Introduction
Severe stress induces anxiety disorders which
manifest as marked, continual, excessive and irrational reactions
to fear (1). Anxiety disorders are
closely associated with an inability to distinguish between learned
fear responses (2). Of the anxiety
disorders, post-traumatic stress disorder (PTSD) exhibits a variety
of symptoms that include exaggerated fear, helplessness and terror
following extremely stressful events. The symptoms of the
re-experience of an earlier traumatic event are panic attack,
phobic avoidance of situations that resemble the traumatic event
and psychic numbing (3).
Pharmacological treatments of PTSD patients are mainly serotonergic
agonists. Delayed onset, poor efficacy and relapse are the main
complications of these drugs (4,5).
Serotonin, also known as 5-hydroxytryptamine (5-HT),
has been implicated in a wide variety of physiological,
sensorimotor and behavioral functions. Malfunction of the
serotonergic systems has been hypothesized to be the main
etiological factor for numerous psychiatric illnesses, including
anxiety, mood and eating disorders, as well as neurovascular
disorders, i.e., migraine (6,7).
Serotonergic systems inhibit proactive coping responses, including
aggression and escape behaviors, and facilitate passive-submissive
behaviors including fear- and anxiety-like behaviors (6,8,9). The
biosynthesis of 5-HT from tryptophan is catalyzed by tryptophan
hydroxylase (TPH), initially producing 5-hydroxytryptophan. The
decarboxylation of 5-hydroxytryptophan into 5-HT is catalyzed by an
aromatic amino acid decarboxylase. As TPH is the rate-limiting
enzyme in serotonin production, its expression is used as the
indicator of serotonin synthesis (10). Depressed suicide victims have
higher TPH immunoreactivity in the dorsal raphe nucleus compared
with nonpsychiatric control subjects (11). Chamas et al(12) proposed that repeated immobilization
stress increases TPH mRNA and protein concentrations.
The immediate-early gene product, c-Fos, is
expressed throughout the brain in response to a variety of stimuli.
The detection of c-Fos is a powerful tool for the study of
intracellular responses of neurons. As c-Fos expression in the
brain represents neuronal activation, increased c-Fos expression
indicates that neurons are activated by stimuli (13–15).
Increased serotonergic neuronal activity induced by social defeat
is followed by increased c-Fos expression, resulting in an
increased extracellular serotonin concentration within the dorsal
raphe nucleus (8,16).
The biological substrates involved in social
interactions and anxiety behaviors remain unclear. Nitric oxide
(NO) has been implicated in social interactions and anxiety-like
behaviors (17,18). NO is synthesized from L-arginine by
a family of isoformic enzymes known as NO synthases (NOSs). Three
isoforms of NOS have been identified: neuronal (nNOS), endothelial
(eNOS) and inducible (iNOS). Of these, nNOS is rich throughout the
limbic system of the brain area, which controls emotional behaviors
(19,20). Inhibition of nNOS alters social
interactions and anxiety-like behaviors (17,18)
and inhibition of NOS causes antidepressive effects (21).
Exercise is known to enhance learning ability and
memory function (22–24). An ameliorating effect of exercise
on depression has also been reported (25–28).
However, the effects of exercise on stress-induced anxiety symptoms
remain unclear. In the present study, the effect of treadmill
exercise on anxiety-like symptoms induced by stress during
pregnancy was investigated in maternal rats. The anxiolytic effects
of treadmill exercise were evaluated by an elevated plus maze (EPM)
test and the expression of 5-HT, TPH, c-Fos and nNOS were
determined by immunohistochemistry.
Materials and methods
Animals
Adult pregnant Sprague-Dawley rats were obtained
from a commercial breeder (Orient Co., Seoul, Korea). The animals
were housed under controlled temperature (23±2°C) and lighting
(08:00–20:00 h) conditions, with food and water available ad
libitum. The pregnant rats were randomly divided into 4 groups
(n=5 per group): control, control exercise, PTSD-induced and
PTSD-induced and exercise. All experimental procedures were
performed in accordance with the animal care guidelines of the
National Institutes of Health and the Korean Academy of Medical
Sciences.
Induction of stress
Stress was induced by exposure of pregnant rats to a
hunting dog in an enclosed room as previously described (29). Exposure time was 10 min, repeated 3
times/day with a 1-h interval. Exposure of maternal rats to the
hunting dog was performed from day 7 following pregnancy until
delivery. The response of each pregnant rat during predator
exposure involved observation from a distance, approaching and
sniffing and chasing with occasional mild attack. The hunting dog
was able to approach, but was not able to injure the pregnant rats.
The same hunting dog was used throughout the experiment. Control
rats were left undisturbed during the pregnancy.
Exercise protocol
Pregnant rats in the exercise groups were forced to
run on a treadmill for 30 min/day starting at day 7 of pregnancy
until delivery. The exercise load consisted of running at a speed
of 2 m/min for 5 min, followed by 5 m/min for 5 min and then 8
m/min for the last 20 min. The rats in the non-exercise groups were
left on the treadmill without running for the same period as the
exercise groups.
Elevated plus maze test
Following delivery, the maternal rats were left
undisturbed together with their neonatal rats for 3 weeks.
Anxiety-like behavior was evaluated using the EPM test as described
previously (30,31). The EPM consisted of two opposing
open arms (45×10 cm) and two closed arms (45×10×50 cm) that
extended from a central platform (10×10 cm) elevated 65 cm above
the floor. Each pregnant rat was placed on the central platform
facing a closed arm and was allowed to freely explore the maze for
5 min. Entry into an arm was defined as entry of all four paws into
the arm. Time spent in the open and closed arms were measured.
Brain preparation
Animals were sacrificed immediately following EPM
testing. To prepare the brain slices, animals were fully
anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac,
Carros, France), transcardially perfused with 50 mM
phosphate-buffered saline (PBS) and then fixed with freshly
prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer
(pH 7.4). Brains were then removed, post-fixed in the same fixative
overnight and transferred into a 30% sucrose solution for
cryoprotection. Coronal sections (40-μm thick) were made using a
freezing microtome (Leica Microsystems Ltd., Nussloch,
Germany).
Immunohistochemistry for 5-HT and
TPH
Immunohistochemistry for 5-HT- and TPH-positive
cells in the dorsal raphe nucleus was conducted as previously
described (28). The dorsal raphe
nucleus spanning from Bregma −7.20 to −8.00 mm were obtained from
each brain. To begin the procedure, the sections were incubated in
PBS for 10 min and then washed three times in the same buffer.
Sections were then incubated in 1% hydrogen peroxide
(H2O2) for 30 min. Next, the sections were
incubated overnight with rabbit anti-5-HT (1:5,000; Immuno Star,
Hudson, WI, USA) or mouse anti-TPH antibodies (1:1,000; Oncogene
Research Products, Cambridge, UK). The sections were then incubated
for 1 h with anti-rabbit secondary antibody (1:200; Vector
Laboratories, Burlingame, CA, USA) for 5-HT immunohistochemistry or
anti-mouse secondary antibody (1:200; Vector Laboratories) for TPH
immunohistochemistry. Next, the sections were incubated with
avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1
h at room temperature. For staining, the sections were incubated in
a solution consisting of 0.02% 3,3′-diaminobenzidine
tetrahydrochloride (DAB) and 0.03% H2O2 in 50
mM Tris-HCl (pH 7.6) for ~5 min, following which they were washed
with PBS and mounted onto gelatin-coated slides. The number of
5-HT- and TPH-positive cells in the dorsal raphe nucleus of the
selected sections were counted using a light microscope (Olympus,
Tokyo, Japan).
Immunohistochemistry for c-Fos and
nNOS
Immunohistochemistry for c-Fos- and nNOS-positive
cells in the hypothalamus (Bregma −1.80 to −1.92 mm) and locus
coeruleus (Bregma −9.84 to −9.96 mm) was conducted as previously
described (13,32). To begin the procedure, sections
were incubated in PBS for 10 min and then washed three times in the
same buffer. The sections were then incubated in 1%
H2O2 for 30 min. Next, sections were
incubated overnight with rabbit anti-c-Fos (1:500; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) or mouse anti-nNOS
antibodies (1:500; BD Biosciences, San Jose, CA, USA). The sections
were then incubated for 1 h with anti-rabbit secondary antibody
(1:200; Vector Laboratories) for c-Fos immunohistochemistry and
with anti-mouse secondary antibody (1:200; Vector Laboratories) for
nNOS immunohistochemistry. Next, sections were incubated with
avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1
h at room temperature. For staining, the sections were incubated in
a solution of 0.02% DAB and 0.03% H2O2 in 50
mM Tris-HCl (pH 7.6) for ~5 min, washed with PBS and mounted onto
gelatin-coated slides. The numbers of c-Fos and nNOS-positive cells
in the hypothalamus and locus coeruleus of the selected sections
were counted using a light microscope (Olympus).
Statistical analysis
Results are presented as the mean ± SEM. Data were
analyzed by one-way analysis of variance, followed by Duncan's
post-hoc test using SPSS software (SPSS, Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of treadmill exercise on
anxiety-like behaviors
The effect of treadmill exercise on anxiety-like
behaviors is presented in Fig. 1.
The percentage of time spent on the open and closed arms during the
total 5-min test were calculated. The open arm values were
46.47±8.20% in control, 74.72±7.93% in control exercise, 6.90±4.09%
in PTSD-induced and 28.02±3.22% in the PTSD-induced and exercise
group. The decreased value obtained for the PTSD-induced group
compared with the control was found to be significant (P<0.05).
By contrast, treadmill exercise increased the percentage of time
spent in the open arms in the control and PTSD-induced groups
(P<0.05).
The percentage of time spent in the closed arms was
39.97±8.04% in control, 11.57±6.27% in control exercise,
76.55±5.64% in PTSD-induced and 40.97±9.11% in the PTSD-induced and
exercise group. The time spent in the closed arms was identified to
be significantly increased in the PTSD-induced group compared with
the control (P<0.05). By contrast, treadmill exercise was
observed to significantly decrease the time spent in the closed
arms in the control and PTSD-induced groups (P<0.05).
Effect of treadmill exercise on 5-HT and
TPH expression in the dorsal raphe nucleus
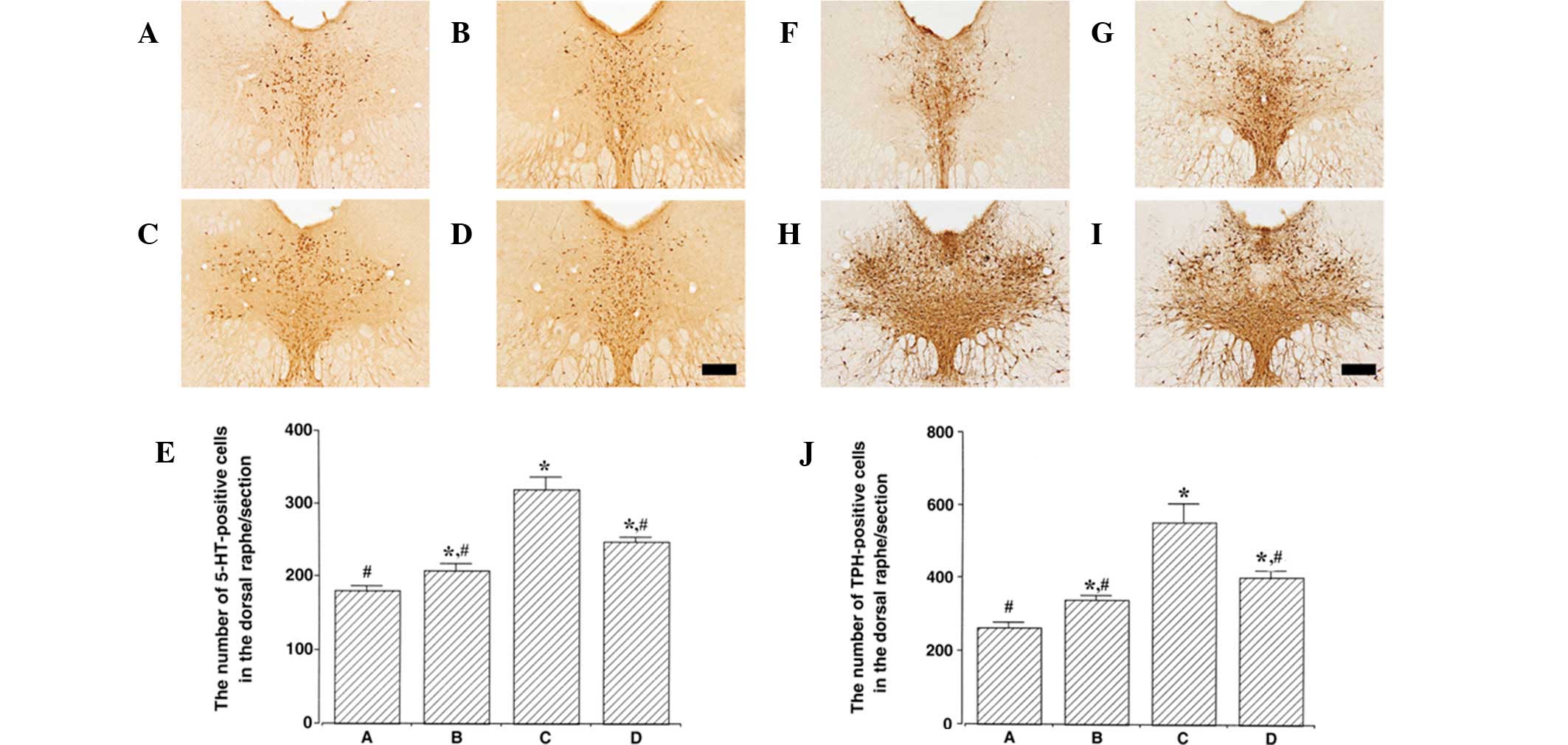
Photomicrographs of 5-HT- and TPH-positive cells in
the dorsal raphe nucleus are presented in Fig. 2. The number of 5-HT-positive cells
was 180.50±6.93 in control, 208.00±9.56 in control exercise,
320.00±16.26 in PTSD-induced and 247.60±6.66 in the PTSD-induced
and exercise group. Expression of 5-HT in the dorsal raphe nucleus
was found to be significantly increased in the PTSD-induced group
compared with the control (P<0.05). Treadmill exercise
suppressed 5-HT expression in the dorsal raphe nucleus of the
PTSD-induced group (P<0.05). In normal rats, treadmill exercise
slightly increased 5-HT expression in the dorsal raphe nucleus
(P<0.05).
The number of TPH-positive cells was 262.00±15.46 in
control, 339.50±11.55 in control exercise, 551.66±50.89 in
PTSD-induced and 402.00±15.74 in the PTSD-induced and exercise
group. TPH expression in the dorsal raphe nucleus was increased in
the PTSD-induced group compared with control (P<0.05). Treadmill
exercise suppressed TPH expression in the dorsal raphe nucleus of
the PTSD-induced group (P<0.05). In normal rats, treadmill
exercise slightly increased TPH expression in the dorsal raphe
nucleus (P<0.05).
Effect of treadmill exercise on c-Fos
expression in the hypothalamus and locus coeruleus
Photomicrographs of c-Fos-positive cells in the
hypothalamus and locus coeruleus are presented in Fig. 3. The number of c-Fos-positive cells
in the hypothalamus was 111.37±16.35 in control, 113.50±8.15 in
control exercise, 170.00±8.47 in PTSD-induced and 117.75±20.16 in
the PTSD-induced and exercise group. c-Fos expression in the
hypothalamus was increased in the PTSD-induced group compared with
control (P<0.05). Treadmill exercise suppressed c-Fos expression
in the hypothalamus of the PTSD-induced group (P<0.05). In
normal rats, treadmill exercise was identified to have no
significant effect on c-Fos expression in the hypothalamus.
The number of c-Fos-positive cells in the locus
coeruleus was 35.58±5.30 in control, 56.40±6.33 in control
exercise, 71.53±7.34 in PTSD-induced and 37.78±5.96 in the
PTSD-induced and exercise group. c-Fos expression in the locus
coeruleus was increased in the PTSD-induced group compared with the
control (P<0.05). Treadmill exercise suppressed c-Fos expression
in the locus coeruleus of the PTSD-induced group (P<0.05). In
normal rats, treadmill exercise increased c-Fos expression in the
locus coeruleus (P<0.05).
Effect of treadmill exercise on nNOS
expression in the hypothalamus and locus coeruleus
Data concerning the nNOS-positive cells in the
hypothalamus and locus coeruleus are presented in Fig. 4. The number of nNOS-positive cells
in the hypothalamus was 64.11±4.82 in control, 63.66±4.13 in
control exercise, 103.64±4.17 in PTSD-induced and 83.60±2.96 in the
PTSD-induced and exercise group. nNOS expression in the
hypothalamus was increased in the PTSD-induced group compared with
the control (P<0.05). Treadmill exercise suppressed nNOS
expression in the hypothalamus of the PTSD-induced group
(P<0.05). In normal rats, treadmill exercise was found to have
no significant affect on nNOS expression in the hypothalamus.
The number of nNOS-positive cells in the locus
coeruleus was 64.11±4.82 in control, 63.66±4.13 in control
exercise, 103.64±4.17 in PTSD-induced and 83.60±2.96 in the
PTSD-induced and exercise group. nNOS expression in the locus
coeruleus was increased in the PTSD-induced group compared with the
control (P<0.05). Treadmill exercise suppressed nNOS expression
in the locus coeruleus of the PTSD-induced group (P<0.05). In
normal rats, treadmill exercise was identified to have no
significant effect on nNOS expression in the locus coeruleus.
Discussion
Exercise is known to reduce symptoms of depression
and anxiety (25–27). In a previous study, aerobic
exercise was reported to reduce childhood PTSD, depression and
anxiety (26). In addition, a
study utilizing an animal model identified that wheel running
reversed a long-lasting interference with shuttle box escape
produced by uncontrollable stress (33). Other studies have documented the
efficacy of aerobic exercise on depressive disorders to be
comparable to antidepressant medication (25,27).
In the present study, the anxiolytic effect of treadmill exercise
on PTSD was investigated through analysis of 5-HT, TPH, c-Fos and
nNOS expression levels in the brain.
PTSD is characterized mainly by symptoms of
re-experiencing, avoidance and hyperarousal as a consequence of
catastrophic and traumatic events that are distinguished from
ordinary stressful life events. The EPM test is an unconditioned
test for anxiety in rodents in which animals exposed to stress
spend a reduced duration of time in the open arms of the test and
increased time in the closed arms (30,31).
In the present study, the rats in the PTSD-induced group were
observed to spend less time in the open arms and more time in the
closed arms compared with the rats in the control group. These
results indicate that exposure to the hunting dog during pregnancy
induced PTSD in the maternal rats following delivery. Treadmill
exercise significantly increased the time spent in the open arms
and decreased the time spent in the closed arms in the PTSD-induced
group, demonstrating that treadmill exercise during pregnancy
reduced the anxiety-like behaviors of the PTSD-induced maternal
rats. Previously, treadmill running in rats was reported to reduce
anxiety-like behavior in the EPM test without a significant change
in total activity in the open field test (34).
5-HT neurons in the dorsal raphe nucleus have been
implicated in the stress-induced changes in behavior. The dorsal
raphe nuclei are the major source of 5-HT innervation of the
forebrain and are critical for response to stress. 5-HT, as well as
noradrenaline and γ-aminobutyric acid, are important
neurotransmitters implicated in anxiety (35). In a competitive study, losing male
Syrian hamsters exhibited increased c-Fos immunoreactivity in the
dorsal raphe nucleus neurons compared with winners or controls,
indicating that social stress activates 5-HT neurons in the dorsal
raphe nucleus, reduces 5-HT1A autoreceptor-mediated
inhibition and induces hyperactivity of 5-HT neurons (36). Inescapable shock and social defeat
increased extracellular levels of 5-HT within the dorsal raphe
nucleus in rats (16). The number
and density of serotonergic neurons was also found to be higher in
suicide victims than controls (11). TPH is the rate-limiting enzyme in
serotonin production and its expression is used as an indicator of
5-HT synthesis (10). A previous
study found that levels of TPH immunoreactivity were higher in
suicides than controls in the dorsal raphe nucleus but were not
identified to be different in the median raphe nucleus (10). The expression of the
5-HT1A receptor in the dorsal raphe nucleus was
increased following prolonged stress exposure, indicating that an
increase in 5-HT1A receptor levels in the dorsal raphe
nucleus may be important in the pathogenesis of PTSD rats (37). Liu et al(38) reported that neuronal apoptosis by
chronic stress induced PTSD, hypothesizing that the disease is
associated with 5-HT1A receptor activity. In the current
study, the expression levels of 5-HT and TPH were increased in the
dorsal raphe nucleus of the PTSD-induced rats, indicating that
exposure to the predator during pregnancy caused stress, which led
to PTSD in maternal rats following delivery. By contrast, treadmill
exercise decreased the expression of 5-HT and TPH in the dorsal
raphe nuclei of the PTSD-induced rats, indicating that treadmill
exercise during pregnancy may alleviate the aforementioned stress.
These observations are consistent with additional studies
demonstrating that voluntary exercise reduces the incidence of
stress-related psychiatric disorders in humans and prevents
serotonin-dependent behavioral consequences of stress in rodents
(39).
The primary component of the stress response is the
activation of the hypothalamic-pituitary-adrenal axis. This axis is
a feedback loop containing a complex set of direct effects and
feedback interactions between the hypothalamus, pituitary and
adrenal glands. The hypothalamic paraventricular nucleus receives
direct and indirect inputs from the hippocampus and amygdala and is
central to orchestration of the physiological response to stress
(40,41).
c-Fos expression has been used to map the pattern of
neural activation following either a single or repeated exposure of
aggressive interaction (14,15,42).
Expression of c-Fos following stress is highly region-specific,
including the paraventricular nucleus and locus coeruleus, and is
involved in adaptation to social stress (14). Noise-induced c-Fos mRNA expression
in the paraventricular nucleus has been markedly correlated with
levels of plasma adrenocorticotropin hormone (43). c-Fos expression in the
paraventricular nucleus and locus coeruleus is increased by single
restraint and these increments are attenuated by repeated restraint
stress (44). Restraint stress
enhances c-Fos expression in neurons of the paraventricular
nucleus, locus coeruleus, supraoptic nucleus, rostral raphe
pallidus, nucleus of the solitary tract and ventrolateral medulla
(45). In the current study,
expression of c-Fos in the hypothalamic paraventricular nucleus and
locus-related stress during pregnancy led to neuronal activation in
stress-related areas of the brain. By contrast, treadmill exercise
decreased the expression of c-Fos in the PTSD-induced rats,
indicating that treadmill exercise during pregnancy suppressed
neuronal activation induced by the stress exposure. These results
are consistent with previous observations that uncontrollable
stress increased c-Fos expression in the dorsal raphe nucleus and
wheel running attenuated uncontrollable stress-induced c-Fos
expression in the dorsal raphe nucleus (39).
A number of studies have indicated that NOS is
involved in anxiety-related behaviors (46,47).
nNOS overexpression in the hippocampus is essential for chronic
stress-induced depression and inhibition of nNOS signaling in the
brain may represent a novel approach for the treatment of
depressive disorders (48).
Inhibition of nNOS was previously reported to prevent
N-methyl-D-aspartate receptor activation-induced
anxiogenic-like effect in mice (46). In the present study, expression of
NOS in the hypothalamic paraventricular nucleus and locus coeruleus
were increased in PTSD-induced rats, demonstrating that exposure to
the hunting dog during pregnancy induced anxiety in the maternal
rats following delivery. By contrast, treadmill exercise decreased
the expression of NOS in PTSD-induced rats, indicating that
treadmill exercise during pregnancy evoked an anxiolytic effect
against stress exposure. Workman et al(47) demonstrated that nNOS inhibition
reduced anxiety-like responses, concluding that NO may be important
for mediating the effects of social interactions on anxiety.
In conclusion, exposure to a predator during
pregnancy induced anxiety-like behaviors with enhancement of
anxiety-related neurochemical parameters, including 5-HT, TPH,
c-Fos and nNOS, in the brains of maternal rats following delivery.
Treadmill exercise during pregnancy had an anxiolytic effect that
alleviated the anxiety-induced increase of 5-HT, TPH, c-Fos and
nNOS expression in the brain of the maternal rats. The present
results are consistent with the hypothesis that exercise during
pregnancy may be suitable as a therapeutic strategy to reduce
anxiety-related disorders, including PTSD.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea funded by the Korean Government
(NRF-2010-327-G00123).
References
|
1
|
Cryan JF and Holmes A: The ascent of
mouse: advances in modelling human depression and anxiety. Nat Rev
Drug Discov. 4:775–790. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Myers KM and Davis M: Mechanisms of fear
extinction. Mol Psychiatry. 12:120–150. 2007. View Article : Google Scholar
|
|
3
|
Yehuda R: Post-traumatic stress disorder.
N Engl J Med. 346:108–114. 2002. View Article : Google Scholar
|
|
4
|
Baker DG, Nievergelt CM and Risbrough VB:
Post-traumatic stress disorder: emerging concepts of
pharmacotherapy. Expert Opin Emerg Drugs. 14:251–272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davis LL, Frazier EC, Williford RB and
Newell JM: Long-term pharmacotherapy for post-traumatic stress
disorder. CNS Drugs. 20:465–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bannai M, Fish EW, Faccidomo S and Miczek
KA: Anti-aggressive effects of agonists at 5-HT1B
receptors in the dorsal raphe nucleus of mice. Psychopharmacology
(Berl). 193:295–304. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barnes NM and Sharp T: A review of central
5-HT receptors and their function. Neuropharmacology. 38:1083–1152.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cooper MA, McIntyre KE and Huhman KL:
Activation of 5-HT1A autoreceptors in the dorsal raphe
nucleus reduces the behavioral consequences of social defeat.
Psychoneuroendocrinology. 33:1236–1247. 2008.
|
|
9
|
Graeff FG and Zangrossi H Jr: The dual
role of serotonin in defense and the mode of action of
antidepressants on generalized anxiety and panic disorders. Cent
Nerv Syst Agents Med Chem. 10:207–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boldrini M, Underwood MD, Mann JJ and
Arango V: More tryptophan hydroxylase in the brainstem dorsal raphe
nucleus in depressed suicides. Brain Res. 1041:19–28. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Underwood MD, Khaibulina AA, Ellis SP,
Moran A, Rice PM, Mann JJ and Arango V: Morphometry of the dorsal
raphe nucleus serotonergic neurons in suicide victims. Biol
Psychiatry. 46:473–483. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chamas FM, Underwood MD, Arango V, Serova
L, Kassir SA, Mann JJ and Sabban EL: Immobilization stress elevates
tryptophan hydroxylase mRNA and protein in the rat raphe nuclei.
Biol Psychiatry. 55:278–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim SU, Ko IG, Kim BK, et al:
Transplantation of human adipose-derived stem cells into the
urethra ameliorates stress urinary incontinence and blunts the
induction of c-Fos immunoreactivities in brain areas related to
micturition in female rats. Anim Cells Syst. 14:237–244. 2010.
View Article : Google Scholar
|
|
14
|
Martinez M, Phillips PJ and Herbert J:
Adaptation in patterns of c-fos expression in the brain associated
with exposure to either single or repeated social stress in male
rats. Eur J Neurosci. 10:20–33. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weinberg MS, Girotti M and Spencer RL:
Restraint-induced fra-2 and c-fos expression in the rat forebrain:
relationship to stress duration. Neuroscience. 150:478–486. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amat J, Aleksejev RM, Paul E, Watkins LR
and Maier SF: Behavioral control over shock blocks behavioral and
neurochemical effects of later social defeat. Neuroscience.
165:1031–1038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Forestiero D, Manfrim CM, Guimarães FS and
de Oliveira RM: Anxiolytic-like effects induced by nitric oxide
synthase inhibitors microinjected into the medial amygdala of rats.
Psychopharmacology (Berl). 184:166–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pokk P and Väli M: The effects of the
nitric oxide synthase inhibitors on the behaviour of
small-platform-stressed mice in the plus-maze test. Prog
Neuropsychopharmacol Biol Psychiatry. 26:241–247. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bredt DS, Glatt CE, Hwang PM, Fotuhi M,
Dawson TM and Snyder SH: Nitric oxide synthase protein and mRNA are
discretely localized in neuronal populations of the mammalian CNS
together with NADPH diaphorase. Neuron. 7:615–624. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dawson TM, Bredt DS, Fotuhi M, Hwang PM
and Snyder SH: Nitric oxide synthase and neuronal NADPH diaphorase
are identical in brain and peripheral tissues. Proc Natl Acad Sci
USA. 88:7797–7801. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jesse CR, Bortolatto CF, Savegnago L,
Rocha JB and Nogueira CW: Involvement of L-arginine- nitric
oxide-cyclic guanosine monophosphate pathway in the
antidepressant-like effect of tramadol in the rat forced swimming
test. Prog Neuropsychopharmacol Biol Psychiatry. 32:1838–1843.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colcombe S and Kramer AF: Fitness effects
on the cognitive function of older adults: a meta-analytic study.
Psychol Sci. 14:125–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Galper DI, Trivedi MH, Barlow CE, Dunn AL
and Kampert JB: Inverse association between physical inactivity and
mental health in men and women. Med Sci Sports Exerc. 38:173–178.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rovio S, Kåreholt I, Helkala EL, Viitanen
M, Winblad B, Tuomilehto J, Soininen H, Nissinen A and Kivipelto M:
Leisure-time physical activity at midlife and the risk of dementia
and Alzheimer's disease. Lancet Neurol. 4:705–711. 2005. View Article : Google Scholar
|
|
25
|
Blumenthal JA, Babyak MA, Doraiswamy PM,
et al: Exercise and pharmacotherapy in the treatment of major
depressive disorder. Psychosom Med. 69:587–596. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Newman CL and Motta RW: The effects of
aerobic exercise on childhood PTSD, anxiety and depression. Int J
Emerg Ment Health. 9:133–158. 2007.PubMed/NCBI
|
|
27
|
Perraton LG, Kumar S and Machotka Z:
Exercise parameters in the treatment of clinical depression: a
systematic review of randomized controlled trials. J Eval Clin
Pract. 16:597–604. 2010.PubMed/NCBI
|
|
28
|
Sung YH, Shin MS, Cho S, Baik HH, Jin BK,
Chang HK, Lee EK and Kim CJ: Depression-like state in maternal rats
induced by repeated separation of pups is accompanied by a decrease
of cell proliferation and an increase of apoptosis in the
hippocampus. Neurosci Lett. 470:86–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Adamec R, Walling S and Burton P:
Long-lasting, selective, anxiogenic effects of feline predator
stress in mice. Physiol Behav. 83:401–410. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kung JC, Chen TC, Shyu BC, Hsiao S and
Huang AC: Anxiety- and depressive-like responses and c-fos activity
in preproenkephalin knockout mice: oversensitivity hypothesis of
enkephalin deficit-induced posttraumatic stress disorder. J Biomed
Sci. 17:29–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sterley TL, Howells FM and Russell VA:
Effects of early life trauma are dependent on genetic
predisposition: a rat study. Behav Brain Funct. 7:11–25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim EK, Lee MH, Kim H, et al: Maternal
ethanol administration inhibits 5-hydroxytryptamine synthesis and
tryptophan hydroxylase expression in the dorsal raphe of rat
offspring. Brain Dev. 27:472–476. 2005. View Article : Google Scholar
|
|
33
|
Greenwood BN, Strong PV, Dorey AA and
Fleshner M: Therapeutic effects of exercise: wheel running reverses
stress-induced interference with shuttle box escape. Behav
Neurosci. 121:992–1000. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fulk LJ, Stock HS, Lynn A, Marshall J,
Wilson MA and Hand GA: Chronic physical exercise reduces
anxiety-like behavior in rats. Int J Sports Med. 25:78–82. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sandford JJ, Argyropoulos SV and Nutt DJ:
The psychobiology of anxiolytic drugs. Part 1: Basic neurobiology.
Pharmacol Ther. 88:197–212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cooper MA, Grober MS, Nicholas CR and
Huhman KL: Aggressive encounters alter the activation of
serotonergic neurons and the expression of 5-HT1A mRNA
in the hamster dorsal raphe nucleus. Neuroscience. 161:680–690.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo FF, Han F and Shi YX: Changes in
5-HT1A receptor in the dorsal raphe nucleus in a rat
model of post-traumatic stress disorder. Mol Med Rep. 4:843–847.
2011.
|
|
38
|
Liu H, Wang HT, Han F and Shi YX: Activity
of 5-HT1A receptor is involved in neuronal apoptosis of
the amygdala in a rat model of post-traumatic stress disorder. Mol
Med Rep. 4:291–295. 2011.
|
|
39
|
Greenwood BN, Foley TE, Burhans D, Maier
SF and Fleshner M: The consequences of uncontrollable stress are
sensitive to duration of prior wheel running. Brain Res.
1033:164–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Herman JP, Ostrander MM, Mueller NK and
Figueiredo H: Limbic system mechanisms of stress regulation:
hypothalamopituitary-adrenocortical axis. Prog Neuropsychopharmacol
Biol Psychiatry. 29:1201–1213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jankord R and Herman JP: Limbic regulation
of hypothalamopituitary-adrenocortical function during acute and
chronic stress. Ann NY Acad Sci. 1148:64–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Mahony CM, Sweeney FF, Daly E, Dinan TG
and Cryan JF: Restraint stress-induced brain activation patterns in
two strains of mice differing in their anxiety behaviour. Behav
Brain Res. 213:148–154. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Burow A, Day HE and Campeau S: A detailed
characterization of loud noise stress: intensity analysis of
hypothalamo-pituitary-adrenocortical axis and brain activation.
Brain Res. 1062:63–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY
and Suh HW: The effect of single or repeated restraint stress on
several signal molecules in paraventricular nucleus, arcuate
nucleus and locus coeruleus. Neuroscience. 142:1281–1292. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goebel M, Stengel A, Wang L and Taché Y:
Restraint stress activates nesfatin-1-immunoreactive brain nuclei
in rats. Brain Res. 1300:114–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miguel TT and Nunes-de-Souza RL:
Anxiogenic-like effects induced by NMDA receptor activation are
prevented by inhibition of neuronal nitric oxide synthase in the
periaqueductal gray in mice. Brain Res. 1240:39–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Workman JL, Trainor BC, Finy MS and Nelson
RJ: Inhibition of neuronal nitric oxide reduces anxiety-like
responses to pair housing. Behav Brain Res. 187:109–115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X,
Zhu XJ, Wang B, Xu JS and Zhu DY: Neuronal nitric oxide synthase
contributes to chronic stress induced depression by suppressing
hippocampal neurogenesis. J Neurochem. 103:1843–1854. 2007.
View Article : Google Scholar : PubMed/NCBI
|