Introduction
The prevalence of obesity or being overweight is on
the increase, particularly in Western countries (1). In children, the prevalence of obesity
or being overweight has nearly doubled in the last 2 decades
(2). In total, 31% of children
aged 6–19 years are either overweight or at risk of being
overweight (NHANES, 1999–2002) (3). It has been well documented that
obesity is a risk factor for the onset of metabolic disorders,
including type 2 diabetes, cardiovascular disease, osteoporosis and
numerous other chronic diseases (4,5).
Clinically, significant health care costs are generated as a result
of obesity-related bone fractures. With burgeoning medical, social
and health care costs, obesity is rapidly becoming the Western
world’s number one health problem.
However, when compared with previously published
data, when genetic factors were precluded, the incidence of hip
fractures was much lower in countries such as China, where less
high-fat diets (HFDs) are consumed compared with Western countries
which consume HFDs on a regular basis (6). Although there is no published
statistical data, the fact that HFDs are increasingly consumed by
children in China has recently become a cause of public alarm. A
substantial number of children are estimated to be becoming obese
in China and therefore the incidence of metabolic syndromes
associated with obesity or being overweight is expected to grow
more rapidly in the country.
With regard to obesity, low bone mass osteopenia or
osteoporosis is considered another disease with an altered body
composition. Osteoporosis and obesity have multifactorial
etiologies, including genetic and environmental factors, with
potential interaction between these two factors. Earlier
epidemiological data showed that a high body weight or BMI was
correlated with a high bone mass and that reductions in body weight
may cause bone loss (7). The basic
mechanism underlying this correlation remains unclear, although
several explanations have been proposed. It is generally accepted
that a larger body mass imposes greater mechanical loading on the
bone and that the bone mass increases to accommodate this greater
load. Body weight and lean body mass are strong determinants of
bone mass that reflect the adaptations of skeletal modeling to
loading (8). However, clinically,
obese individuals usually perform fewer physical activities and the
bone mass is therefore not accommodated. Zhao et al
previously demonstrated that body fat mass is negatively correlated
with bone mass when the mechanical loading effect of body weight is
statistically removed (9). It has
consistently been observed that if the confounding factor of body
weight is adjusted, a strong but inverse association between
percent fat mass and bone mass is evident (10). Moreover, children who have
increased body fat or are obese have a significantly increased
fracture risk (11). The exact
correlation and mechanisms behind obesity and bone acquisition and
the increased fracture risk in obese children remain poorly
understood.
Bone marrow surrounds the trabecular elements of the
skeleton and is composed of pluripotent stromal cells. Stromal
cells are regulated by a number of factors. When osteoblast
differentiation signals, including Runt-related transcription
factor 2 (Runx2) and Wnt/β-catenin, are activated, stromal cells
enter into the osteoblast lineage (12). By contrast, entry of the stromal
cells into the adipocyte lineage occurs through activation of the
nuclear receptor peroxisome proliferator-activated receptor-γ
(PPARγ). Since bone and fat cells share a common origin, a
switching mechanism in the mesenchymal stromal cells may explain a
few of the previous observations in which factors enhanced
adipogenesis at the expense of osteoblast differentiation (13). It is not known how dietary-induced
obesity affects this process or osteoblast and adipocyte
differentiation.
In the present study, a HFD-induced pediatric
obesity mouse model was utilized to show that a reduced bone
quality occurs in HFD mice. The model also showed that HFD-induced
obesity may favor the activation of adipogenic genes, but suppress
osteoblastic cell differentiation. Ex vivo bone marrow cell
cultures showed that the number of colony-forming unit osteoblasts
(CFU-OBs) per bone was significantly reduced in the samples from
the HFD mice compared with those from the controls. These
observations suggested that HFD-induced obesity in growing animals
may affect the total available osteoblastic cell differentiation
progenitors in the bone, while increasing adipogenesis. This may
result in negative consequences for the bone later on in life.
Materials and methods
Animal experiments
Male C57BL mice (23–24 g) were provided by the
animal center of China Medical University, Shenyang, China. Mice
(n=10/group) were randomly assigned to one of two diets containing
either a high-fat (45% total calories, HFD) or low-fat (14%, chow
diet control) mixture. The diet of the HFD group contained 25%
protein, 45% fat (corn oil) and 30% carbohydrate and was fed at 375
kcal/kg/3/4 days. The control low-fat chow diet group
was fed ad libitum with the standard AIN-93G diet made with
casein as the sole protein source and 14% fat (14). The body weights were monitored for
8 weeks. At the completion of the experiment the mice were
anesthetized with an injection of 100 mg Nembutal/kg body weight,
followed by decapitation and collection of the serum, left tibia,
femur and gonadal and abdominal fat. Peripheral quantitative
computerized tomography (pQCT) was performed on the formalin-fixed
left tibia for the bone mineral density measurement using a
previously well-established method (15). Animal procedures were approved by
the Animal Care and Use Committee of the China Medical
University.
Bone pQCT scanning and three-point
bending
The body compositions of the mice were evaluated
according to a previously published method (15). Indices of the percent fat mass,
including abdominal and gonadal fat, were derived using this
procedure. pQCT scans were performed on individual bones (left
tibia) from each mouse. The scanning was performed with a XCT unit
(XCT Research SA, Stratec Biomedical Systems, Birkenfeld, Germany)
specifically configured for small bone specimens. Software version
5.4 was used and the threshold of 570 mg/cm3 was used to
distinguish the cortical bone, while 214 mg/cm3 was used
to distinguish the trabecular from the cortical and sub-cortical
bone. Ex vivo pQCT analysis was conducted on each bone. The
tibial bone mineral density (BMD) and bone mineral content (BMC)
were automatically calculated by the software and color images were
generated. The coefficient of variation (CV) for these measurements
were <2%. The position for the pQCT scan was defined as the
distance from the proximal tibia growth palate corresponding to ~7%
of the total length of the tibia. The distance between each scan
was 1 mm and a total of 5 scans (5 slices) were carried out.
Three-point bending of the left femur was performed at room
temperature using a miniature bending apparatus with the posterior
femoral surface lying on the lower supports (7 mm apart) and the
left support immediately proximal to the distal condyles. A load
was applied to the anterior femoral surface by an actuator midway
between the two supports and moving at a constant rate of 3 mm/min
to produce a physiological in vivo strain rate of 1% to
mimic the average mouse femur. The mechanical properties, including
ultimate strength/stress and stiffness, were recorded with a
digital caliper.
RNA isolation and real-time PCR
array
The mouse femurs were harvested, followed by removal
of the marrow cells by aspiration according to the methods
previously described (16). RNA
from the femur tissue was extracted using TRIzol reagent (MRC Inc.,
Cincinnati, OH, USA) according to the manufacturer’s instructions,
followed by DNase digestion and column cleanup using Qiagen mini
columns (Qiagen GmbH, Hilden, Germany) using a previously described
procedure (16). Reverse
transcription (RT) was performed using an iScript cDNA Synthesis
kit purchased from Bio-Rad (Hercules, CA, USA). Primers for the
real-time PCR analysis used in the present study were designed
using Primer Express software 2.0.0 (Applied Biosystems, Foster
City, CA, USA) and are listed in Table
I.
 | Table IReal-time reverse transcription
polymerase chain reaction (RT-PCR) primer sequences. |
Table I
Real-time reverse transcription
polymerase chain reaction (RT-PCR) primer sequences.
| Gene | Forward primer | Reverse primer |
|---|
| Runx2 |
CCGTGGCCTTCAAGGTTGTA |
ATTTCGTAGCTCGGCAGAGTAGTT |
| aP2 |
CCAAGCCCAACTTGATCATCAG |
TGGTCGACTTTCCATCCCACT |
| PPARγ |
GCTTCCACTATGGAGTTCATGCT |
CCGGCAGTTAAGATCACACCTAT |
| β-catenin |
GATATTGACGGGCAGTATGCAA |
AACTGCGTGGATGGGATCTG |
| GAPDH |
GTATGACTCCACTCACGGCAAA |
GGTCTCGCTCCTGGAAGATG |
Cell cultures
The bone marrow cells were aspirated and harvested
from each mouse femur at the end of each experiment as previously
described (17). For
quantification of the CFU-OBs, the cells were seeded in six-well
cell culture plates at a density of 1.5×106 cells per
well. The cell cultures were maintained in the presence of minimal
essential medium (Invitrogen, Calsbad, CA, USA) with 15% fetal
bovine serum (FBS; Hyclone Laboratories, Logan, UT, USA), 1 mM
ascorbyl-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), 4 mM
L-glutamine and 100 U/ml each of penicillin and streptomycin
(Sigma-Aldrich). The cell cultures were stopped at day 12 and then
the cells were fixed and stained with alkaline phosphatase for the
CFU-OBs.
Western blot analysis
The tibia bone tissue proteins were extracted using
a cell lysate buffer as previously described (18). β-catenin, PPARγ and β-actin
expression in the bone tissue was assessed by western
immunoblotting using goat polyclonal antibodies recognizing
β-catenin (Cell Signaling Technology, Beverly, MA, USA), rabbit
polyclonal antibodies recognizing PPARγ (Cell Signaling Technology)
and mouse polyclonal antibodies recognizing β-actin (Sigma-Aldrich
Co., Oakville, ON, Canada), followed by incubation with either an
anti-rabbit or anti-mouse antibody conjugated with horseradish
peroxidase (Santa Cruz Inc., Santa Cruz, CA, USA). A SuperSignal
West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA)
was used for developing the blots. Quantification of the intensity
of the bands in the autoradiograms was performed using a ChemiDoc
XRS imaging system (Bio-Rad).
Statistical analysis
Data were presented as the mean ± standard error. A
one-way or two-way analysis of variance (ANOVA) followed by a
Student-Newman-Keuls post-hoc analysis was used to compare the
treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Body weight and body fat mass in the
HFD-induced obese mice
To examine the effect of diet-induced obesity on
bone, the majority of the previously studied ad libitum
diet-based animal models of obesity either focused on adults or
utilized long-term (3- to 4-month) diets started following puberty.
In the present study, postnatal day 17 (PND17) mice were weaned on
a HFD or control diet for only 8 weeks. During the period of the
experiment, a diet with 45% fat was provided to the HFD group every
day to induce obesity. The chow diet group was defined as the
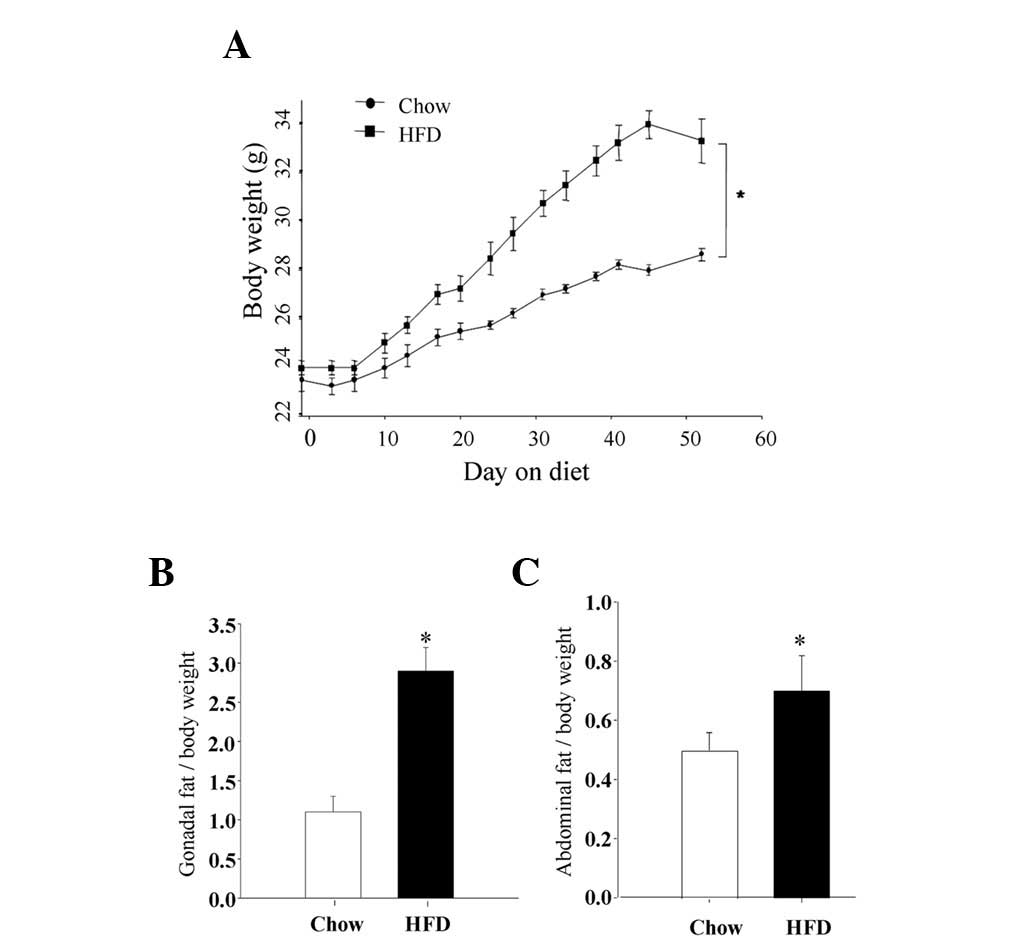
control group. As the data show in Fig. 1A, the mean body weight gains were
significantly higher after 2 weeks and thereafter in the HFD
animals. At sacrifice, the mean body weight in the HFD group was
33.6±0.6 g versus 28.3±0.5 g in the control animals (P<0.01).
This was accompanied by a significantly increased retroperitoneal
adipose tissue mass (percent body weight; gonadal adipose) of
2.9±0.4 g in the HFD group versus 1.1±0.3 g in the control diet
animals (Fig. 1B; P<0.01).
Similarly, the mean abdominal fat mass in the HFD group was
0.78±0.02 g versus 0.5±0.01 g in the control diet animals
(P<0.01; Fig. 1C). These data
clearly indicated that obesity was induced in the young mice by
feeding them ad libitum with a diet containing 45% fat for 8
weeks.
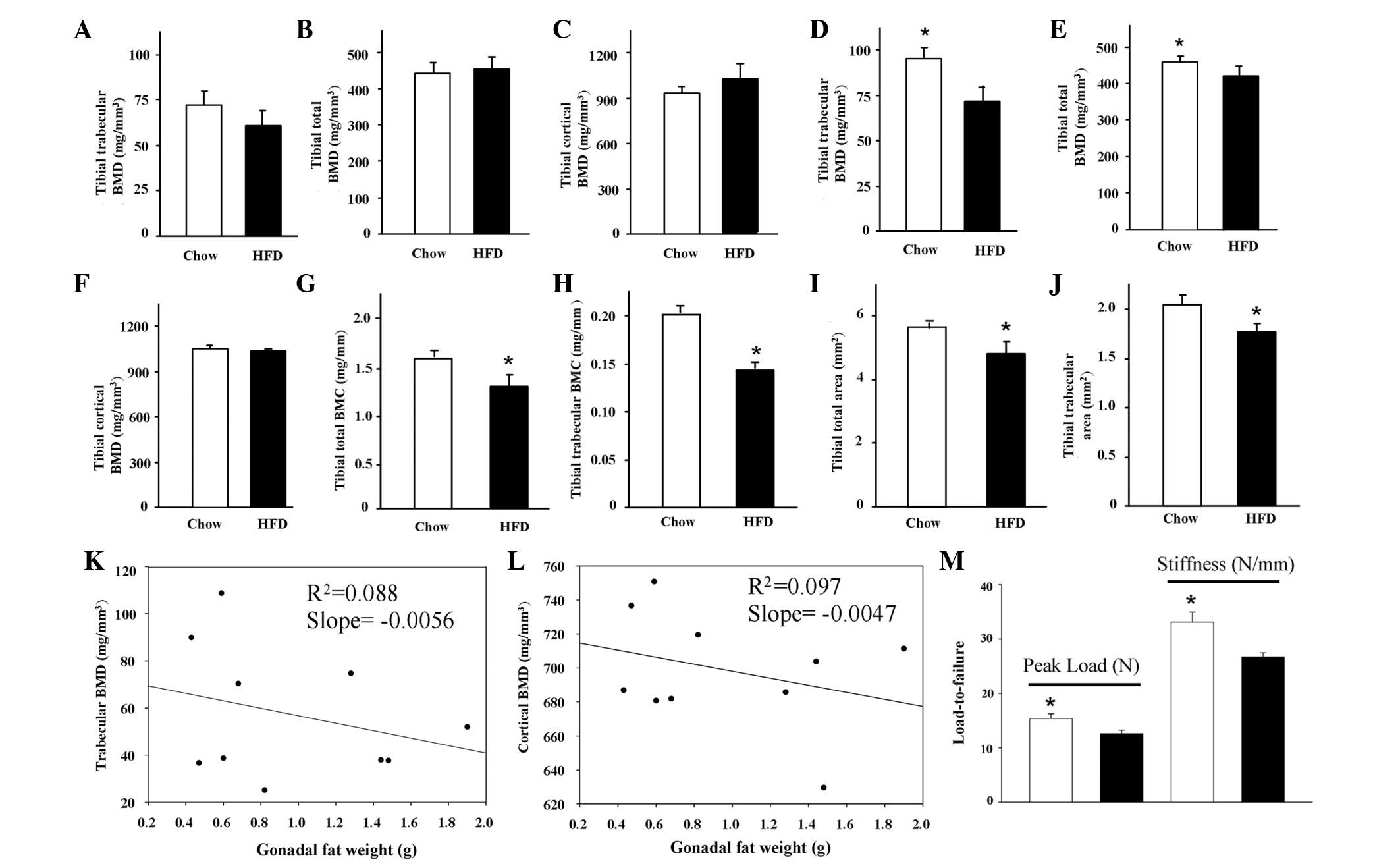
Bone quality
To obtain a better idea of the bone quality, pQCT
was first used to measure the tibial BMD and BMC in the two groups.
The total, trabecular and cortical BMDs showed no significant
changes between the two groups (Fig.
2A-C). However, other parameters from the pQCT measurement,
including the total and trabecular BMCs and the total bone and
trabecular areas were all significantly decreased (P<0.05) in
the HFD group compared with the control group (Fig. 2G-J). Notably, when the BMD was
normalized by body weight, the total, trabecular and cortical BMDs
were significantly lower in the HFD mice compared with those from
the control mice (Fig. 2D-F).
Moreover, in the HFD group, the trabecular BMD in particular was
inversely (negatively) correlated with the observed
retroperitoneal/gonadal fat accumulation (Fig. 2K), while the correlation of the
cortical BMD with the fat mass was not clearly observed (Fig. 2L). Most significantly, a femur bone
fracture (bone strength) test was carried out using a three-point
bending analysis. The peak load and stiffness were significantly
lower in the HFD group compared with the control group (P<0.05;
Fig. 2M). These data indicated for
the first time that there is an inverse correlation between fat and
bone mass in a short-term HFD-induced growing obese mouse
model.
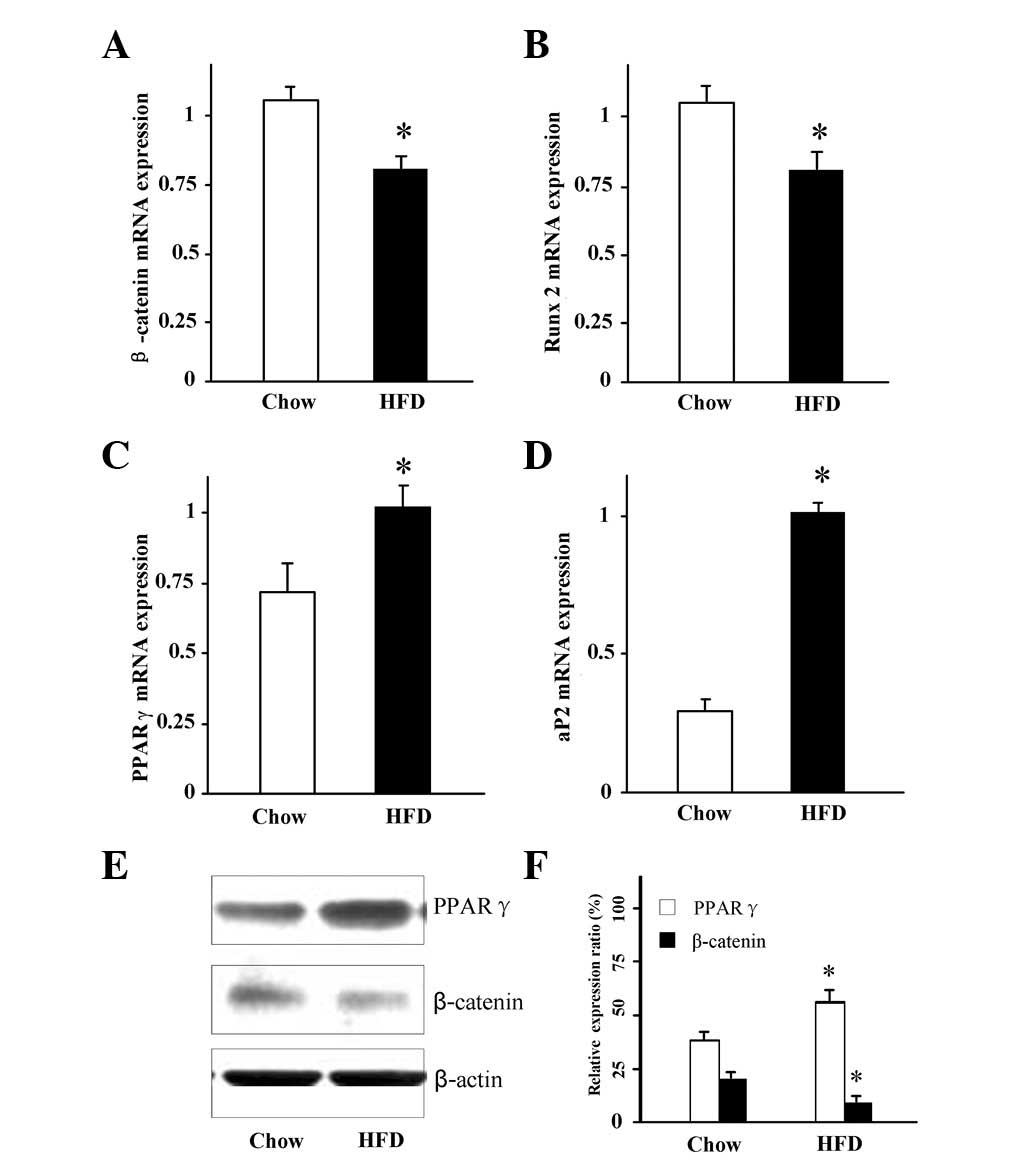
Changes in the expression of osteogenic
and adipogenic markers in the bone
A previous study in rats have suggested that
HFD-induced obesity may favor adipogenesis whereas bone formation
may be reduced (19). To determine
whether obesity affected the balance between osteoblastogenesis and
adipogenesis in the bone in the present mouse model, RNA and
protein were isolated from the bone following aspiration of the
bone marrow cells. The expression of the markers for
osteoblastogenesis and adipogenesis were then measured. Consistent
with the increased adiposity in the HFD mice, real-time RT-PCR
analysis of the mRNA levels of the adipocyte-specific genes PPARγ
and aP2 were significantly increased in the bone (Fig. 3C and D). By contrast, the mRNA
levels of the bone-forming gene β-catenin and the osteoblast
differentiation transcription factor Runx2 were significantly lower
in the HFD mice compared with the controls(Fig. 3A and B; P<0.05). The protein
expression of β-catenin and PPARγ in the bone was further confirmed
by western blotting (Fig. 3E and
F). Consistent with the mRNA expression, the β-catenin protein
expression was significantly lower, but the PPARγ protein
expression was significantly higher in the HFD mice compared with
the control animals (Fig. 3E and
F). These data suggest that increased adipogenesis but
decreased osteoblast differentiation in the bone occurred in the
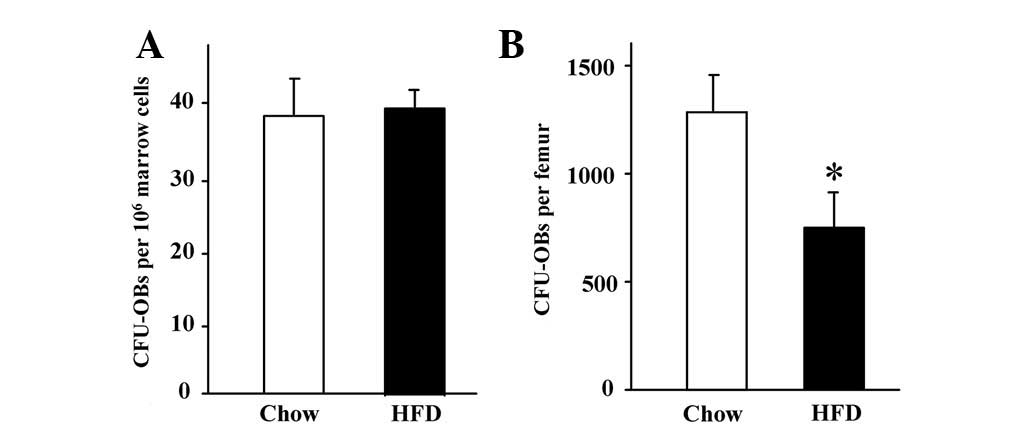
HFD obese mice. The bone marrow cells were then aspirated and
1.5×106 cells per well were cultured ex vivo in 6
well plates. After 14 days of culture in the presence of an
osteoblast differentiation medium, alkaline phosphatase activity
staining was performed. There were no differences in the number of
CFU-OBs between the HFD obese mice and the controls (Fig. 4A). However, the mean number of
isolated total bone marrow cells per femur was significantly lower
in the HFD obese mice compared with that in the control animals
(20.8±1.2×106 versus 30.0±1.0×106,
respectively; P<0.05). Therefore, the recalculated CFU-OBs per
femur were significantly reduced in the HFD obese mice compared
with their controls (Fig. 4B),
indicating that there were lower numbers of available osteoblast
progenitor cells in bone marrow of the HFD mice compared with their
controls.
Discussion
Chronic overconsumption of a HFD with fewer physical
activities is well known to cause obesity. However, the effects of
such HFD-induced obesity on postnatal and pre-natal bone
development are not well studied. The present study demonstrated
that feeding mice a high-fat ad libitum diet for eight weeks
starting from PND17 induced obesity. The HFD-induced adiposity was
inversely associated with the BMD and BMC. A mechanical loading
test indicated that there may be a significantly increased fracture
rate in HFD-induced obese animals compared with the control diet
animals. This is consistent with recent clinical data that showed
there was an increased fracture risk in obese children and
adolescents (4) and a higher
prevalence of obesity among diagnosed osteoporotic patients
(20). Of note, the present study
suggests that HFD-induced obesity in growing animals may affect the
total available osteoblastic cell differentiation progenitors in
bone, while increasing adipogenesis. These data provided a
potential mechanistic explanation for the imbalance between
adipogenesis and osteogenesis in HFD-induced obesity and further
suggested HFD-induced obesity in the negative consequences that may
arise in the bone later on in adult life.
It has generally been accepted that postnatal body
composition is affected by diet intake, intrinsic hormonal milieus
and physical activities, although genetics or pre-natal uterine
conditions may also be significant. Obesity or being overweight was
considered to be correlated with an increased bone mass based upon
observation of the correlations between BMD and weight and body
mass index, particularly at weight-bearing sites (21). The majority of these previous
analyses on BMD did not consider the mechanical loading of the
total body weight as one of the confounding factors, therefore, the
true association between obesity and bone development is not well
understood. The majority of more recent studies have emphasized a
negative association between obesity and bone development or
quality (22) if the confounding
factors are assumed to have been removed. This observation is
consistent with the data shown in the present study, which
indicated that the BMD normalized by body weight was significantly
lower in the HFD obese mice compared with their controls, however,
the difference was not clear when the actual BMD was compared.
Moreover, in the present diet-induced mouse model, the BMD was
observed and confirmed to be negatively correlated with the
visceral fat mass, particularly when using the trabecular BMD.
Although there was no difference in the BMD without weight
normalization between the groups, the mechanical loading test
showed significantly decreased long bone strength in the HFD
animals compared with their controls, which further indicated a
poor bone quality in the HFD obese animals. Although it has been
reported that rats fed on high-vegetable oil diets showed no
significant effects in their bone parameters, a diet which was rich
in saturated fatty acids had decreased digestibility and adversely
affected energy and bone metabolism in growing, healthy male rats
(23). Such a saturated fatty acid
diet was used in the present study.
We particularly believe that data of this study on
markers for osteoblastogenesis and adipogenesis, support our
knowledge and understanding of the changes in the signaling
mechanisms that lead to increased adipocyte differentiation, but
also to decreased osteoblast differentiation of the mesenchymal
stromal cells in the bone, as occurs in diet-induced pediatric
obesity. It is therefore possible that shifting the differentiation
program from osteogenesis to favor adipogenesis may lead to a
deficit in bone formation. This is fully supported by previously
observed clinical data, which indicated that populations with more
body fat may have a lower bone mass (24) and may result in fractures more
easily later in life. It also agreed with the data from studies in
rodents which reported that a low-carbohydrate/HFD may result in
significantly more visceral and bone marrow fat, induce a lower BMD
and reduce bone formation (25,15).
The present study indicated that PPARγ and Wnt/β-catenin were
expressed in opposite directions in the bone from obese animals.
The causal mechanism leading to the increased expression of PPARγ
but decreased expression of β-catenin remains unclear. Further
molecular mechanisms responsible for the downregulation of
β-catenin but upregulation of PPARγ in the bone from obese subjects
remain to be elucidated.
In summary, the present study demonstrated that an
ad libitum HFD resulted in obesity in mice and was
correlated with a lower bone quality compared with the control diet
group. The impaired bone growth in the HFD-induced obese mice may
have been due to an altered stromal cell differentiation potential
towards either the adipocytes or osteoblasts. In addition, an ex
vivo bone marrow cell culture showed that the number of CFU-OBs
per bone was significantly lower in the samples from the HFD mice
compared with the controls. The results suggested that HFD-induced
obesity in growing mice may affect the total available osteoblastic
cell differentiation progenitors in the bone, while increasing
adipogenesis. This may result in negative consequences for the bone
later on in adult life.
References
|
1
|
Faeh D, Braun J, Tarnutzer S and Bopp M:
Obesity but not overweight is associated with increased mortality
risk. Eur J Epidemiol. 26:647–655. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ritchie LD, Ivey SL, Woodward-Lopez G and
Crawford PB: Alarming trends in pediatric overweight in the United
States. Soz Preventivmed. 48:168–177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weiler HA, Janzen L, Green K, Grabowski J,
Seshia MM and Yuen KC: Percentage body fat and bone mass in healthy
Canadian females 10 to 19 years of age. Bone. 27:203–207.
2000.PubMed/NCBI
|
|
4
|
Taylor ED, Theim KR, Mirch MC, et al:
Orthopedic complications of overweight in children and adolescents.
Pediatrics. 117:2167–2174. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao LJ, Liu YJ, Liu PY, Hamilton J,
Recker RR and Deng HW: Relationship of obesity with osteoporosis. J
Clin Endocrinol Metab. 92:1640–1646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reinwald S and Weaver CM: Soy isoflavones
and bone health: a double-edged sword? J Nat Prod. 69:450–459.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid IR: Relationships among body mass,
its components and bone. Bone. 31:547–555. 2002.PubMed/NCBI
|
|
8
|
Leonard MB, Shults J, Wilson BA,
Tershakovec AM and Zemel BS: Obesity during childhood and
adolescence augments bone mass and bone dimensions. Am J Clin Nutr.
80:514–523. 2004.PubMed/NCBI
|
|
9
|
Zhao LJ, Jiang H, Papasian CJ, Maulik D,
Drees B, Hamilton J and Deng HW: Correlation of obesity and
osteoporosis: effect of fat mass on the determination of
osteoporosis. J Bone Miner Res. 23:17–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao JJ, Gregoire BR and Gao H: High-fat
diet decreases cancellous bone mass but has no effect on cortical
bone mass in the tibia in mice. Bone. 44:1097–1104. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chan G and Chen CT: Musculoskeletal
effects of obesity. Curr Opin Pediatr. 21:65–70. 2009. View Article : Google Scholar
|
|
12
|
Krishnan V, Bryant HU and MacDougald OA:
Regulation of bone mass by Wnt signaling. J Clin Invest.
116:1202–1209. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smas CM, Chen L, Zhao L, Latasa MJ and Sul
HS: Transcriptional repression of pref-1 by glucocorticoids
promotes 3T3-L1 adipocyte differentiation. J Biol Chem.
274:12632–12641. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reeves PG, Nielsen FH and Fahey GC Jr:
AIN-93 purified diets for laboratory rodents: final report of the
American Institute of Nutrition ad hoc writing committee on the
reformulation of the AIN-76A rodent diet. J Nutr. 123:1939–1951.
1993.PubMed/NCBI
|
|
15
|
Chen JR, Lazarenko OP, Wu X, et al:
Obesity reduces bone density associated with activation of PPARγ
and suppression of Wnt/β-catenin in rapidly growing male rats. PLoS
One. 5:e137042010.PubMed/NCBI
|
|
16
|
Chen JR, Lazarenko OP, Wu X, et al:
Dietary-induced serum phenolic acids promote bone growth via p38
MAPK/β-catenin canonical Wnt signaling. J Bone Miner Res.
25:2399–2411. 2010.PubMed/NCBI
|
|
17
|
Di Gregorio GB, Yamamoto M, Ali AA, Abe E,
Roberson P, Manolagas SC and Jilka RL: Attenuation of the
self-renewal of transit-amplifying osteoblast progenitors in the
murine bone marrow by 17 beta-estradiol. J Clin Invest.
107:803–812. 2001.PubMed/NCBI
|
|
18
|
Chen JR, Lazarenko OP, Haley RL, Blackburn
ML, Badger TM and Ronis MJ: Ethanol impairs estrogen receptor
signaling resulting in accelerated activation of senescence
pathways, whereas estradiol attenuates the effects of ethanol in
osteoblasts. J Bone Miner Res. 24:221–230. 2009. View Article : Google Scholar
|
|
19
|
Kyung TW, Lee JE, Phan TV, Yu R and Choi
HS: Osteoclastogenesis by bone marrow-derived macrophages is
enhanced in obese mice. J Nutr. 139:502–506. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Premaor MO, Pilbrow L, Tonkin C, Parker RA
and Compston J: Obesity and fractures in postmenopausal women. J
Bone Miner Res. 25:292–297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Felson DT, Zhang Y, Hannan MT and Anderson
JJ: Effects of weight and body mass index on bone mineral density
in men and women: the Framingham study. J Bone Miner Res.
8:567–573. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akridge M, Hilgers KK, Silveira AM, Scarfe
W, Scheetz JP and Kinane DF: Childhood obesity and skeletal
maturation assessed with Fishman’s hand-wrist analysis. Am J Orthod
Dentofacial Orthop. 132:185–190. 2007.PubMed/NCBI
|
|
23
|
Macri EV, Gonzales Chaves MM, Rodriguez
PN, Mandalunis P, Zeni S, Lifshitz F and Friedman SM: High-fat
diets affect energy and bone metabolism in growing rats. Eur J
Nutr. 51:399–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yajnik CS and Yudkin JS: The Y-Y paradox.
Lancet. 363:1632004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bielohuby M, Matsuura M, Herbach N,
Kienzle E, Slawik M, Hoeflich A and Bidlingmaier M: Short term
exposure to low-carbohydrate, high-fat diets induces low bone
mineral density and reduces bone formation in rats. J Bone Miner
Res. 25:275–284. 2010. View Article : Google Scholar : PubMed/NCBI
|