Introduction
Cerebral arteriovenous malformation (AVM) is a
vascular disease that occurs primarily in the young. The most
common clinical manifestations are hemorrhage, seizures and limb
dysfunction. Hemorrhage is the main cause of death and disability
in AVM patients and accounts for 30–86% of mortalities (1–4). At
present, the causes and mechanisms of hemorrhage are unclear. In
addition to hemodynamic factors, the inflammatory response is
considered to be a major factor in hemorrhage. Recent studies on
AVM have focused on matrix metalloproteinases (MMPs) and
inflammatory factors (5–9). MMP-9 is a Zn2+-dependent
protease, the main functions of which are the degradation and
remodeling of the extracellular matrix, which is closely associated
with vascular remodeling and growth. MMP-9 expression levels
increase in areas surrounding tumor-associated intracranial and
cerebral AVM hemorrhages and with structural instability of the
vessel walls in certain lesions, including cerebral and abdominal
aortic aneurysms and carotid artery atherosclerosis. Excessive
MMP-9 expression results in the degradation of the vascular matrix,
which may weaken the vessel walls and cause blood vessels to
rupture (10,11). Interleukin (IL)-6 has been
associated with cardiovascular disease and its expression levels
were shown to be significantly higher in hemorrhagic cerebral AVM
tissues than in non-hemorrhagic tissues (5,6). In
mouse brain tissues and human umbilical vein endothelial cells,
IL-6 is able to stimulate the expression and activity of MMP-9
(7). However, the association of
plasma IL-6 and MMP-9 with hemorrhage in AVM has rarely been
studied. Therefore, the present study aimed to explore the
potential association of IL-6, NF-κB and MMP-9 with AVM
hemorrhage.
Materials and methods
Materials
Tissue samples were collected from 31 AVM patients
who underwent surgical treatment. The criteria to be allocated to
the ruptured group were as follows: i) A history of hemorrhage
before surgery, confirmed by computed tomography or magnetic
resonance imaging; ii) a consistent hemorrhage and AVM location;
iii) blood clot or hematoma was observed in the tissue during
surgery; and iv) post-surgical pathology confirmed hemosiderin
deposition. The criteria to be allocated to the unruptured group
were as follows: i) No preoperative hemorrhage symptoms; ii)
radiographic evidence showed no hemorrhaging; iii) no blood clot or
hematoma was observed during surgery; and iv) no pathological
hemosiderin deposition was noted after the surgery. Samples for the
control group (n=31) were obtained from epilepsy patients
undergoing temporal lobectomy (23–24).
Blood samples were collected from all 31 AVM and 30 control
patients during a normal physical examination. Consent was obtained
from the patients and their families for the collection of
specimens and blood. Patient information is presented in Table I.
 | Table ICharacteristics of the patients with
AVM. |
Table I
Characteristics of the patients with
AVM.
| No. of patients
(%) |
|---|
| Gender |
| Male | 17 (55) |
| Female | 14 (45) |
| Hemorrhage |
| Yes | 14 (45) |
| No | 17 (55) |
| Location |
| Temporal | 12 (39) |
| Occipital | 7 (23) |
| Frontal | 10 (32) |
| Other | 2 ( 6) |
| Symptoms |
| Headache | 14 (45) |
| Epilepsy | 9 (29) |
| Limb
dysfunction | 2 ( 6) |
| Other | 5 (20) |
| Size (cm) |
| <3 | 6 (19) |
| 3–6 | 21 (68) |
| >6 | 4 (13) |
| Drainage |
| Single | 10 (32) |
| >1 | 21 (68) |
| Spetzler-Martin
G |
| 1 | 8 (26) |
| 2 | 10 (32) |
| 3 | 11 (35) |
| 4 | 2 ( 7) |
| 5 | 0 (0) |
| With aneurysm | 1 (3) |
Antibodies against human MMP-9 (cat. no. 3852),
NF-κB p65 (no. 3034), IκBα (no. 9242) and β-actin (no. 4967) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The human IL-6 enzyme-linked immunosorbent assay (ELISA) kit was
purchased from Beijing 4A Biotech Co., Ltd. (CHE0009; Beijing,
China). The MMP zymography assay kit (for MMP-2 and MMP-9) was
purchased from China Puli Lai Gene Technology Co., Ltd. (P1700;
China).
Immunofluorescence
Slice preparation, fixation and blocking was
achieved in 5% bovine serum albumin at room temperature for 10 min,
followed by washing in PBS (three times, 3 min each). Primary
antibody incubation and rewarming was followed by secondary
antibody incubation. Fluorescein isothiocyanate-conjugated
secondary antibody was added dropwise. The sections were incubated
at 37°C in the dark for 30 min and then washed with PBS three times
for 3 min each. The coverslips were then sealed and images were
captured.
ELISA
Sample kits were prepared by adding samples to a
plate and incubating at 37°C for 90 min, after which the plate was
washed four times (Beijing 4A Biotech Co., Ltd., CHE0009).
Biotinylated antibody (100 μl) was added to the incubation
solution. The plate was incubated at 37°C for 60 min and then
washed. Enzyme conjugate working solution (100 μl/well) was added
and incubated at 37°C for 60 min. After washing, color reagent (100
μl/well) was added. The plate was incubated in the dark at 37°C for
10 min. A stop solution (100 μl/well) was added, each well was
mixed and absorbance was measured immediately at 450 nm.
Gelatin zymography
Firstly, the positive control and test samples were
pretreated. SDS-PAGE was run at a constant current of 30 mA for 45
min. After washing and incubation, Buffer B was discarded and
Coomassie blue was added to stain the protein. The mixture was
agitated in a horizontal shaker for 120 min. Bleaching solution
(25% methanol, 10% acetic acid) was applied to the gel for 60 min.
Band scanning was carried out by placing the gel on the scanner and
scanning with non-transmitted light.
Western blot analysis
The vascular tissue total protein was isolated and
an equal volume of 2X SDS sample buffer was added. After mixing,
the sample was boiled at 100°C in water for 10 min. After the
samples were added, electrophoresis was carried out. Following
gel-membrane transfer and blocking, primary antibody was used for
an overnight incubation (dilution, 1:2000) at 4°C. After
incubation, the membrane was washed with PBS three times for 15 min
each. Incubation with secondary antibody (1:1000 dilution) at room
temperature for 1 h was followed by three 15-min washes in PBS.
Band scanning was then carried out and all the specimens underwent
routine hematoxylin and eosin (H&E) staining.
Statistical analysis
The results were expressed as the mean ± standard
deviation. Experimental data were analyzed with GraphPad Prism 4.0
software. P<0.05 was considered to indicate a statistically
significant difference.
Results
Plasma IL-6 level
Plasma samples were obtained during the surgical
treatment of AVM patients and from healthy controls. In the plasma
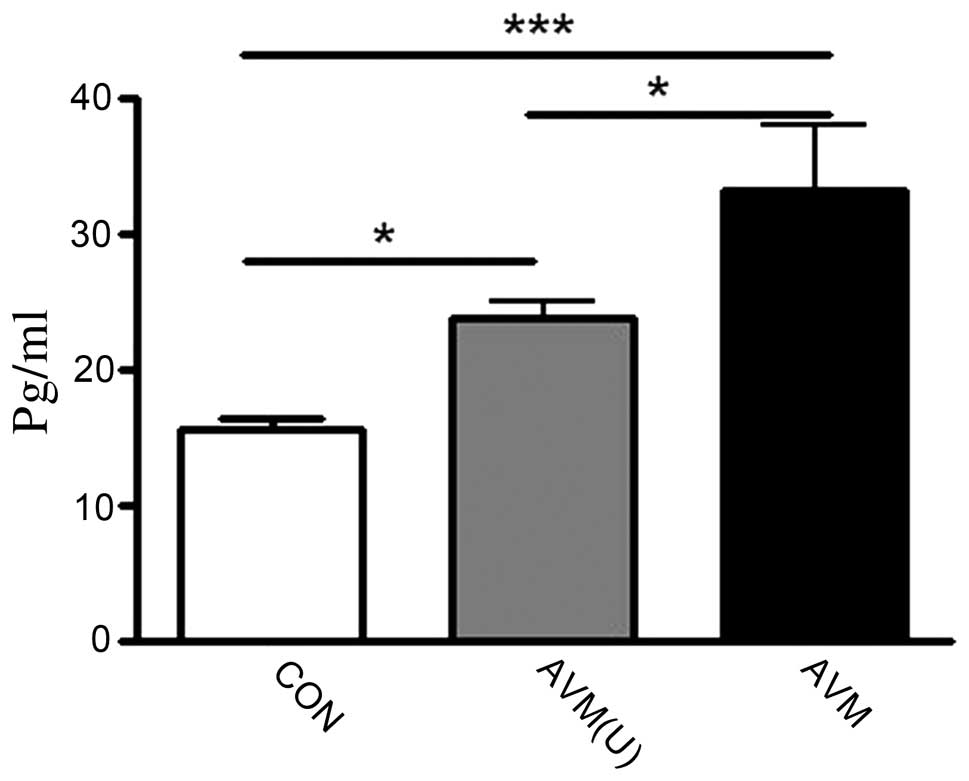
of the AVM ruptured group, the mean level of IL-6 was 33.25±4.77
pg/ml, which was significantly higher than that of the unruptured
(23.79±1.20 pg/ml) and control groups (15.56±0.97 pg/ml; Fig. 1), indicating that the plasma IL-6
levels increased in the AVM ruptured group.
Tissue NF-κB and IκBα expression
Tissue samples were obtained from the surgical
resection of AVM specimens. The specimens of the control group were
obtained from the surgical resection of brain tissue vascular
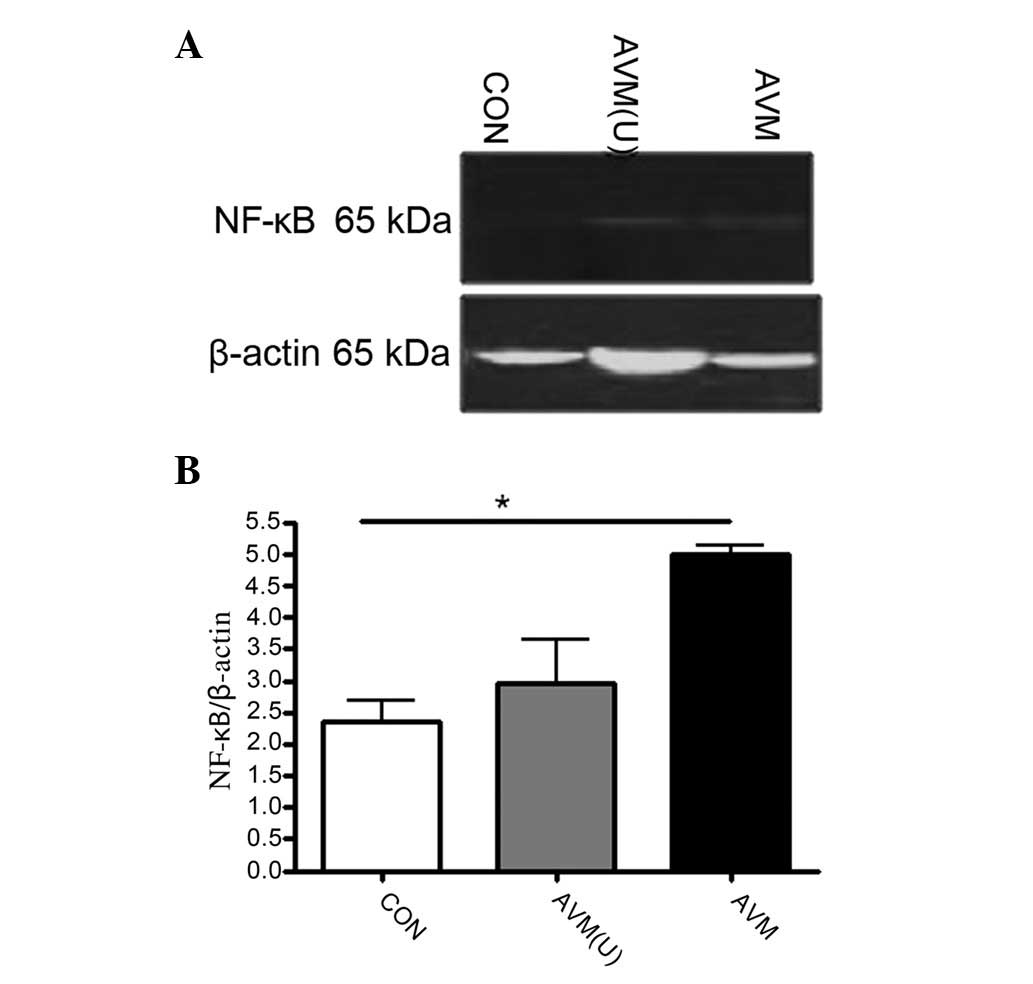
contusions in patients undergoing temporal lobectomy. The NF-κB
levels in the AVM ruptured group (5.00±0.12 kDa) were not
significantly different to those of the unruptured group (5.00±0.12
vs. 2.96±0.69; Fig. 2). The IκBα
levels were similar in the ruptured and unruptured groups, however,
they were significantly lower than those of the control group
(0.12±0.02 vs. 1.27±0.06; and 0.45±0.15 vs. 1.27±0.06,
respectively; Fig. 3). These data
suggest that the nuclear transcription factor NF-κB was actively
expressed in the AVM ruptured group.
MMP-9 protein level and activity
MMP-9 protein expression levels in the unruptured
group were higher than those in the normal and ruptured groups
(1.21±0.34 vs. 0.35±0.06; and l1.21±0.34 vs. 0.32±0.08,
respectively; Fig. 4). The gelatin
zymography assay indicated that MMP-9 activity in the ruptured
group was significantly higher than in the unruptured and control
groups (0.97±0.08 vs.0.40±0.09; and 0.97±0.08 vs. 0.30±0.07,
respectively). The expression levels of MMP-2 in the ruptured group
was significantly higher than those in the unruptured and control
groups (1.36±0.17 vs. 0.55±0.12; and 1.36±0.17 vs. 0.36±0.09,
respectively; Fig. 5), indicating
that MMP-9 expression levels and activity are higher under AVM
conditions than under normal conditions.
Tissue immunofluorescence
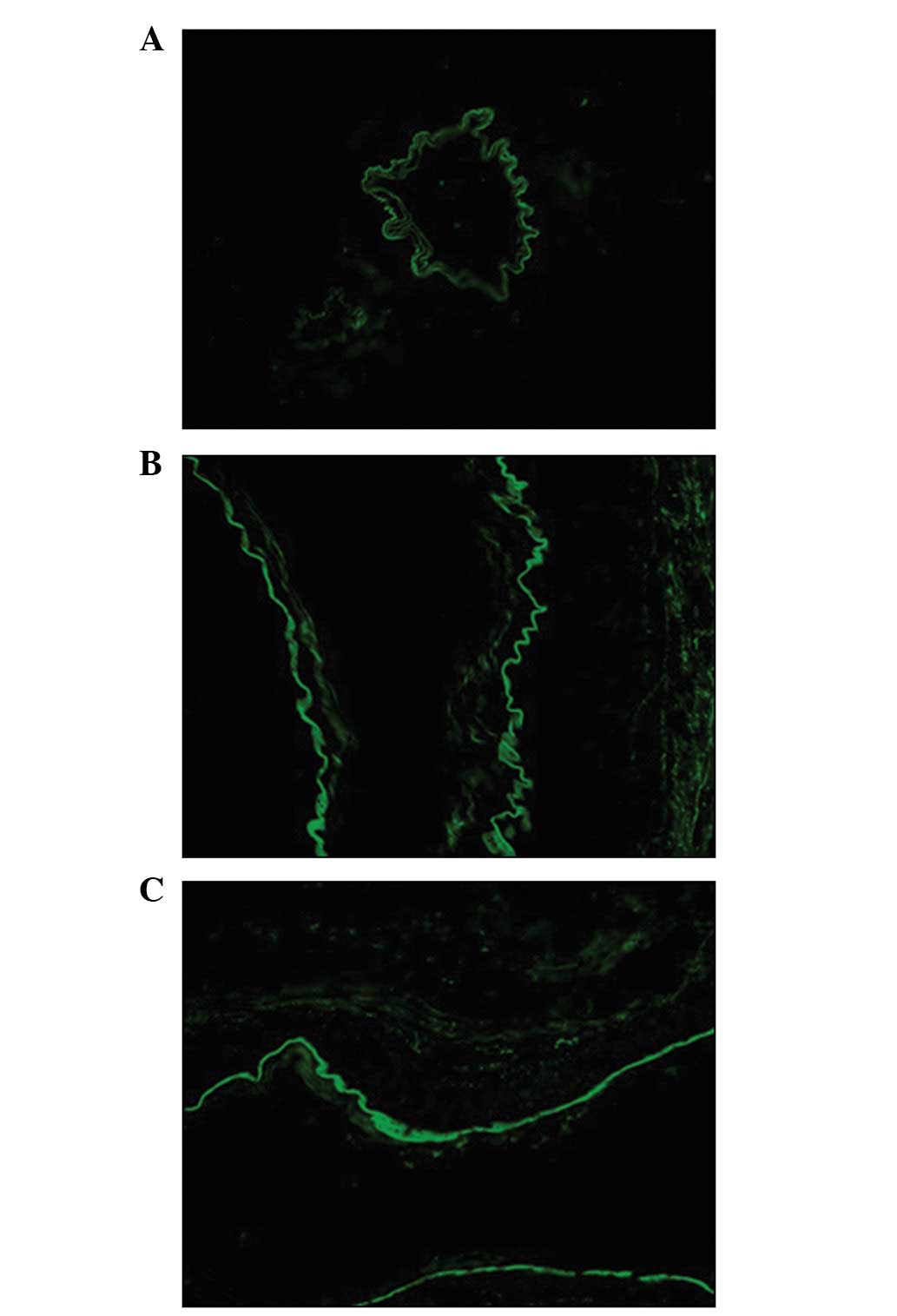
MMP-9 was expressed in endothelial cells, the
extracellular matrix and vascular adventitia in the AVM ruptured
and unruptured groups, whereas it was only expressed in endothelial
cells in the control group. In the ruptured group, MMP-9
fluorescence was more intense and widespread than in the AVM
tissues of the unruptured group (Fig.
6).
The ruptured and unruptured AVM vessels had a
disorderly structure of the smooth muscles, uneven wall thickness
and partial/incomplete endothelial cells compared with the control
vessel (Fig. 7). The blood levels
of IL-6 were correlated with the tissue levels of activated MMP-9
(r=0.1691, P=0.0240; Fig. 8).
Discussion
The present study has demonstrated that MMP-9 is
activated in AVM tissues, which leads to abnormal vascular
extracellular matrix degradation and increases the risk of cerebral
AVM hemorrhage. Plasma IL-6 levels were significantly increased in
AVM patients and this correlated with tissue-activated MMP-9
protein levels, indicating that plasma IL-6 concentration level
changes may be an index for brain AVM hemorrhage.
Hemorrhage is a major cause of death and disability
in cerebral AVM. H&E staining demonstrated that the ruptured
and unruptured AVM vessels had a disorderly structure of the smooth
muscles, uneven wall thickness and partial/incomplete endothelial
cells compared with the control vessels (Fig. 7). Hemorrhages are caused by
hemodynamic effects, however, the local inflammatory response and
angiogenic processes are also important factors. The blood vessels
near AVMs often experience inflammatory cell infiltration (8) and MMPs are important in local
inflammatory responses.
AVM has historically been associated with angiogenic
factors, including vascular endothelial growth factor (VEGF), CD34
and CD31 (12,13); however, MMPs have been the main
focus in recent studies. MMPs are a group of Zn2+- and
Ca2+-dependent proteases that modify the extracellular
matrix. Their substrates include collagen, fibronectin, laminin and
proteoglycans. Under normal circumstances, MMPs exist in an
inactive zymogene form. After activation by plasminogens, they are
rapidly degraded, which ensures a low level of active MMPs in
tissues. MMP-9 and -2 are important in neovascularization and the
remodeling and degradation of the extracellular matrix. Activated
MMP-9 and -2 are able to degrade extracellular matrix components,
including collagen IV, resulting in damage to the stability and
integrity of blood vessels and a weakened blood-brain barrier,
which leads to an increased risk of hemorrhage (9,14,15).
MMP-9 or -2 activity also promotes a loose connection between
vascular endothelial cells, causing damage to vascular smooth
muscle and adventitia. We demonstrated that the MMP-9 total protein
level in the unruptured group was significantly higher than that in
the ruptured and control groups. Gelatin zymography showed that
MMP-9 expression levels in the ruptured group were significantly
higher than those in the unruptured and control groups, suggesting
that more MMP-9 was activated and released in the form of active
plasminogen in the ruptured group. The MMP-9 tissue
immunofluorescence results showed that MMP-9 was expressed in the
extracellular matrix and vascular adventitia in AVM patients,
whereas it was only present in the endothelial cells in the control
group. Furthermore, the distribution and fluorescence intensity of
MMP-9 in the ruptured group were greater than in the unruptured
group. This suggests that more MMP-9 was secreted and activated in
ruptured AVM tissue, allowing it to degrade the extracellular
matrix and basement membrane, resulting in damage to the stability
and integrity of blood vessels and increasing the risk of AVM
hemorrhage.
Findings of recent studies have shown that AVM
patients with subarachnoid hemorrhage expressed higher levels of
IL-6 protein in tissue compared with patients without rupture
(6). IL-6 protein levels have been
associated with the IL6 174GG genotype (16–20),
which may be an upstream promoter in the angiogenic cascade
(12,21–24).
By contrast, other studies have shown that lower IL-6 levels exist
in abdominal aortic aneurysm and an increased IL-6 level was
associated with a lower incidence of ischemic events in giant cell
arteritis (25–27). In the present study, we compared
the plasma levels of IL-6 in the control, ruptured and unruptured
groups and showed that IL-6 levels were significantly higher in AVM
groups than in the control group. Additionally, IL-6 levels in the
ruptured group were significantly higher than in the unruptured
group. This result was consistent with previous observations that
IL-6 may be associated with vascular disease. Chen et
al(27) showed that IL-6
induced MMP-3 and -9 expression and activity in the mouse brain and
increased the proliferation and migration of cerebral endothelial
cells in AVM. IL-6 mRNA levels were associated with MMP-3 and -9
mRNA levels (27,28). These studies suggest an association
between IL-6 and AVM. Therefore, to further investigate the
association between IL-6 and hemorrhage in AVM, we examined the
plasma IL-6 concentration and analyzed the correlation between the
plasma level of IL-6 and activated MMP-9 in AVM tissues. Analysis
of tissue MMP-9 protein levels and the plasma IL-6 level using
linear regression identified that the plasma levels of IL-6 were
correlated with the tissue levels of activated MMP-9 (r=0.1691,
P=0.0240), as shown in Table I.
This result was in collaboration the with outcome of a study by
Chen et al(27), mentioned
above. All these results appear to suggest plasma IL-6 level as a
predictor of hemorrhage risk. Results from the study carried out by
Kim et al showed that tumor necrosis factor-α and IL-1 may
also be associated with AVM hemorrhage (29,30).
In this study, we also observed NF-κB protein expression in each
group. NF-κB expression in the ruptured group was significantly
higher than in the unruptured and control groups. IκBα, an
inhibitor of NF-κB, was expressed at lower levels in the ruptured
and unruptured groups than in the control group; this exhibits the
same trend as IL-6.
Taken together, IL-6 levels in the plasma of
patients with AVM showed similar increases regardless of whether
they were in the ruptured or unruptured group and were
significantly higher than those in the control group. Considering
the hemorrhagic predicting characteristic of MMP-9, the correlation
of plasma IL-6 and tissue MMP-9 and the significant differences in
plasma IL-6 levels between the control, ruptured and unruptured
groups, our study was consistent with the hypothesis that IL-6 is
associated with the MMP-9 level and hemorrhage in AVM. Therefore,
plasma IL-6 may offer a therapeutic intervention for cerebral AVM
and is a possible predictor of hemorrhage risk.
There were limitations in the present study.
Quantitative measures should have been carried out on the H&E
staining and immunofluorescence to increase the relevance of
results. Furthermore, future studies are required to explore the
plasma levels of IL-6 in patients before surgery and after, for one
day, one week and one month until three months, to define the
variation in IL-6 levels during this time for AVM patients.
References
|
1
|
ApSimon HT, Reef H, Phadke RV and Popovic
EA: A population-based study of brain arteriovenous malformation:
long-term treatment outcomes. Stroke. 33:2794–2800. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crawford PM, West CR, Chadwick DW and Shaw
MD: Arteriovenous malformations of the brain: natural history in
unoperated patients. J Neurol Neurosurg Psychiatry. 49:1–10. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fults D and Kelly DL Jr: Natural history
of arteriovenous malformations of the brain: a clinical study.
Neurosurgery. 15:658–662. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Martin NA and Vinters HV: Arteriovenous
malformations. Neurovascular Surgery. Carter LP, Spetzler RF and
Hamilton MG: McGraw-Hill; New York: pp. 875–903. 1994
|
|
5
|
Chen Y, Pawlikowska L, Yao JS, et al:
Interleukin-6 involvement in brain arteriovenous malformations. Ann
Neurol. 59:72–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okazaki S, Furukado S, Abe Y, et al:
Association of inflammatory markers and carotid intima-media
thickness with the risk of cardiovascular events in high-risk
patients. Cerebrovasc Dis. 30:180–187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Zhu W, Bollen AW, et al: Evidence
of inflammatory cell involvement in brain arteriovenous
malformations. Neurosurgery. 62:1340–1350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fishman D, Faulds G, Jeffery R, et al: The
effect of novel polymorphisms in the interleukin-6 (IL-6) gene on
IL-6 transcription and plasma IL-6 levels, and an association with
systemic-onset juvenile chronic arthritis. J Clin Invest.
102:1369–1376. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feiler S, Plesnila N, Thal SC, et al:
Contribution of matrix metalloproteinase-9 to cerebral edema and
functional outcome following experimental subarachnoid hemorrhage.
Cerebrovasc Dis. 32:289–295. 2011. View Article : Google Scholar
|
|
10
|
Loftus IM, Naylor AR, Goodall S, et al:
Increased matrix metalloproteinase-9 activity in unstable carotid
plaques. A potential role in acute plaque disruption. Stroke.
31:40–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chyatte D and Lewis I: Gelatinase activity
and the occurrence of cerebral aneurysms. Stroke. 28:799–804. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sandalcioglu IE, Asgari S, Wende D, et al:
Proliferation activity is significantly elevated in partially
embolized cerebral arteriovenous malformations. Cerebrovasc Dis.
30:396–401. 2010. View Article : Google Scholar
|
|
13
|
Choi EJ, Walker EJ, Shen F, et al: Minimal
homozygous endothelial deletion of Eng with VEGF stimulation is
sufficient to cause cerebrovascular dysplasia in the adult mouse.
Cerebrovasc Dis. 33:540–547. 2012. View Article : Google Scholar
|
|
14
|
Terry CF, Loukaci V and Green FR:
Cooperative influence of genetic polymorphisms on interleukin 6
transcriptional regulation. J Biol Chem. 275:18138–18144. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rundek T, Elkind MS, Pittman J, et al:
Carotid intima-media thickness is associated with allelic variants
of stromelysin-1, interleukin-6, and hepatic lipase genes: the
Northern Manhattan Prospective Cohort Study. Stroke. 33:1420–1423.
2002. View Article : Google Scholar
|
|
16
|
Jones KG, Brull DJ, Brown LC, et al:
Interleukin-6 (IL-6) and the prognosis of abdominal aortic
aneurysms. Circulation. 103:2260–2265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brull DJ, Leeson CP, Montgomery HE, et al:
The effect of the Interleukin-6-174G>C promoter gene
polymorphism on endothelial function in healthy volunteers. Eur J
Clin Invest. 32:153–157. 2002.
|
|
18
|
Acalovschi D, Wiest T, Hartmann M, et al:
Multiple levels of regulation of the interleukin-6 system in
stroke. Stroke. 34:1864–1869. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hernández-Rodríguez J, Segarra M,
Vilardell C, et al: Elevated production of interleukin-6 is
associated with a lower incidence of disease-related ischemic
events in patients with giant-cell arteritis: angiogenic activity
of interleukin-6 as a potential protective mechanism. Circulation.
107:2428–2434. 2003.
|
|
20
|
Hashimoto T, Lawton MT, Wen G, et al: Gene
microarray analysis of human brain arteriovenous malformations.
Neurosurgery. 54:410–425. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aoki Y, Jaffe ES, Chang Y, et al:
Angiogenesis and hematopoiesis induced by Kaposi’s
sarcoma-associated herpesvirus-encoded interleukin-6. Blood.
93:4034–4043. 1999.PubMed/NCBI
|
|
22
|
Gaudino M, Andreotti F, Zamparelli R, et
al: The-174G/C interleukin-6 polymorphism influences postoperative
interleukin-6 levels and postoperative atrial fibrillation. Is
atrial fibrillation an inflammatory complication? Circulation.
108(Suppl 1): II195–II199. 2003. View Article : Google Scholar
|
|
23
|
Pola R, Flex A, Gaetani E, et al:
Synergistic effect of -174G/C polymorphism of the interleukin-6
gene promoter and 469 E/K polymorphism of the intercellular
adhesion molecule-1 gene in Italian patients with history of
ischemic stroke. Stroke. 34:881–885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Flex A, Gaetani E, Pola R, et al: The -174
G/C polymorphism of the interleukin-6 gene promoter is associated
with peripheral artery occlusive disease. Eur J Vasc Endovasc Surg.
24:264–268. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sternlicht MD and Werb Z: How matrix
metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol.
17:463–516. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brinckerhoff CE and Matrisian LM: Matrix
metalloproteinases: a tail of a frog that became a prince. Nat Rev
Mol Cell Biol. 3:207–214. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Pawlikowska L, Yao JS, et al:
Interleukin-6 involvement in brain arteriovenous malformations. Ann
Neurol. 59:72–80. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hashimoto T, Wu Y, Lawton MT, et al:
Coexpression of angiogenic factors in brain arteriovenous
malformations. Neurosurgery. 56:1058–1065. 2005.PubMed/NCBI
|
|
29
|
Achrol AS, Pawlikowska L, McCulloch CE, et
al: Tumor necrosis factor-alpha-238G>A promoter polymorphism is
associated with increased risk of new hemorrhage in the natural
course of patients with brain arteriovenous malformations. Stroke.
37:231–234. 2006.
|
|
30
|
Kim H, Hysi PG, Pawlikowska L, et al:
Common variants in interleukin-1-Beta gene are associated with
intracranial hemorrhage and susceptibility to brain arteriovenous
malformation. Cerebrovasc Dis. 27:176–182. 2009. View Article : Google Scholar : PubMed/NCBI
|