Introduction
Bioactive glasses have attractive behaviors for
biomedical substitutes, and provide a biological alternative for
bone and tissue regeneration (1,2). For
example, silicate-based bioglass, such as 45S5 glass (3), has been most widely studied for its
biomedical applications (4). Due
to its incomplete degradation behaviors (5), 45S5 glass has gradually been replaced
by the bioactive borate glass (6),
which has a similar composition to 45S5 glass, but all the
SiO2 is replaced with B2O3. The
new composition has a faster degradation ability and a higher
conversion rate than silicate 45S5 glass. It has been suggested
that borate glass could be applied in the field of tissue
engineering (7,8).
To the best of our knowledge, strontium is a trace
element in humans and has been applied as a medication, such as
strontium ranelate in the treatment of osteoporosis.
Sr2+ has been found to promote osteogenic
differentiation of mesenchymal stem cells (MSCs) (9–11),
stimulate osteoblast proliferation (12,13)
and accelerate osteoclast apoptosis (14). Moreover, incorporating strontium
into bioactive glass improves the bioactivity of bone repair
(15) and bone formation (16). Therefore, we hypothesized that
strontium-containing bioactive borate glass would have improved
cell compatibility in tissue engineering.
The purpose of our study was to evaluate the
proliferation and differentiation effects of strontium in borate
glass on MSCs in vitro. Additionally, we attempted to
determine an appropriate composition of strontium in borate glass
for future applications in vivo.
Materials and methods
Sample preparation
Biomaterials synthesis
The preparation of the glass samples by melting and
casting processes was performed as described previously (16,17).
Generally, the glass samples with the composition of
Na2O-K2O-MgO-CaO-B2O3-SiO2-SrO-P2O5
(Table I) were prepared by melting
each analytical chemical in a platinum crucible at 1,500°C for 2 h
with stirring, and then quenching the melt between cold stainless
steel plates into glass flakes. These flakes were used as glass
wafers (1-mm thick) in the assay experiments, while some of the
glass flakes were crushed into fine particles (105–125 μm in size)
and used for the extraction experiments.
 | Table IComposition of the groups (mol%). |
Table I
Composition of the groups (mol%).
| Group |
Na2O | K2O | MgO | CaO | SrO |
SiO2 |
B2O3 |
P2O5 |
|---|
| A: 0Sr | 6 | 8 | 8 | 22 | 0 | 18 | 36 | 2 |
| B: 3Sr | 6 | 8 | 5 | 22 | 3 | 18 | 36 | 2 |
| C: 6Sr | 6 | 8 | 2 | 22 | 6 | 18 | 36 | 2 |
| D: 9Sr | 6 | 8 | 0 | 21 | 9 | 18 | 36 | 2 |
| E: 12Sr | 6 | 8 | 0 | 18 | 12 | 18 | 36 | 2 |
Prior to all the experiments, the particles or
flakes were soaked in 0.025 M K2HPO4 solution
for 10 days to allow for the superficial reaction and to avoid the
fast borate-releasing stage occurring during the following the
experimental processes (18,19).
Scanning electron microscope (SEM) observation was performed
following the soaking of samples and the partial conversion to
hydroxyapatite on the surface of borate glass particles, and prior
to commencing the experiments (Fig.
1).
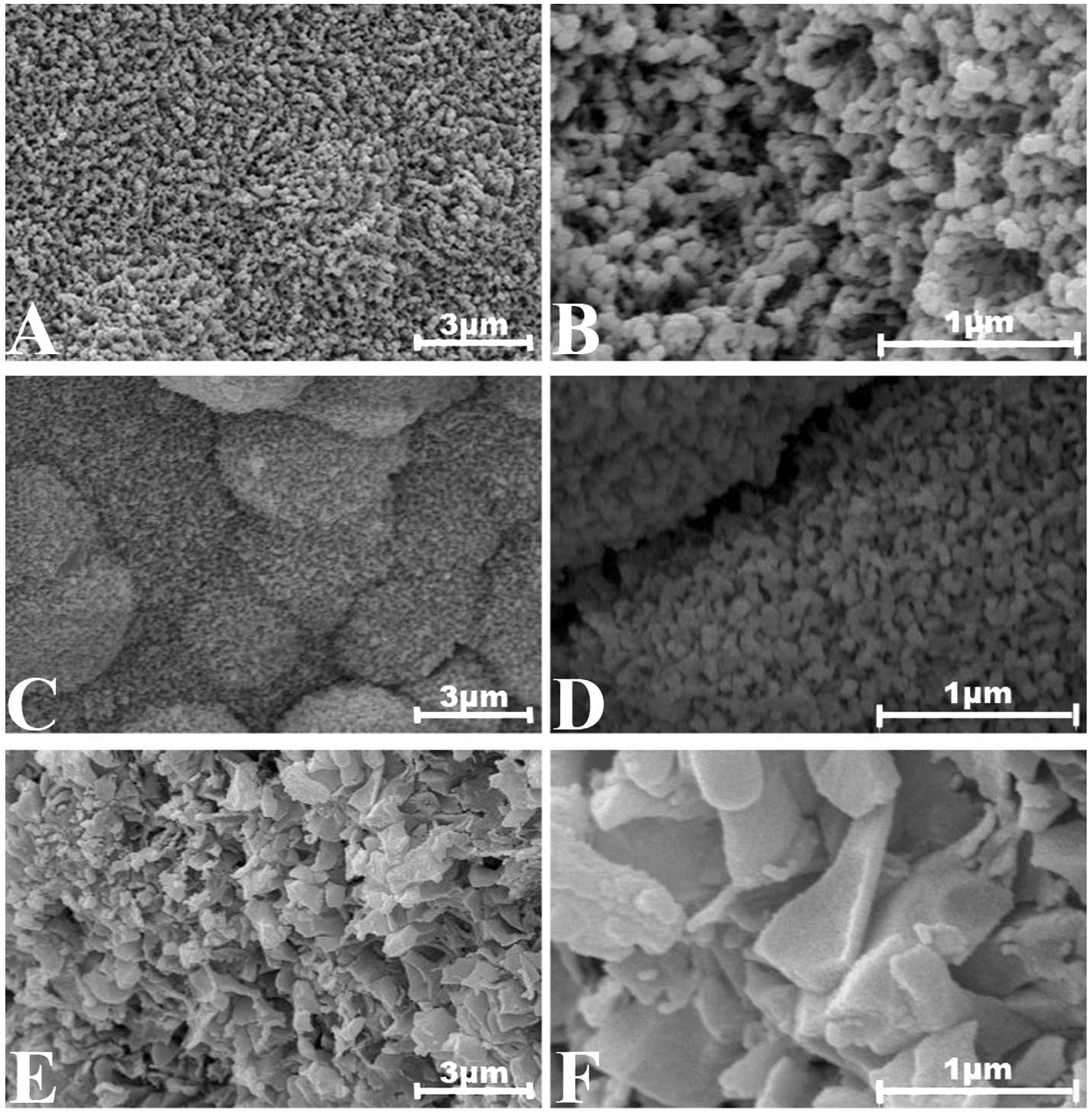 | Figure 1Scanning electron microscope (SEM)
micrographs of the surfaces of the borate glass particles used in
the cell culture experiments after soaking for 10 days at 37°C in
the 0.025 M K2HPO4 solutions: (A) 3Sr group,
×10,000; (B) 3Sr group, ×40,000 times; (C) 6Sr group, ×10,000; (D)
6Sr group, ×40,000; (E) 12Sr group, ×10,000; (F) 12Sr group,
×40,000. |
Sample degradation
Assessment of the in vitro degradation of
strontium in borate glass for each group was conducted by
statically immersing the glass particles of each group (1 g) in
0.02 M K2HPO4 solution (100 ml; pH 7.0) at
37°C for 28 days. The strontium in the immersion solution at
different time points was analyzed by ion chromatography (DX 500
Dionex, USA). The average values were obtained from three parallel
experiment samples. The accumulated released irons of strontium at
different time points were calculated by the weight of the
strontium in the solution divided by that in the original
samples.
MSC culture
Canine bone marrow-derived mesenchymal stem cells
(MSCs) were used in the present experiment. The animal study was
approved by the Committee of Medical Ethics and the Institutional
Review Board of Shanghai Sixth People’s Hospital, Shanghai Jiaotong
University (China). MSCs were isolated by density gradient
centrifugation following iliac bone marrow aspiration. The cells
were resuspended in Dulbecco’s modified Eagle’s medium (DMEM;
Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS; Thermo Scientific, HyClone, Logan, UT, USA), 100 IU/ml
penicillin (Gibco-BRL) and 100 lg/ml streptomycin (Gibco-BRL), and
cultured at 37°C in 5% CO2 and 95% humidity. After
culturing for 7 days in the 75-cm2 flask, the adherent
cells (passage 0) were washed with phosphate-buffered saline (PBS),
and then fresh medium was added every 3–4 days. Following the
initiation of cultures for 2 weeks, the cells were washed with PBS,
and then lifted by incubation in 0.5 ml of 0.25% trypsin and 1 mM
ethylenediaminetetraacetic acid for 2 min at room temperature.
Trypsin was neutralized by adding 2 ml complete medium. Each flask
of cells was passaged into 75-cm2 culture flasks every
3–4 days. After primary culturing for 2 weeks, the second passage
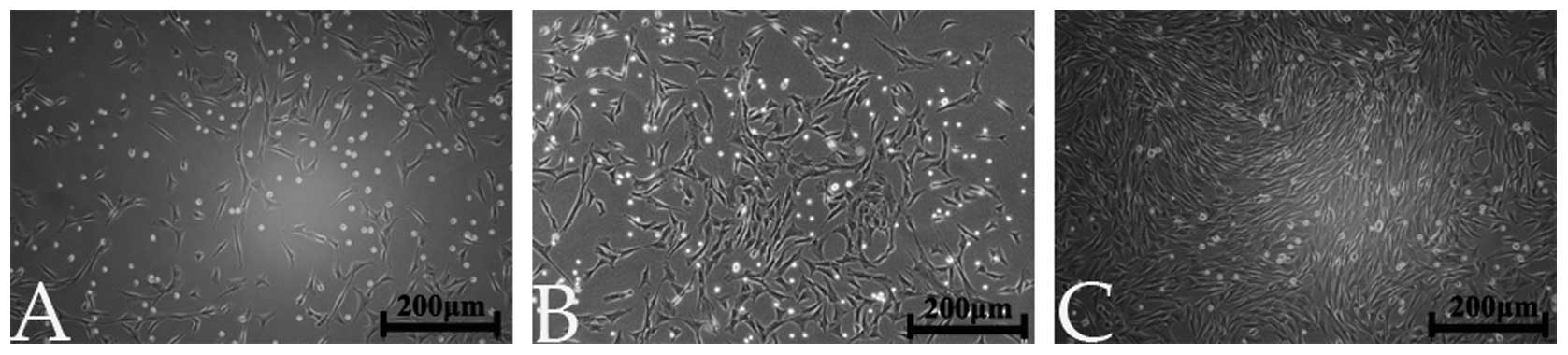
MSCs were successfully harvested and used in this study (Fig. 2).
Proliferation
Cell Counting Kit-8 (CCK-8) assay
The extraction solutions were prepared by immersing
75 mg partially converted glass particles (average diameter,
105–125 μm) in 10 ml culture medium in a CO2 incubator
at 37°C for 24 h, to ensure the specific surface areas reached 6
cm2/ml (according to DS/EN ISO 10993-12:2009).
Subsequently, MSCs (passage 2) were seeded in 96-well plates at a
density of 1×104 cells/well in 100 μl FBS culture
medium, in a CO2 incubator at 37°C for 24 h. The medium
in the well was then replaced with the prepared extraction
solution. After 3 days, the CCK-8 (Dojindo Molecular Technologies,
Inc., Rockville, MD, USA) with 10 μl CCK-8 was added to each well
using a repeating pipettor, and then mixed on an orbital shaker for
1 min. Following incubation for 2 h, the absorbance of each well
was measured spectrophotometrically at 450 nm. Three parallel
replicates of each sample at each time point were prepared during
the cell viability assay. Statistical analyses were performed using
a one-way ANOVA.
Cell morphology
The 60-mm petri dishes with affixed partially
converted glass particles (105–125 μm; 6 cm2/ml) for
each group (A-E, as in Table I)
were placed on a small platform rocker in the CO2
incubator to gently mix in 10 ml culture medium prior to
incubation. The rocking cycle consisted of 1 min on and 5 min off.
Passage 2 MSCs cells were seeded with culture medium at an initial
density of 15,000 cells/cm2 and incubated at 37°C in a
humidified atmosphere of 5% CO2. The phase contrast
images of MSCs at the interface of each group were observed after 3
and 7 days of culturing.
Live-Dead cell staining
Similarly, MSCs cells were seeded in the medium
containing each group of glass particles (6cm2/ml) at a
density of 15,000 cells/cm2. After culturing for 7 days,
the cells mixed with particles were rinsed with warm PBS twice and
incubated for an additional 30 min in serum-free DMEM containing 2
mM calcein acetoxymethyl ester (calcein AM; Biotium, Hayward, CA,
USA) and 1 μg/ml propidium iodide (Sigma-Aldrich, USA). The
fluorochrome-labeled cultures on the glass wafers were then rinsed
with PBS and examined under an epifluorescent microscope fitted
with appropriate exciter and emitter filters to detect live (green
fluorescent) and dead (red fluorescent) cells.
DNA quantification
The global effects of the glass particles with
different compositions on the proliferation of MSC cells were
assessed by measuring the total cellular DNA content in cultures
incubated with the glass particles in place. Triplicate samples of
each glass group were added to MSC cultures in 60-mm petri dishes
and incubated for 7 days. Following incubation, the cultures were
rinsed twice with PBS. The DNA on all dishes was extracted using
the TIANamp Genomic DNA kit (Tiangen Biotech, Beijing, China)
according to the manufacturer’s instructions. The quantification of
DNA concentration in each group was measured by a UV
spectrophotometer (Eppendorf, Hamburg, Germany).
Differentiation
MSCs were cultured in 60-mm petri dishes affixed
with partially converted glass particles for each group (A-E, as in
Table I) at an initial seeding
density of 15,000 cells/cm2. DMEM supplemented with 10%
fetal bovine serum and 1% antibiotics mixture (100 μg/ml of
penicillin and streptomycin) were used as the culture medium. After
being grown to confluence, the culture medium of each group was
replaced with osteogenic induction medium, containing
10−8 M dexamethasone, 0.2 mM ascorbic acid-phosphate and
10 mM β-glycerophosphate (Sigma-Aldrich, Co., St. Louis, MO, USA).
Cells were incubated at 37°C, 5% CO2 and 95% humidity,
and the medium was replaced every 2 days.
Real-time reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA of each group was extracted from cells in
the 60-mm petri dishes using TRIzol reagent (Invitrogen, Carlsbad,
CA, USA). Total RNA (2 μg) was reverse transcribed into cDNA using
the cDNA kit (Takara Biotechnology, Dalian, China) according to the
manufacturer’s instructions. Real-time PCR was then performed to
examine the expression of osteogenic genes and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels in the
MSCs. The expression levels of each gene were standardized by the
internal control levels of GAPDH. Amplification was denatured at
94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1
min; then annealed at 54°C for 30 sec and 72°C for 1 min. The
temperature was then returned to 95°C and samples were stored at
4°C overnight. These procedures were performed in a PCR machine
(Eppendorf).
The primer sequences used were: Forward: 5′-CCGCACGA
CAACCGCACCAT-3′ and reverse: 5′-CGCTCCGGCCCA CAAATCTC-3′ for core
binding factor α1 (Cbfa1); forward: 5′-CAGTAGTGACTCATCCGAAG-3′ and
reverse: 5′-CTCCTCTTCTTCTTCATCAC-3′ for bone sialoprotein (BSP);
forward: 5′-CACCGAGACACCATGAGAGC-3′ and reverse:
5′-TGGTCAGCCAACTCGTCAC-3′ for osteocalcin (OCN); forward:
5′-CTTTTAACTCTGGTAAAGTGG-3′ and reverse:
5′-TTTTGGCTCCCCCCTGCAAAT-3′ for GAPDH.
Alkaline phosphatase (ALP)
activity
After 14 and 21 days of culturing, the cells were
washed twice with PBS, placed in 500 μl of 1% Triton X-100, and
lysed using two freeze-thaw cycles (−80/37°C). Aliquots of the
lysate were placed in a 96-well plate for spectrophotometric
measurement of ALP activity with p-nitrophenyl phosphate (PNPP)
substrate, as previously described (17). ALP activity was normalized with
respect to the total protein content obtained from the same cell
lysate, and was expressed as nanomoles of PNPP formed per microgram
of protein per minute. Total protein content was determined using
the Micro-Bicinchoninic acid (BCA) Protein Assay kit (Pierce
Biotechnologies, Rockford, IL, USA), according to the
manufacturer’s instructions.
Alizarin Red S staining
Alizarin Red S staining of MSC cultures was
conducted after 21 days of culturing. The culture medium was
removed from each well and cells were rinsed three times with PBS.
After fixing with 4% formaldehyde solution for 30 min at room
temperature, the cells were rinsed twice with PBS and fixed cells
were stained with 1 ml of 2% Alizarin Red S working solution
(Sigma-Aldrich) and incubated for 10–20 min at 37°C. Cells were
then rinsed three times and visualized under an optical microscope.
The number of mineralized nodules per unit was counted.
Results
Sample preparation
Characterization of degradation
During immersion in phosphate solution, the ions of
strontium changed over time. As shown in Fig. 3, the concentration reached a stable
level after ≥20 days. From the curve, we found that in the
solution, the levels of strontium released from the borate glass
were relative to its composition in each group, the more strontium
the particles contained, the higher the concentration in the
corresponding soaking solution.
Proliferation
CCK-8 assay
Results of the CCK-8 assay demonstrated the growth
rates of the different groups relative to that of the control group
with DMEM+FBS. After culturing for three days, the relative growth
rates in the groups containing strontium were between 1.0 and 1.5.
The 6Sr group demonstrated a significant difference. However, the
0Sr group demonstrated the lowest relative growth rate (Fig. 4).
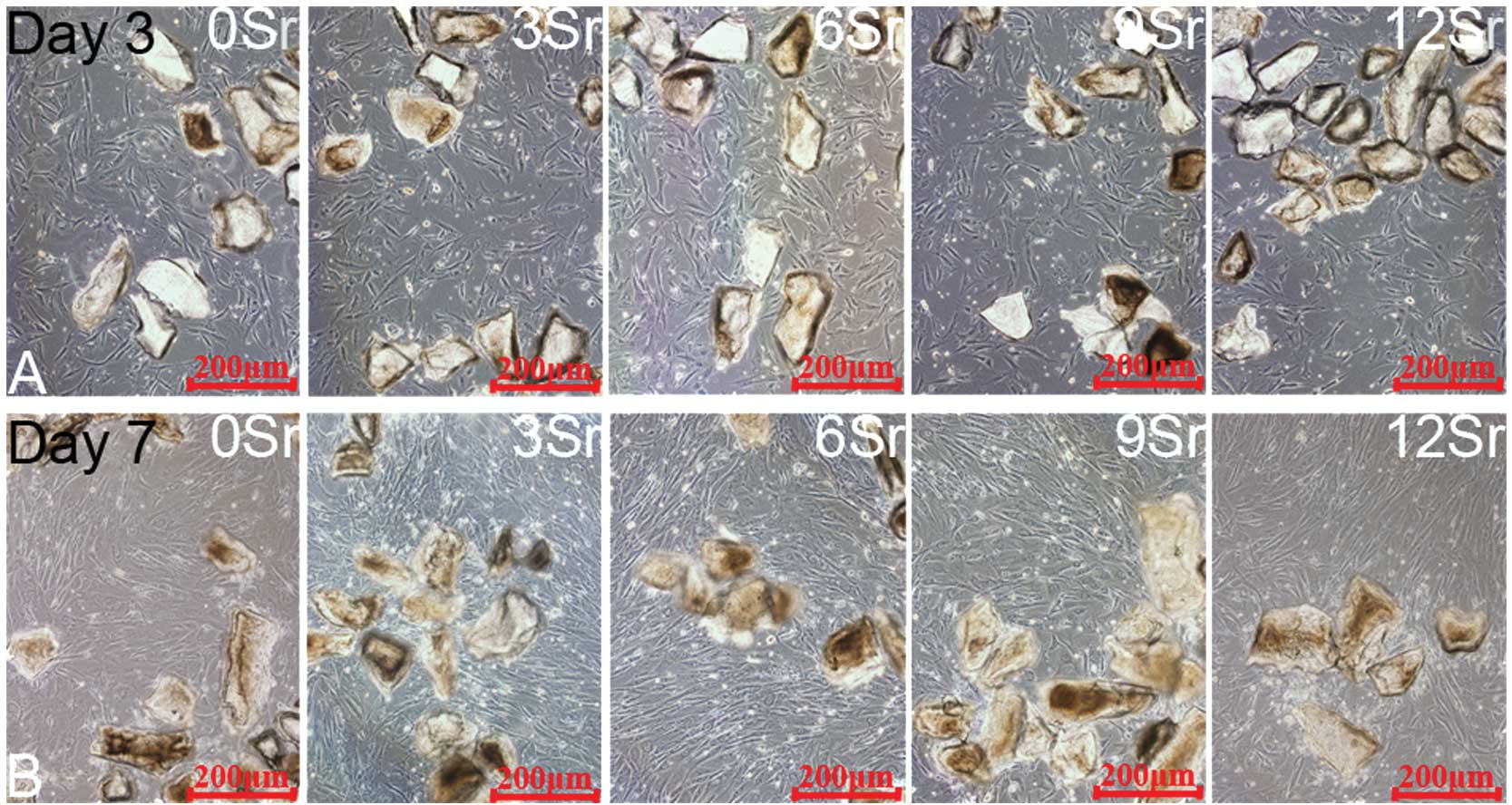
Cell morphology
Fig. 5 shows the
cell morphology (on days 3 and 7) of MSCs incubated with the
strontium-containing borate glass particles of different
compositions. The optical microscope revealed that all cells
proliferated well along the borate glass particles. Additionally,
the glass particles and the MSCs were 70% confluent, which was
consistent with the results of the CCK-8 assay. On day 7, the 6Sr
group showed the greatest amount of proliferation of MSCs among all
the groups.
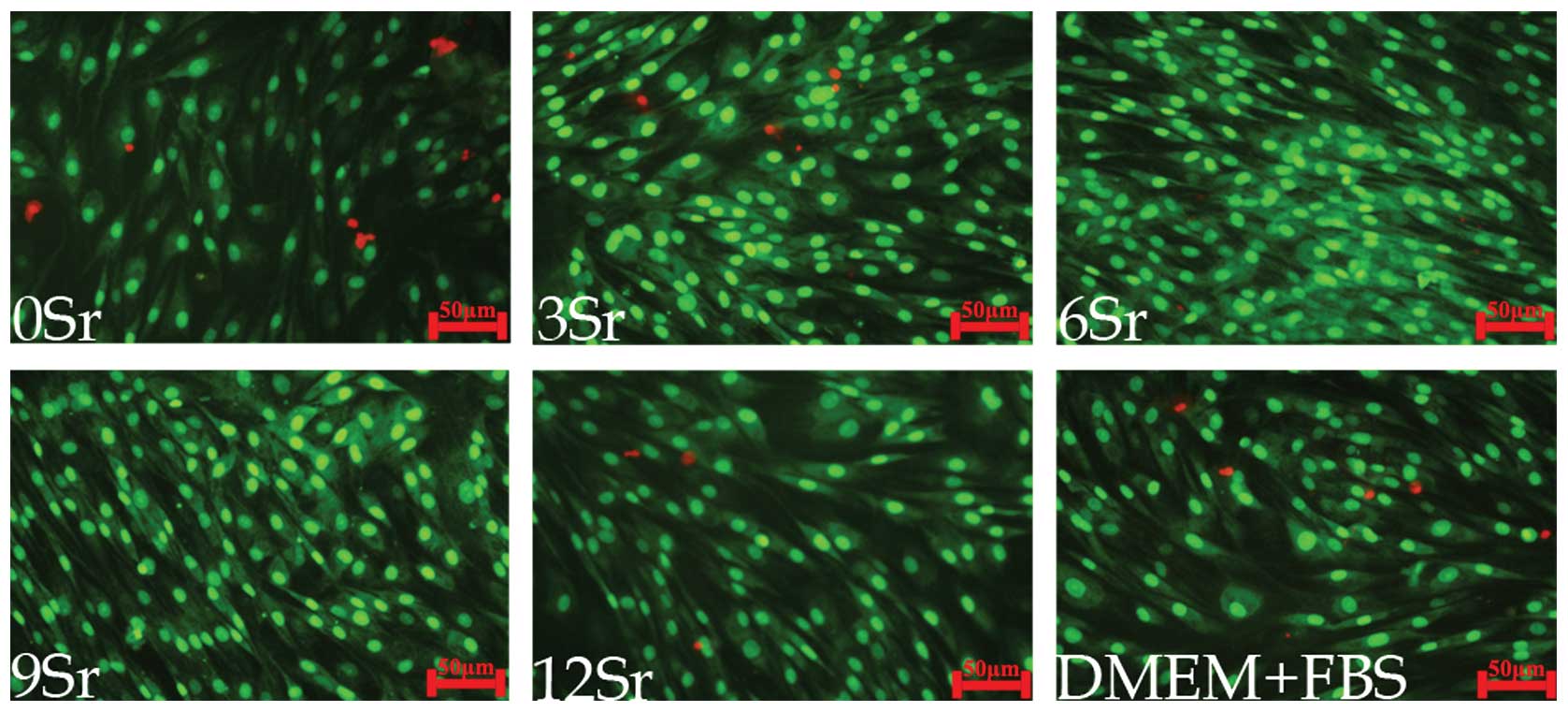
Live-Dead assay
The Live-Dead assay (Fig. 6) provided a direct observation of
the proportion of living and dead cells. All the groups showed
increased cell proliferation on day 7. The 6Sr group exhibited the
least number of dead cells and the greatest number of living cells,
on staining. Other groups showed a relatively higher proportion of
dead cells than the 6Sr group.
Total DNA concentration
Total DNA concentration, cell growth at the genetic
level, indicated that the 6Sr group demonstrated the highest
concentration, statistically, than any other group (Fig. 7).
Differentiation
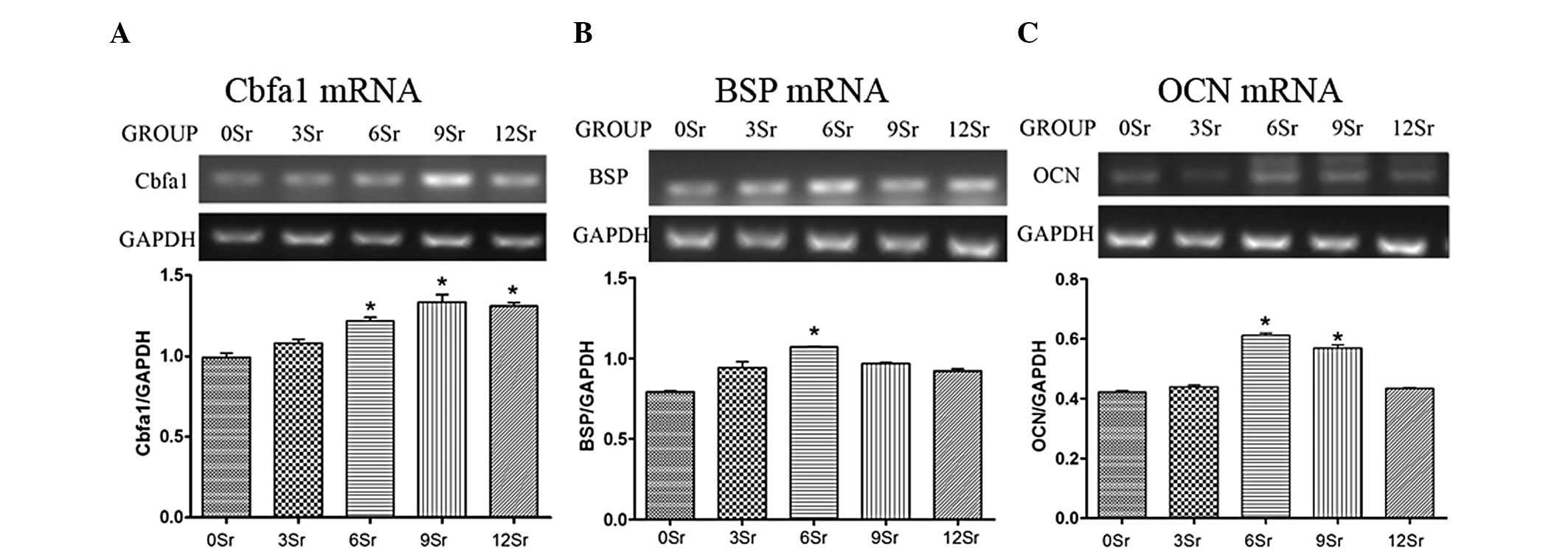
Real-time RT-PCR
In our experiment, the expression of early, middle
and late osteogenic genes in primary canine bone marrow MSCs was
examined using real-time RT-PCR on days 7, 14, and 21,
respectively, to analyse MSC maturation. It was found that on day
7, compared with the 0Sr group, the Cbfa1 mRNA expression level was
significantly higher in the 6Sr, 9Sr and 12Sr groups (P<0.01).
On day 14, compared with the 0Sr group, the BSP mRNA level was
significantly higher in the 6Sr group (P<0.01). In addition, on
day 21, similar to BSP expression, groups 6Sr and 9Sr exhibited
higher OCN mRNA expression levels than the 0Sr group (P<0.01)
(Fig. 8).
ALP activity
The results of the spectrophotometric measurement of
ALP activity of MSCs in all the groups are presented in Fig. 9. On day 14, ALP levels in groups
with strontium showed no significant differences compared with the
0Sr group. However, on day 21, the 6Sr group demonstrated a higher
ALP activity than the 0Sr group (P<0.01). Moreover, at both time
points, the groups containing strontium had a higher ALP level than
the 0Sr group. However, the difference was only statistically
significant in the 6Sr group on day 21.
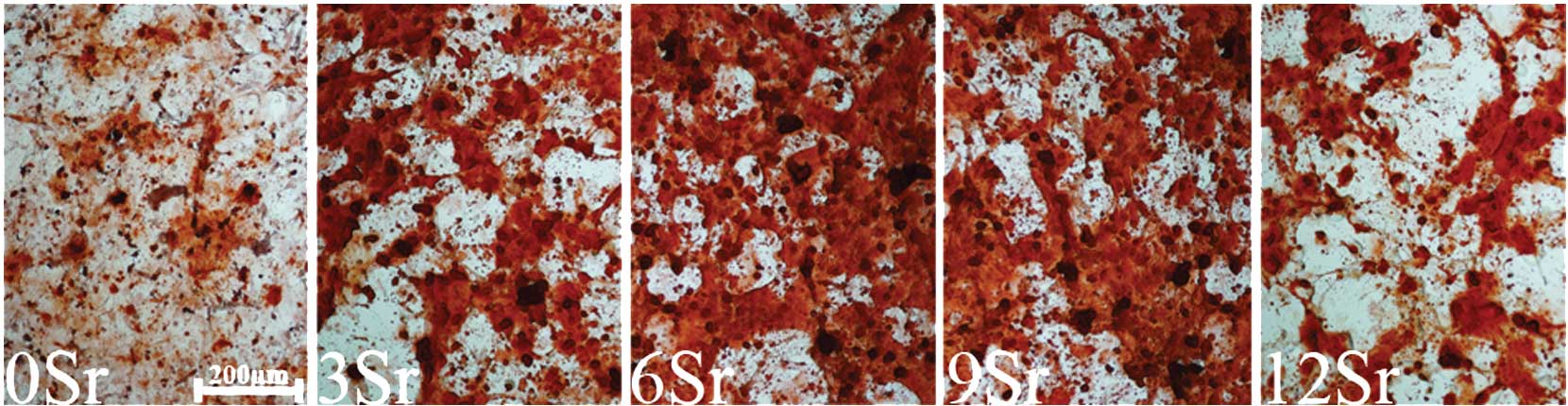
Alizarin Red S staining
Alizarin Red S staining was used to examine the
mineralized nodule formation by MSCs cultured with different
contents of strontium-incorporated borate medium for 21 days
(Fig. 10). Measured by Alizarin
Red S staining, the number of mineralized nodules per unit in MSCs
was also counted. The result showed that groups 6Sr and 9Sr had the
greatest number of mineralized nodules (Fig. 11).
Discussion
Bioglass has attracted increasing attention since it
was produced in 1971 (3,6). Bioactive glasses and glass-ceramic
systems are famous for their excellent biocompatibility and
bioactivity in bone tissue engineering (20). After advances in industrial
techniques and preparation configuration, silicate bioactive glass
has gradually been replaced by borate bioactive glass, which has a
wider range of bioactivity and degradation rates, with stronger
bone formation in combination with hydroxyapatite for tissue
regeneration. However, silicate bioactive glass has certain
disadvantages: It converts slowly and incompletely to hyaluronic
acid (HA) when placed in body fluid, and it is difficult to sinter
45S5 particles into a porous three-dimensional (3D) network
(7). Moreover, borate-based
bioactive glasses have been developed for their potential
biomedical advantages (21).
Borosilicate scaffolds with the fastest degradation
rate are toxic to cells, likely due to the high concentration of
boron ions released into the medium (15). Increased cell proliferation has
been demonstrated to occur when <2/3 of the SiO2 in
45S5 glass was replaced by B2O3 in a
borosilicate glass composition, or by partially converting the
borate glass to HA prior to cell culture (17). Alternatively, dynamic culturing
(17) and minor element
incorporation would both achieve a biologically acceptable rate of
borate release. Notably, strontium is a trace element in the human
body and has been demonstrated to play a unique role in bone
remodeling by stimulating bone formation and reducing bone
resorption (22,23). As a result, we proposed that
strontium-containing borate glass particles would have a higher
superficial degradation rate, and that an improved compatibility
and proliferation of the MSCs may be observed.
Cytocompatibility behavior with stem cells is an
important procedure to assess a novel material prior to application
in tissue engineering. In the present study, all the 6Sr groups
demonstrated a high relative growth rate after culturing for 3
days. The optical microscopy results revealed that following mixed
culturing with MSC cells for 7 days, all groups showed good
proliferation. However, the 6Sr group demonstrated the optimum
growth performance, both under optical observation and the
Live-Dead cell staining. It has been demonstrated that the
concentration of boron released from glass incorporating strontium
was 2-fold lower than that released from borate glass (16). As the radius of the strontium ion
is larger than that of a magnesium or calcium ion, the strontium
ion occupies more space in the glass network, and effectively
inhibits the movement and release of other ions. Therefore, borate
release from the glass particles can be controlled by altering the
strontium oxide content in the glass composition. This explains why
different compositions of strontium have different growth rates. To
investigate the proliferation ability of the strontium-containing
borate glass among the five different compositions of strontium,
the total DNA concentration following mixed culturing for 7 days
was examined in each group. The results indicated that the 6Sr
group had the highest DNA quantification, providing evidence of
improved proliferation activity in this group. From a molecular
biological perspective, a greater amount of proliferation activity
and improved duplicate ability may be evident in the
strontium-containing groups, particularly in the 6Sr group.
However, the detailed underlying mechanism requires further study.
Consequently, the cytotoxicity of the rapid release of boron was
minimized, and incorporating strontium into borate glass may be
regarded as an effective way to promote MSC proliferation.
During differentiation towards mature osteoblasts, a
number of extracellular matrix genes are expressed by MSCs. For
instance, Cbfa1 is a transcription factor in the early osteogenetic
stage. BSP and OCN are regarded as the middle and late stage gene
markers of bone formation, respectively. In the present study, the
expression of these early, middle and late osteogenic genes in
primary canine bone marrow MSCs was examined using real-time RT-PCR
on days 7, 14 and 21, respectively, to analyze MSC maturation. On
day 7, compared with the 0Sr group, the Cbfa1 mRNA level was
significantly higher in groups 6Sr, 9Sr and 12Sr (P<0.01). The
results were consistent with a previous study that demonstrated the
higher Cbfa1 mRNA performance of the 5–10% strontium-substituted HA
(Sr-HA) ceramics (9). Furthermore,
on day 14, compared with the 0Sr group, the BSP mRNA expression
level was significantly higher in the 6Sr group. In addition, on
day 21, groups 6Sr and 9Sr exhibited higher OCN mRNA expression
levels than the 0Sr group (P<0.01). It was found that 6–9%
strontium-substituted borate glass provided an improved
osteogenetic ability for MSCs compared with 0–3%
strontium-substituted borate glass. It has also been demonstrated
that strontium may promote the differentiation of MSCs through
activation of the BSP and OCN genes in the middle and late stages
of osteogenesis. However, the 12Sr group demonstrated poor ability
in the process of bone formation, suggesting a negative feedback
mechanism may affect the regulation of strontium in osteoblastic
differentiation when the concentration of strontium is at a
relatively higher level (9).
ALP is an important marker for osteogenic cell
derivatives of MSCs. Previously, it demonstrated elevated levels
when grown on 45S5 bioactive glass compared with tissue culture
(24). As shown in the present
study, the 6Sr group exhibited the highest ALP excretion after the
21 days of culturing. The data demonstrated that the 6Sr group is a
reliable indication for the differentiation of MSCs into the
osteoblastic lineage. Similarly, Alizarin Red S staining is an
intuitionistic method to examine the mineralization capacity. In
our experiments, the medium in all the groups was stained red under
a microscope. It may be that the strontium-containing borate glass
is able to promote MSC secretion of the osteogenetic substance. In
particular, in the 6Sr group, the obvious mineralized nodules were
observed. These results were consistent with previous in
vitro studies regarding the stimulatory effect of various
strontium-incorporated materials, that Sr-HA ceramics may enhance
osteoblastic cell differentiation and mineralization (25,26).
Strontium stimulates bone formation through its
positive action on osteoblastic differentiation and function, as
well as by decreasing osteoclast differentiation and function
(27). The potential mechanism may
involve the fact that both Sr2+ and SiO4−
ions enhance ALP activity in human bone MSCs (15). Furthermore, it has been
demonstrated that strontium ranelate increases the replication of
cells of the osteoblastic lineage by two distinct cell mechanisms
involving CaSR, and triggering mitogenic signals, such as p38, in
C3H10T1/2 cells. It has been suggested that the release of an
autocrine growth factor by strontium is another potential mechanism
for inducing osteoblastic cell replication (28). The detailed mechanism of
strengthened bone formation by strontium requires further
study.
In conclusion, borate glasses containing strontium
oxide of 0, 3, 6, 9 and 12 mol% demonstrate significant levels of
proliferation when interacting with MSCs. The borate glass
containing 6 mol% strontium oxide has the best characteristic of
proliferation when cultured with MSCs. The borate glass containing
6 and 9 mol% strontium oxide facilitates improved bone formation
ability compared with the remaining two compositions. Overall, it
may be concluded that borate glass containing strontium provides a
promising material in tissue engineering, and that 6 mol% strontium
oxide in borate glass could facilitate maximum proliferation and
differentiation abilities.
Acknowledgements
The study was supported by the National Natural
Science Foundation projects (Nos. C160802, 81000788 and 51072133).
The authors would like to thank Professor Wenhai Huang (Institute
of Bioengineering and Information Technology Materials, Tongji
University, Shanghai, China) for his instructions and reviewing of
the study.
References
|
1
|
Rahaman MN, Brown RF, Bal BS and Day DE:
Bioactive glasses for nonbearing applications in total joint
replacement. Semin Arthroplasty. 17:102–112. 2006. View Article : Google Scholar
|
|
2
|
Jones JR, Gentleman E and Polak J:
Bioactive glass scaffolds for bone regeneration. Elements.
3:393–399. 2007. View Article : Google Scholar
|
|
3
|
Hench LL, Splinter RJ, Allen WC and
Greenlee TK: Bonding mechanisms at the interface of ceramic
prosthetic materials. J Biomed Mater Res. 5:117–141. 1971.
View Article : Google Scholar
|
|
4
|
Hench LL and Wilson J: Surface-active
biomaterials. Science. 226:630–636. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamadouche M, Meunier A, Greenspan DC, et
al: Long-term in vivo bioactivity and degradability of bulk
sol-gel bioactive glasses. J Biomed Mater Res. 54:560–566.
2001.
|
|
6
|
Richard MNC: Bioactive Behavior of a
Borate Glass. MS Thesis. University of Missouri-Rolla; 2000
|
|
7
|
Fu Q, Rahaman MN, Fu H and Liu X:
Silicate, borosilicate, and borate bioactive glass scaffolds with
controllable degradation rate for bone tissue engineering
applications. I Preparation and in vitro degradation. J Biomed
Mater Res A. 95:164–171. 2010. View Article : Google Scholar
|
|
8
|
Fu Q, Rahaman MN, Bal BS, Bonewald LF,
Kuroki K and Brown RF: Silicate, borosilicate, and borate bioactive
glass scaffolds with controllable degradation rate for bone tissue
engineering applications. II In vitro and in vivo biological
evaluation. J Biomed Mater Res A. 95:172–179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sila-Asna M, Bunyaratvej A, Maeda S,
Kitaguchi H and Bunyaratavej N: Osteoblast differentiation and bone
formation gene expression in strontium-inducing bone marrow
mesenchymal stem cell. Kobe J Med Sci. 53:25–35. 2007.PubMed/NCBI
|
|
10
|
Yang F, Yang D, Tu J, Zheng Q, Cai L and
Wang L: Strontium enhances osteogenic differentiation of
mesenchymal stem cells and in vivo bone formation by activating
Wnt/catenin signaling. Stem Cells. 29:981–991. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peng S, Zhou G, Luk KD, et al: Strontium
promotes osteogenic differentiation of mesenchymal stem cells
through the Ras/MAPK signaling pathway. Cell Physiol Biochem.
23:165–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Verberckmoes SC, De Broe ME and D’Haese
PC: Dose-dependent effects of strontium on osteoblast function and
mineralization. Kidney Int. 64:534–543. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu K, Zhao XJ, Wan CX, Zhao CS and Chen
YW: Effect of strontium ions on the growth of ROS17/2.8 cells on
porous calcium polyphosphate scaffolds. Biomaterials. 27:1277–1286.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hurtel-Lemaire AS, Mentaverri R,
Caudrillier A, et al: The calcium-sensing receptor is involved in
strontium ranelate-induced osteoclast apoptosis. New insights into
the associated signaling pathways. J Biol Chem. 284:575–584. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu C, Fan W, Gelinsky M, et al: Bioactive
SrO-SiO2 glass with well-ordered mesopores: characterization,
physiochemistry and biological properties. Acta Biomater.
7:1797–1806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pan HB, Zhao XL, Zhang X, et al: Strontium
borate glass: potential biomaterial for bone regeneration. J R Soc
Interface. 7:1025–1031. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown RF, Rahaman MN, Dwilewicz AB, et al:
Effect of borate glass composition on its conversion to
hydroxyapatite and on the proliferation of MC3T3-E1 cells. J Biomed
Mater Res A. 88:392–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang W, Day DE, Kittiratanapiboon K and
Rahaman MN: Kinetics and mechanisms of the conversion of silicate
(45S5), borate, and borosilicate glasses to hydroxyapatite in
dilute phosphate solutions. J Mater Sci Mater Med. 17:583–596.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Huang W, Fu H, et al: Bioactive
borosilicate glass scaffolds: in vitro degradation and bioactivity
behaviors. J Mater Sci Mater Med. 20:1237–1243. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahaman MN, Day DE, Bal BS, et al:
Bioactive glass in tissue engineering. Acta Biomater. 7:2355–2373.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Day DEWJ, Brown RF and McMenamin KD:
Transformation of borate glasses into biologically useful
materials. Glass Technol. 44:75–81. 2003.
|
|
22
|
Reginster JY: Strontium ranelate in
osteoporosis. Curr Pharm Des. 8:1907–1916. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buehler J, Chappuis P, Saffar JL,
Tsouderos Y and Vignery A: Strontium ranelate inhibits bone
resorption while maintaining bone formation in alveolar bone in
monkeys (Macaca fascicularis). Bone. 29:176–179. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reilly GC, Radin S, Chen AT and Ducheyne
P: Differential alkaline phosphatase responses of rat and human
bone marrow derived mesenchymal stem cells to 45S5 bioactive glass.
Biomaterials. 28:4091–4097. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ni GX, Yao ZP, Huang GT, Liu WG and Lu WW:
The effect of strontium incorporation in hydroxyapatite on
osteoblasts in vitro. J Mater Sci Mater Med. 22:961–967. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gentleman E, Fredholm YC, Jell G, et al:
The effects of strontium-substituted bioactive glasses on
osteoblasts and osteoclasts in vitro. Biomaterials. 31:3949–3956.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonnelye E, Chabadel A, Saltel F and
Jurdic P: Dual effect of strontium ranelate: stimulation of
osteoblast differentiation and inhibition of osteoclast formation
and resorption in vitro. Bone. 42:129–138. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caverzasio J: Strontium ranelate promotes
osteoblastic cell replication through at least two different
mechanisms. Bone. 42:1131–1136. 2008. View Article : Google Scholar : PubMed/NCBI
|