Introduction
Pancreatic cancer is one of the most malignant
tumors. The morbidity and mortality rates caused by pancreatic
cancer are on the increase in China and Western countries. Due to
its poor prognosis and obscure etiology, pancreatic cancer is
becoming a focus for medical science.
In humans, ARHI is a maternally imprinted suppressor
encoding a 26-kDa small G protein. In ovarian and breast cancer,
the expression of ARHI is usually downregulated (1), and in pancreatic tumors, recent
studies (2) have shown that ARHI
may be a tumor suppressor, however, the mechanism of its action has
not been elucidated.
The progression of pancreatic cancer involves
various stages and a number of genes. Although the signaling
pathway involved during tumor development has not been elucidated,
studies (3,4) have shown that the overactivation and
expression of the NF-κB signaling pathway correlates with tumor
formation, i.e., malignant tumors with increased NF-κB expression
have been observed. The present study aimed to investigate the
inhibitory effect of ARHI in the pancreatic cancer PANC-1 cells and
identify the correlation between ARHI and the NF-κB signaling
pathway.
Materials and methods
Cell culture
The human pancreatic cancer cell line, PANC-1, was
cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA)
supplemented with 10% fetal calf serum (Hyclone, Waltham, MA, USA)
at 5% CO2 and 37°C. The study was approved by the ethics
committee of Zhongshan Hospital Affiliated to Xiamen
University.
Reverse transcription (RT)-PCR
The total RNA was extracted from normal pancreatic
tissue with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA)
according to the manufacturer's instructions. The coding sequence
of ARHI was obtained by PCR using the primers: forward, 5′-CGG
AATTCCGTATCTCCCCTCCGAATCC-3′ and reverse,
5′-CGGGATCCGCCCAGGGCTCACATGATT-3′. The PCR protocol was as follows:
35 cycles of amplification, each cycle consisting of denaturation
at 94°C for 40 sec, annealing at 55°C for 40 sec and extension at
72°C for 1 min, prior to an additional extension at 72°C for 10
min. The 745 bp PCR product was purified using a DNA purifying
kit.
Construction of the recombinant plasmid
pIRES2-EGFP- ARHI and transient transfection
The eukaryotic expression vector pIRES2-EGFP and the
ARHI PCR product were digested with EcoRI and BamHI.
The digested products were purified and ligated with T4 ligase and
transiently transfected into thePANC-1 cells using
Lipofectamine™ 2000 according to the standard protocol.
The transfected cells utilized in the various experiments were
cultured for 24, 48, 72, 96 and 120 h.
Analysis of MTT cell growth assay
The PANC-1 cells utilized for transfection were
divided into 4 groups: cells transfected with pIRES2-EGFP-ARHI,
cells containing the empty vector pIRES2-EGFP, untreated PANC-1
cells as a control and the Lipofectamine™ 2000
transfection control group. For each group, 2×105 cells
were seeded in triplicate in 6-well plates and cultured for 24, 48,
72, 96 and 120 h, followed by digestion with trypsinogen. The cell
number was calculated each day and used to plot the growth
curve.
Analysis of cell morphology and apoptosis
by flow cytometry
At the 120 h time-point, the transfected PANC-1
cells were digested and washed 3 times with phosphate-buffered
saline, collected by centrifugation and permeabilized in 70%
ethanol (4°C) overnight. The permeabilized cells were then
incubated for 30 min in 50 μg/ml propidium iodide, 0.1 μg/ml of
RNase A, 0.1% Nonidet P-40 and 50 μg/ml sodium citrate and analyzed
in a Becton-Dickinson FACSort analyzer (Franklin Lakes, NJ, USA).
The transfected cells were also stained with Hoechst 33258 dye to
observe the cellular morphology.
Western blot analysis
The transfected cells were harvested at the various
time-points by scraping them from the tissue culture wells and then
centrifugation was performed. The cells were resuspended in a lysis
buffer and the lysates were centrifuged for 30 min. The entire
handling process was carried out on ice. The samples were loaded on
10% SDS-PAGE for separation and then transferred
electrophoretically to a Hybond polyvinylidene difluoride (PVDF)
membrane. Each membrane was incubated for 24 h at 4°C with a
monoclonal antibody against the nuclear phosphorylated p65 or
β-actin proteins (Cell Signaling, Danvers, MA, USA). The blots were
washed with phosphate buffer containing 0.05% Tween 20 and
incubated for 0.5 h with the appropriate secondary antibodies
conjugated with peroxidase (Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) in Tris-buffered saline with Tween 20 (TBS-T;
1:5000) and a 5% blocking reagent. The proteins were visualized
using the enhanced chemiluminescence (ECL) western blot analysis
system (Amersham Pharmacia Biotech, Inc, Piscataway, NJ, USA). The
expression of the nuclear phosphorylated p65 protein at 24, 48, and
72 h post-transfection was compared with that of the normal
pancreatic tissue. β-actin was used as the loading control. A
similar procedure was followed to detect the expression of
ARHI.
Statistical analysis
The experiments were performed in triplicate. A
one-way ANOVA was used to compare the data between groups and
Dunnett's t-test was used to compare the data within each group
using the SPSS 12.0 statistical software.
Results
Transient transfection of the PANC-1
cells
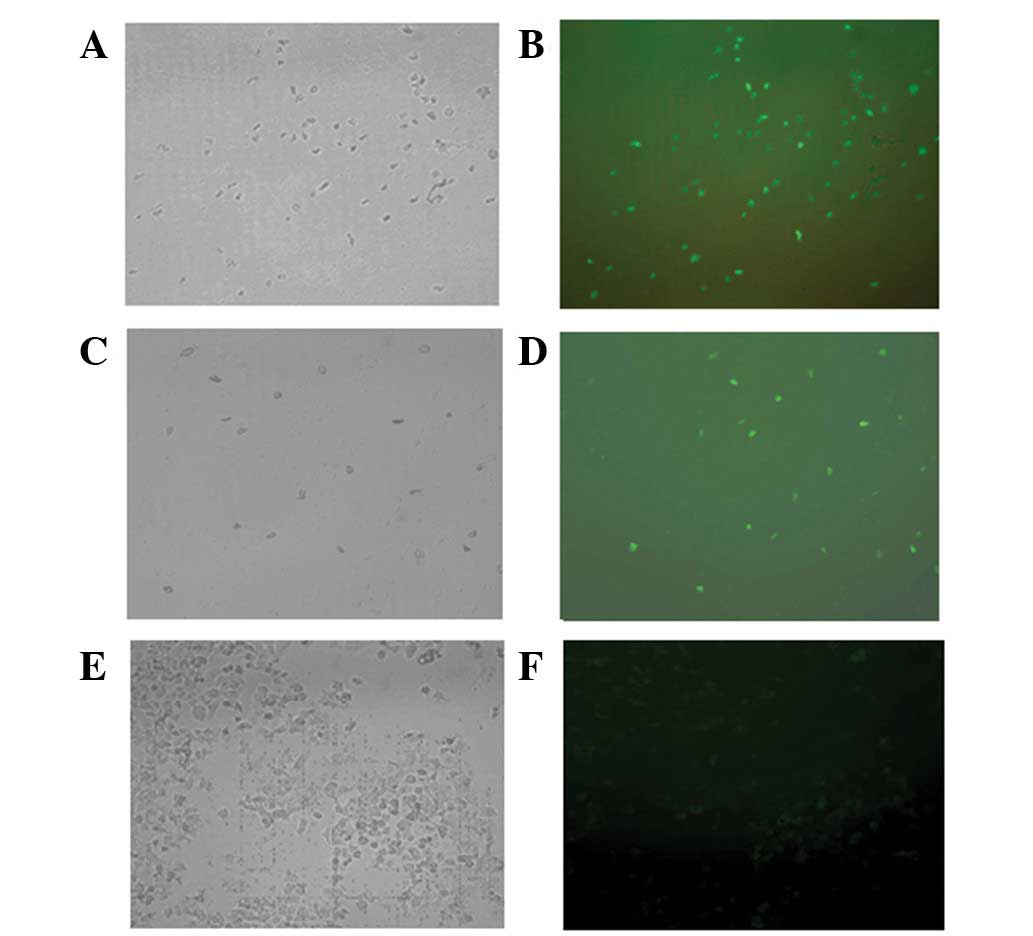
The cells containing the recombinant plasmid
pIRES2-EGFP-ARHI, the pIRES2-EGFP vector or the control group were
examined under UV and visible light. Green fluorescence was
observed in the cells containing the recombinant plasmid
pIRES2-EGFP-ARHI and the pIRES2-EGFP vector, but not in the control
group (Fig. 1).
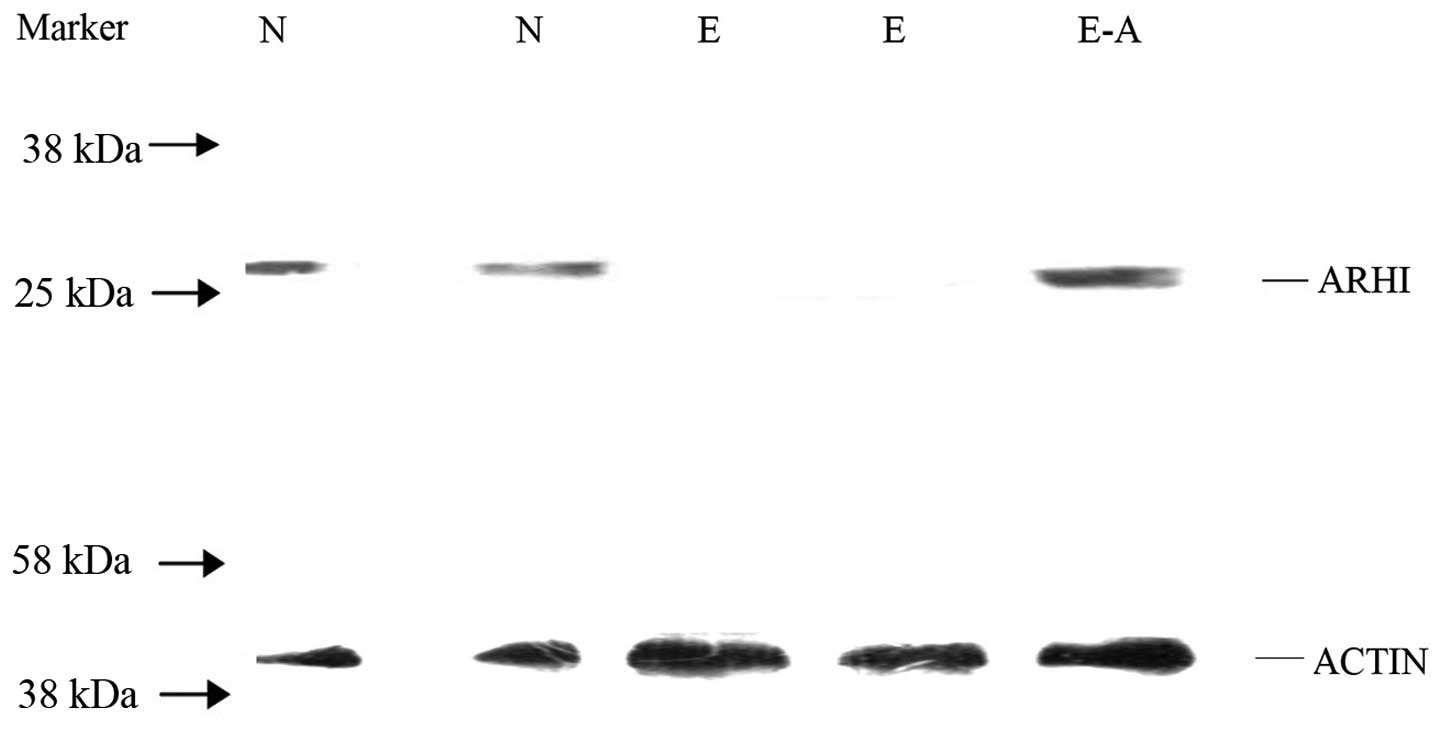
ARHI protein expression following
transient transfection
Only the cells with a high transfection efficiency
for containing the recombinant plasmid pIRES2-EGFP-ARHI, the
pIRES2-EGFP vector and the control group were lysed to detect the
expression of the ARHI protein by western blot analysis. Normal
pancreatic tissue was used as a positive control and β-actin as an
internal control. The ARHI protein was detected in the PANC-1 cells
containing pIRES2-EGFP-ARHI and in the normal pancreatic tissue.
However, ARHI expression was not detected in the PANC-1 cells
containing pIRES2-EGFP (Fig.
2).
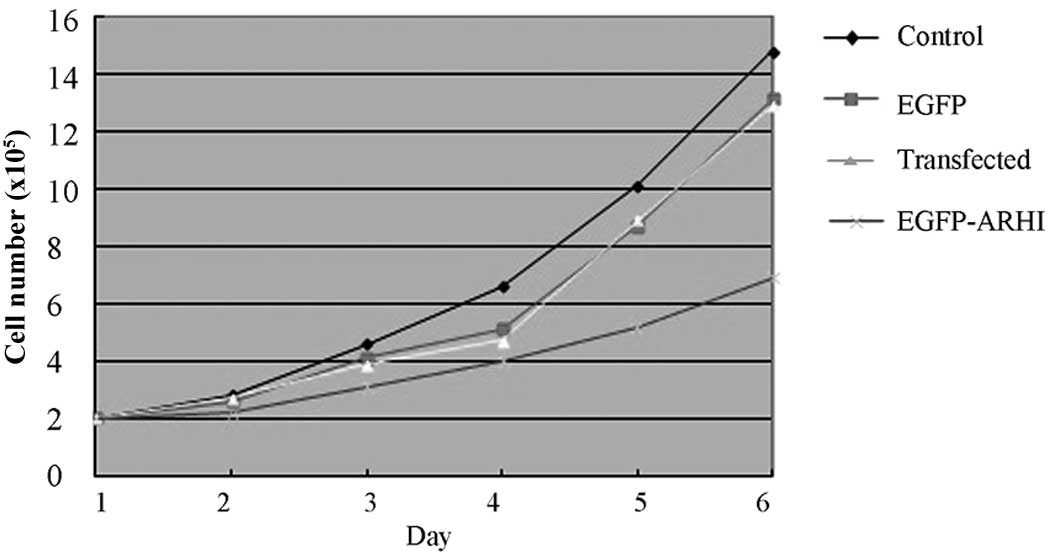
Effect of ARHI on the proliferation of
the PANC-1 cells
The analysis of the cell number at the various
post-transfection time-points (24, 48, 72, 96, and 120 h) revealed
that the PANC-1 cells containing pIRES2-EGFP-ARHI had a decreased
rate of proliferation compared with the other 3 control groups
(P<0.05). These data indicate that ARHI inhibits the growth of
the PANC-1 cells (Fig. 3).
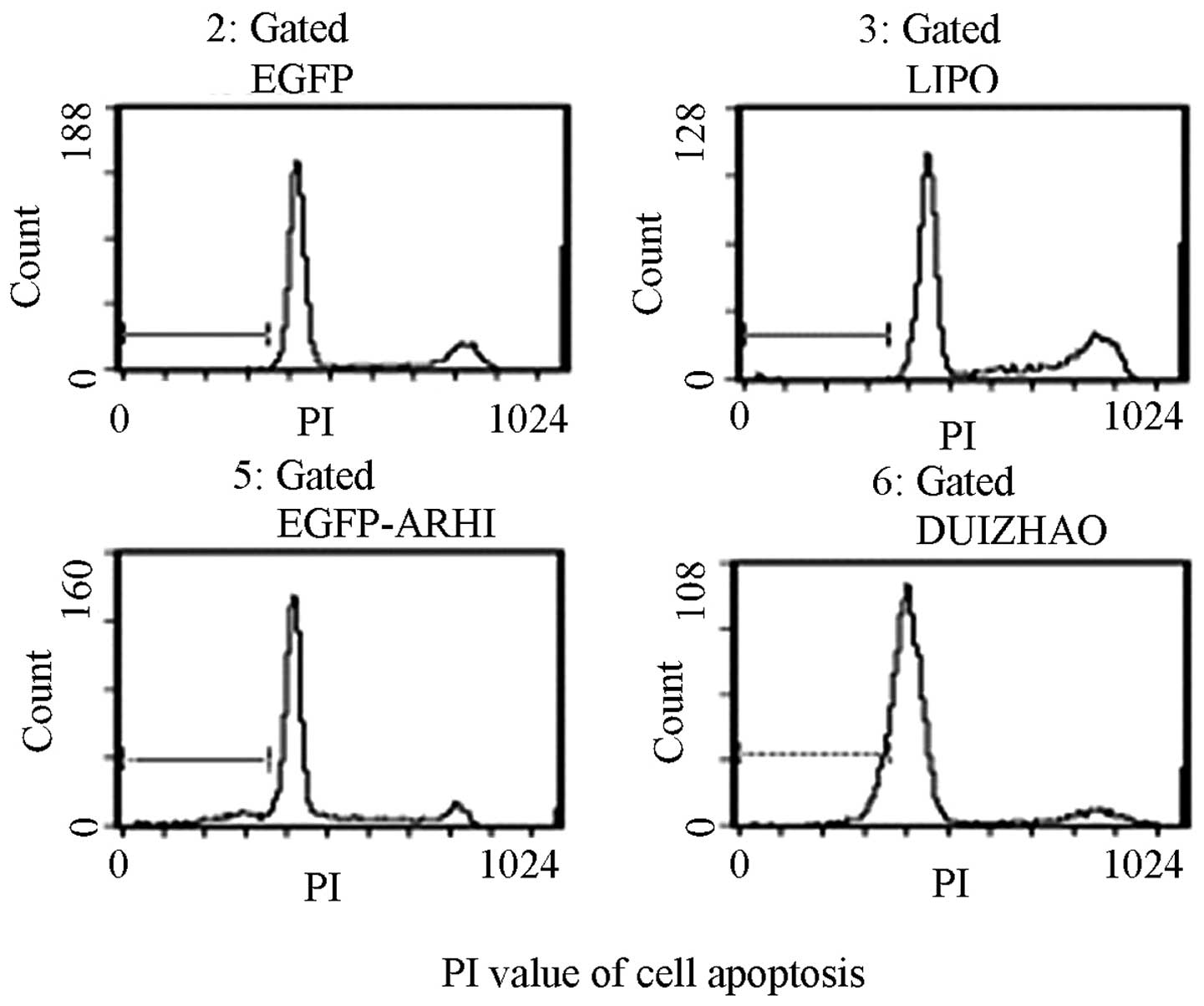
Analysis of the PANC-1 cell cycle and
apoptosis by flow cytometry
Following transfection, a cell cycle analysis was
performed in the PANC-1 cells containing pIRES2-EGFP-ARHI and in
the other 3 control groups. The number of cells in the G1 phase in
the pIRES2-EGFP-ARHI group increased to 68.94±1.35%, whereas the
number of cells in the S phase decreased to 17.31±3.27%. This
difference was also observed in the other control groups (G1 phase,
55.72±2.01% in the blank control group, 64.71±4.26% in the PANC-1
cells containing pIRES2-EGFP and 57.37±3.98% in the control group
transfected with Lipofectamine™ 2000; S phase,
22.94±2.01, 4.9±1.80 and 25.27±3.72%, respectively).
From the time course studies of transfected PANC-1
cell proliferation, the 120-h post-transfection time-point was
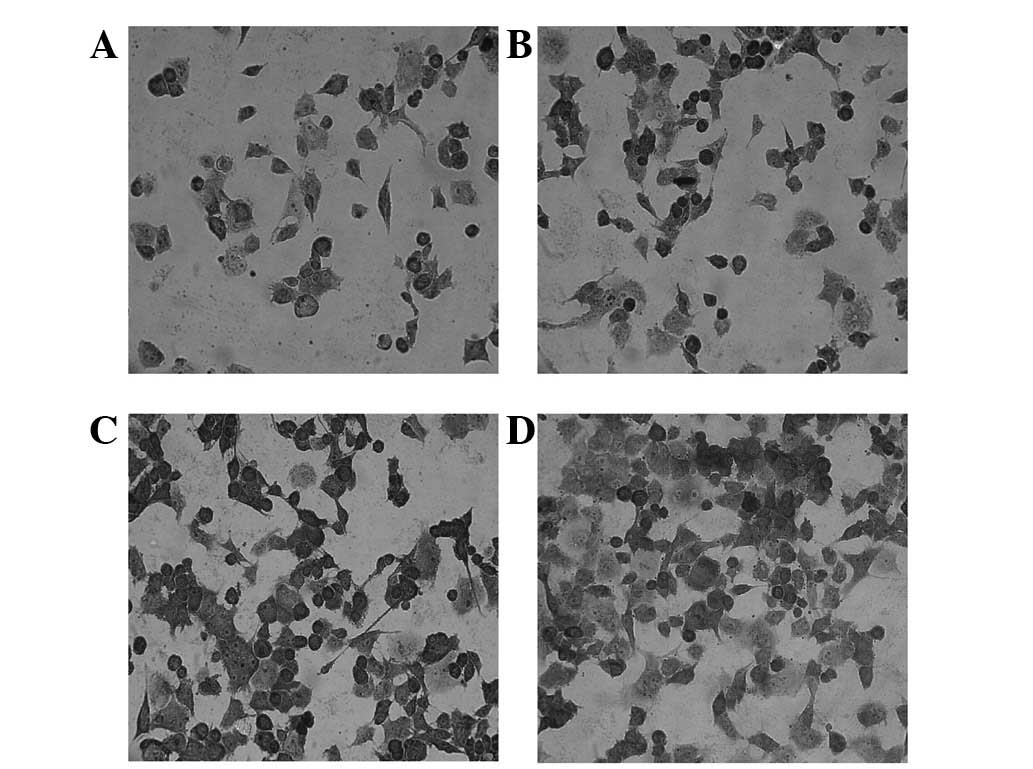
selected in which to observe apoptosis. The results demonstrated
that the PANC-1 cells containing pIRES2-EGFP-ARHI showed a
significant increase in apoptosis compared with the other 3 control
groups (P<0.01; Fig. 4). The
addition of Hoechst 33258 dye to these cells enabled the
examination of their cell morphology, karyopyknosis and apoptotic
bodies were observed in the apoptotic cells.
Analysis of PANC-1 cell growth
post-transfection
Growth analysis of the cells containing
pIRES2-EGFP-ARHI revealed that these cells were less active than
those of the other 3 groups and that the optimum time-point was at
120 h (Fig. 5).
NF-κB protein expression the in PANC-1
cells following transient transfection
The total proteins were extracted from the PANC-1
cells containing pIRES2-EGFP-ARHI, the 3 control groups at the
various time-points (24, 48, and 72 h) post-transfection and the
normal pancreatic tissue, which was used as an additional control.
Expression of the nuclear phosphorylated p65 protein was analyzed
by western blotting and β-actin was used as an internal control.
The nuclear phosphorylated p65 protein was highly expressed in the
control group containing the untreated PANC-1 cells. Expression of
the nuclear phosphorylated p65 protein post-transfection gradually
decreased with time and was not detected in the normal pancreatic
tissue (Fig. 6).
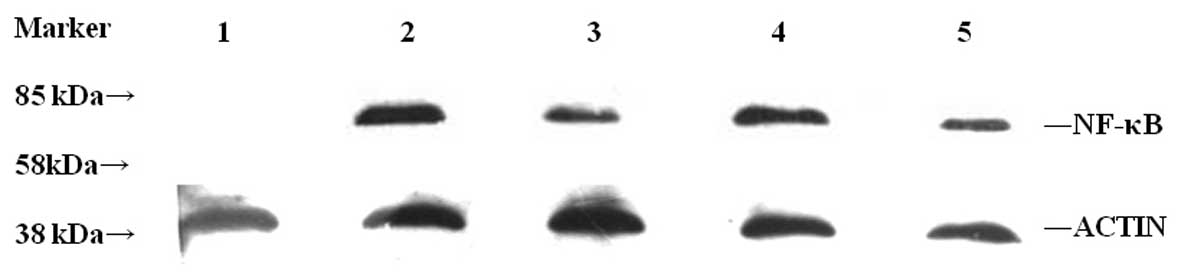 | Figure 6Expression of NF-κB protein following
transient transfection. Expression of the nuclear phosphorylated
p65 protein was detected by western blotting in the cells
transfected with pIRES2-EGFP-ARHI at various time-points (24, 48,
and 72 h). β-actin protein was used as an internal control.
Expression of the nuclear phosphorylated p65 protein gradually
decreased with time and increased in the control group containing
untreated the PANC-1 cells. In normal pancreatic tissue, expression
of the nuclear phosphorylated p65 protein was not detected. Lanes:
1, normal pancreatic tissue; 2, control group consisting of
untreated PANC-1 cells; 3, cells 24 h post-transient transfection;
4, cells 48 h post-transient transfection; 5, cells 72 h
post-transient transfection; NF-κB, nuclear factor-κB; ARHI,
aplasia ras homolog member I; PANC-1, pancreatic cancer-1. |
Discussion
Pancreatic cancer is the fourth leading cause of
mortality in cancer patients in the U.S. The one year survival rate
is <8% and the five-year survival rate is <3%. In China, the
morbidity and mortality rates of pancreatic cancer have been
steadily increasing in recent years. As pancreatic cancer has
highly malignant tumors and obscure symptoms, it has the worst
5-year survival rate of any cancer (2), despite significant advances in our
understanding, diagnosis and access to conventional and novel
treatments (3). The treatment for
pancreatic cancer is a global problem and its early diagnosis is
the most important factor for the prognosis and treatment of
patients.
Human ARHI is located on chromosome 1p31 and is a
potential maternally imprinted tumor suppressor encoding a 26 kDa
GTP-binding protein. Previous studies have revealed that ARHI
inhibited cell growth and the loss of its expression through
promoter methylation (4,5) was related to the evolution of breast
and ovarian cancers. A loss of heterozygosity (LOH) of ARHI and
other mechanisms may stimulate clonogenic growth and contribute to
the pathogenesis of the majority of ovarian cancers (6,7).
Previous studies have also observed that ARHI expression is
downregulated in >60% of ovarian cancers (5) and that ARHI acts as a tumor
suppressor in the development and progression of malignant
pancreatic endocrine tumors (PETs). The mRNA expression of ARHI is
significantly decreased in oligodendrogliomas with a 1p deletion in
oligodendroglial tumors (8).
As earlier studies demonstrated that the
pathogenesis of breast and ovarian cancers was correlated with the
loss of ARHI expression (9), we
examined whether ARHI existed as a suppressor in pancreatic
carcinomas. Previous experiments in our laboratory indicated that
in certain pancreatic carcinoma tissues (10) there was a loss of expression of the
ARHI protein and hyper-methylation of the CpG islands (CpGI 45.5%;
CpGII 27.3%) in its promoter region.
In the present study, a eukaryotic expression vector
with wild-type ARHI was constructed and transfected into the PANC-1
cells, which did not express ARHI. We explored the association of
ARHI and pancreatic carcinoma and the putative mechanisms involved
in its pathogenesis. The results indicated that proliferation
decreased, while the rate of apoptosis increased in the PANC-1
cells containing the vector pIRES2-EGFP-ARHI compared with the
other groups. This demonstrated that ARHI is able to inhibit
pancreatic tumors and that it may act as a suppressor to a certain
degree. However, the observed outcome of the cell cycle assays were
different from the expected results (10). Although a higher percentage of
PANC-1 cells containing pIRES2-EGFP-ARHI were in the G1 phase, with
fewer cells in the S phase, this difference was not significant
when compared with the corresponding phases in the other control
groups. This result suggests that ARHI acts as a tumor suppressor
in pancreatic carcinoma (11),
although its activity is not as potent as other anti-tumor genes,
including p53.
It is well-known that tumor progression involves
multiple genes and that the signal transduction pathway that is
involved is essential to its pathogenesis (12). Previous studies revealed that NF-κB
was constitutively activated in primary pancreatic adenocarcinoma
and the pancreatic cancer cell lines (13). The chemoresistance of the tumor
cells was apparently the major cause and contributing factor for
the failure of conventional chemotherapy in the treatment of
pancreatic cancer. NF-κB plays a crucial role in inflammation,
immunity, cell proliferation and apoptosis (14–16).
The current treatment for patients with pancreatic cancer is not
optimal and new anticancer compounds are required. It is speculated
that a novel drug capable of inhibiting NF-κB activity may be a
potential future approach. Consequently, numerous laboratories
worldwide are searching for a potent inhibitor of NF-κB
transcription (17). To
investigate the correlation between ARHI and NF-κB activity in the
present study, the essential components of NF-κB activation were
examined at various time-points subsequent to transfecting the
PANC-1 cells with pIRES2-EGFP-ARHI. The expression of nuclear
phosphorylated p65 was observed to gradually decrease in these
cells. This downregulation was mediated by the acceleration of
protein degradation. Therefore, we concluded that the suppression
of NF-κB was partly caused by ARHI, which was able to degrade
nuclear phosphorylated p65 and thus affect the function of
NF-κB.
The role of ARHI in the pancreatic tissues and cells
is unknown. In the present study, the transfection experiments in
the PANC-1 cells revealed that ARHI suppressed cell proliferation
and increased apoptosis. However, the ARHI-mediated effects,
including the rate of apoptosis, were not as potent as those of
other tumor suppressors. This indicates that the genesis and
progression of pancreatic tumors is regulated by multiple genes
involving several steps (18) and
that ARHI is just one regulating component. In the cell cycle
assay, the characteristics of the experimental group were similar
to the other control groups. This was in contrast to previous
studies (2,22). This result may be attributed to the
experimental strategy. The transient transfection may have been
affected by a number of factors, including the use of lipofectamine
or the presence of untransfected cells, whereas the majority of the
previous studies involved gynecological neoplasms. In addition,
cells and tissues may be differentially regulated in pancreatic
tumors (19). The findings of this
study should be confirmed in future studies by applying different
experimental strategies.
The present study also revealed the activation and
overexpression of phosphorylated p65 in the pancreatic tumor cells.
This further confirmed that the NF-κB signaling pathway is
upregulated in tumors and that it may also be involved in tumor
progression. We also hypothesize that the NF-κB signaling pathway
may be successfully utilized for cancer therapy (20). A similar mechanism was identified
in a study reporting that apoptosis was stimulated by ellagic acid
through the inhibition of the prosurvival transcription factor
NF-κB (21) and that the
inhibition of NF-κB, particularly the phosphorylated NF-κB protein
or receptor, was able to block tumor progression (22). This is of therapeutic potential as
it renders the tumor more sensitive to chemotherapeutic agents
(23).
ARHI is a potential maternally imprinted tumor
suppressor. The present study confirmed that ARHI is important in
inhibiting the proliferation of pancreatic cancer cells and
promoting apoptosis to a certain degree. ARHI was also demonstrated
to downregulate the activation of the NF-κB protein (phosphorylated
p65). Thus, the NF-κB signaling pathway and ARHI exhibit
intercommunication, which is likely to be examined in subsequent
studies.
Acknowledgements
This study was supported by the Key Science Research
Project Natural Science Foundation of the Xiamen Health Bureau No.
3502z20077038.
References
|
1
|
Yu Y, Xu F, Peng H, et al: NOEY2 (ARHI),
an imprinted putative tumor suppressor gene in ovarian and breast
carcinomas. Proc Natl Acad Sci USA. 96:214–219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herreros-Villanueva M, Hijona E, Cosme A
and Bujanda L: Spontaneous regression of pancreatic cancer: real or
a misdiagnosis? World J Gastroenterol. 18:2902–2908. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strimpakos A, Saif MW and Syrigos KN:
Pancreatic cancer: from molecular pathogenesis to targeted therapy.
Cancer Metastasis Rev. 27:495–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hisatomi H, Nagao K, Wakita K and Kohno N:
ARHI/NOEY2 inactivation may be important in breast tumor
pathogenesis. Oncology. 62:136–140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu Z, Luo RZ, Lu Y, et al: The tumor
suppressor gene ARHI regulates autophagy and tumor dormancy in
human ovarian cancer cells. J Clin Invest. 118:3917–3929.
2008.PubMed/NCBI
|
|
6
|
Feng WU, Marquez R, Lu Z, Liu J, Lu KH,
Issa JP, Fishman DM, Yu Y and Bast RC Jr: Imprinted tumor
suppressor genes ARHI and PEG3 are the most frequently
down-regulated in human ovarian cancers by loss of heterozygosity
and promoter methylation. Cancer. 112:1489–1502. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weber F, Aldred MA, Morrison CD, et al:
Silencing of the maternally imprinted tumor suppressor ARHI
contributes to follicular thyroid carcinogenesis. J Clin Endocrinol
Metab. 90:1149–1155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riemenschneider MJ, Reifenberger J and
Reifenberger G: Frequent biallelic inactivation and transcriptional
silencing of the DIRAS3 gene at 1p31 in oligodendroglial tumors
with 1p loss. Int J Cancer. 122:2503–2510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amaravadi RK: Autophagy-induced tumor
dormancy in ovarian cancer. J Clin Invest. 118:3837–3840.
2008.PubMed/NCBI
|
|
10
|
Lu X, Qian J, Yu Y, Yang H and Li J:
Expression of the tumor suppressor ARHI inhibits the growth of
pancreatic cancer cells by inducing G1 cell cycle arrest. Oncol
Rep. 22:635–640. 2009.PubMed/NCBI
|
|
11
|
Yang H, Lu X, Qian J, et al: Imprinted
tumor suppressor gene ARHI induces apoptosis correlated with
changes in DNA methylation in pancreatic cancer cells. Mol Med Rep.
3:581–587. 2010.PubMed/NCBI
|
|
12
|
Wickremasinghe RG, Prentice AG and Steele
AJ: p53 and Notch signaling in chronic lymphocytic leukemia: clues
to identifying novel therapeutic strategies. Leukemia.
25:1400–1407. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujioka S, Sclabas GM, Schmidt C,
Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C and Chiao
PJ: Function of nuclear factor kappaB in pancreatic cancer
metastasis. Clin Cancer Res. 9:346–354. 2003.PubMed/NCBI
|
|
14
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hayden MS and Ghosh S: Signaling to
NF-kappaB. Genes Dev. 18:2195–2224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma X, Yang L, Xiao L, et al:
Down-regulation of EBV-LMP1 radio-sensitizes nasal pharyngeal
carcinoma cells via NF-κB regulated ATM expression. PLoS One.
6:e246472011.PubMed/NCBI
|
|
17
|
Perona R and Sánchez-Pérez I: Control of
oncogenesis and cancer therapy resistance. Br J Cancer. 90:573–577.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung S, Yi L, Kim J, Jeong D, Oh T, Kim
CH, Kim CJ, Shin J, An S and Lee MS: The role of vimentin as a
methylation biomarker for early diagnosis of cervical cancer. Mol
Cells. 31:405–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Janssen EA, Øvestad I, Skaland I, Søiland
H, Gudlaugsson E, Kjellevold KH, Nysted A, Søreide JA and Baak JP:
LOH at 1p31 (ARHI) and proliferation in lymph node-negative breast
cancer. Cell Oncol. 31:335–343. 2009.PubMed/NCBI
|
|
20
|
Freise C, Ruehl M, Erben U, et al: A
hepatoprotective Lindera obtusiloba extract suppresses
growth and attenuates insulin like growth factor-1 receptor
signaling and NF-kappaB activity in human liver cancer cell lines.
BMC Complement Altern Med. 11:392011.PubMed/NCBI
|
|
21
|
Edderkaoui M, Odinokova I, Ohno I,
Gukovsky I, Go VL, Pandol SJ and Gukovskaya AS: Ellagic acid
induces apoptosis through inhibition of nuclear factor kappa B in
pancreatic cancer cells. World J Gastroenterol. 14:3672–3680. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
MacKenzie L, McCall P, Hatziieremia S, et
al: Nuclear factor κB predicts poor outcome in patients with
hormone-naive prostate cancer with high nuclear androgen receptor.
Hum Pathol. 43:1491–1500. 2012.
|
|
23
|
Aggarwal BB and Sung B: NF-κB in cancer: a
matter of life and death. Cancer Discov. 1:469–471. 2011.
|