF patients treated
with carvedilol, self-identified as black and homozygous for
Ser49Ser may have reduced survival compared to non-black patients
with the same genotype. Additionally, the genotypes ADRB2 Arg16Arg
and Glu27Glu and ADRB1 Gly49Gly had the highest prevalence in the
group of HF patients compared to the control group. However,
further information is required to confirm these results.
Acknowledgements
This study was supported by the Rio de Janeiro
Research Assistance Foundation (FAPERJ) and the Science and
Technology Department of the Brazilian Health Ministry in the
Molecular Genetics Program applied to the Brazilian Health System.
Carvedilol was donated by the pharmaceutical Co., Baldacci Inc.,
which, however, did not participate directly or indirectly in the
design, analysis or publication of the results in this study.
References
|
1
|
El Armouche A and Eschenhagen T:
β-Adrenergic stimulation and myocardial function in the failing
heart. Heart Fail Rev. 14:225–241. 2009.
|
|
2
|
Cohn JN, Levine TB and Olivari MT: Plasma
norepinephrine as a guide to prognosis in patients with chronic
congestive heart failure. N Engl J Med. 311:819–823. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Triposkiadis F, Karayannis G, Giamouzis G,
Skoularigis J, Louridas G and Butler J: The sympathetic nervous
system in heart failure physiology, pathophysiology, and clinical
implications. J Am Coll Cardiol. 54:1747–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liggett SB, Mialet-Perez J, Thaneemit-Chen
S, et al: A polymorphism within a conserved beta(1)-adrenergic
receptor motif alters cardiac function and betablocker response in
human heart failure. Proc Natl Acad Sci USA. 103:11288–11293. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McNamara DM: Emerging role of
pharmacogenomics in heart failure. Curr Opin Cardiol. 23:261–268.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lang RM, Bierig M, Devereux RB, et al:
Recommendations of chamber quantification. Eur J Echocardiogr.
7:79–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Groote P, Lamblin N, Helbecque N, et
al: The impact of beta-adrenoreceptor gene polymorphisms on
survival in patients with congestive heart failure. Eur J Heart
Fail. 7:966–973. 2005.PubMed/NCBI
|
|
8
|
Maqbool A, Hall A, Ball S and Balmforth
AJ: Common polymorphisms of beta1-adrenoceptor: identification and
rapid screening assay. Lancet. 353:8971999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie HG, Dishy V, Sofowora G, et al:
Arg389Gly beta 1-adrenoceptor polymorphism varies in frequency
among different ethnic groups but does not alter response in vivo.
Pharmacogenetics. 11:191–197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore JD, Mason DA, Green SA, Hsu J and
Liggett SB: Racial differences in the frequencies of cardiac
beta(1)-adrenergic receptor polymorphisms: analysis of c145A>G
and c1165G>C. Hum Mutat. 14:2711999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Small KM, McGraw DW and Liggett SB:
Pharmacology and physiology of human adrenergic receptor
polymorphisms. Annu Rev Pharmacol Toxicol. 43:381–411. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Small KM, Wagoner LE, Levin AM, Kardia SL
and Liggett SB: Synergistic polymorphisms of β1- and α2C-adrenergic
receptors and the risk of congestive heart failure. N Engl J Med.
347:1135–1142. 2002.
|
|
13
|
Tesson F, Charron P, Peuchmaurd M, et al:
Characterization of a unique genetic variant in the
beta1-adrenoceptor gene and evaluation of its role in idiopathic
dilated cardiomyopathy. Cardigene Group. J Mol Cell Cardiol.
31:1025–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Börjesson M, Magnusson Y, Hjalmarson A and
Andersson B: A novel polymorphism in the gene coding for the
beta(1)-adrenergic receptor associated with survival in patients
with heart failure. Eur Heart J. 21:1853–1858. 2000.PubMed/NCBI
|
|
15
|
Podlowski S, Wenzel K, Luther HP, et al:
Beta1-adrenoceptor gen variations: a role in idiopathic dilated
cardiomyopathy? J Mol Med. 78:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liggett SB, Wagoner LE, Craft LL, Hornung
RW, Hoit BD, McIntosh TC and Walsh RA: The Ile164 beta2-adrenergic
receptor polymorphism adversely affects the outcome of congestive
heart failure. J Clin Invest. 102:1534–1539. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Forleo C, Resta N, Sorrentino S, et al:
Association of β-adrenergic receptor polymorphisms and progression
of heart failure in patients with idiopathic dilated
cardiomyopathy. Am J Med. 117:451–458. 2004.
|
|
18
|
Moraga F, Troncoso R, Mellado R, et al:
Interactions between beta1 and beta2 adrenergic receptor
polymorphisms as risk factors for chronic heart failure. Rev Med
Chil. 136:1371–1380. 2008.(In Spanish).
|
|
19
|
Bray MS, Krushkal J, Li L, et al:
Positional genomic analysis identifies the beta(2)-adrenergic
receptor gen as a susceptibility locus for human hypertension.
Circulation. 101:2877–2882. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Terra SG, Hamilton KK, Pauly DF, et al:
β1-Adenergic receptor polymorphisms and left ventricular remodeling
changes in response to β-blocker therapy. Pharmacogenet Genomics.
15:227–234. 2005.
|
|
21
|
White HL, de Boer RA, Maqbool A, et al: An
evaluation of the beta-1 adrenergic receptor Arg389Gly polymorphism
in individuals with heart failure: a MERIT-HF sub-study. Eur J
Heart Fail. 5:463–468. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magnusson Y, Levin MC, Eggertsen R,
Nyström E, Mobini R, Schaufelberger M and Andersson B: Ser49Gly of
β1-adrenergic receptor is associated with effective β-blocker dose
in dilated cardiomyopathy. Clin Pharmacol Ther. 78:221–231.
2005.
|
|
23
|
Latado AL, Lopes MB, Passos LC and Lopes
AA: Does any evidence exist to treat heart failure based on race or
ethnicity? Rev Assoc Med Bras. 55:110–116. 2009.(In
Portuguese).
|
|
24
|
McNamara DM, Tam W, Sabolisnski ML, et al:
Endothelial nitric oxide synthase (NOS3) polymorphisms in African
Americans with heart failure: results from the A-HeFT trial. J Card
Fail. 15:191–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beta-Blocker Evaluation of Survival Trial
Investigators. A trial of the beta-blocker bucindolol in patients
with advanced chronic heart failure. N Engl J Med. 344:1659–1667.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cohn JN, Fowler MB, Bristow MR, et al:
Safety and efficacy of carvedilol in severe heart failure. The US
Carvedilol Heart Failure Study Group. J Card Fail. 3:173–179. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Packer M, Fowler MB, Roecker EB, et al:
Effect of carvedilol on the morbidity of patients with severe
chronic heart failure: results of the carvedilol prospective
randomized cumulative survival (COPERNICUS) study. Circulation.
106:2194–2199. 2002. View Article : Google Scholar
|
|
28
|
Yancy CW, Laskar S and Eichhorn E: The use
of beta-adrenergic receptor antagonists in the treatment of African
Americans with heart failure. Congest Heart Fail. 10:34–37. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
López-Sendón J, Swedberg K, McMurray J, et
al: Expert consensus document on beta-adrenergic receptor blockers.
Eur Heart J. 25:1341–1362. 2004.PubMed/NCBI
|
|
30
|
Wood AJ: Racial differences in the
response to drugs-pointers to genetic differences. N Engl J Med.
344:1394–1396. 2001. View Article : Google Scholar : PubMed/NCBI
|