Introduction
Colorectal cancer (CRC) is the joint name for colon
and rectal cancer, one of the most common malignant tumors.
Numerous scholars have observed that the occurrence and development
of CRC is closely correlated with diabetes mellitus (DM) (1–4). The
studies observed that the mechanism of DM-induced CRC may include
the following: i) hyperglycemia, ii) the role of hyperinsulinemia
and insulin resistance, iii) the role of insulin-like growth
factor-1 (IGF-1), iv) the role of metabolic syndrome-related
inflammatory factors (including adipokines) (5) for cell proliferation, v) the role of
the immune system and vi) the increase of plasma endothelin level
in diabetics, as well as the abnormalities of various trace
elements, including chrome, zinc, manganese, selenium, magnesium
and ferrum. The incidence of colorectal tumors caused by the
increased insulin secretion from certain hypoglycemic agents may
also be involved. In the present study, in order to further
demonstrate the correlation between these factors, a type 2 DM
(T2DM) model was established based on a mouse model of a
transplanted colorectal tumor. The changes in the mouse serum IGF-1
levels and the vascular endothelial growth factor (VEGF) expression
were observed in the tumor in order to discuss whether type 2
diabetes was a significant risk factor for promoting the
development and progression of CRC and to explain the possible
mechanism behind this. The present study was conducted in the hope
of providing a research basis for the prevention of CRC.
Materials and methods
Experimental groups
ICR mice were purchased from the Shanghai SLAC
Laboratory Animal Center (Shanghai, China). The mice were 4–6 weeks
old, weighed 22±2.0 g and were half males and half females. The
mice were fed in a clean animal room [Certification Number SCXK
(Shanghai) 2008-0005], at a room temperature of 22–25°C. The
bedding, drinking water and food were all sterilized. The mice were
randomly divided into four groups; 8 mice comprising group A were
the blank control group, 8 mice in group B were the DM group, 12
mice in group C were the CRC group and 12 mice in group D were the
CRC-DM group. Groups B and D were provided a high-fat and
high-sugar diet for four weeks and the other groups were provided a
normal diet for four weeks. The present study was carried out in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee (IACUC) of the Third
Affiliated Hospital of Nantong University, Wuxi, Jiangsu,
China.
Animal model
CT26 cells were suspended in 20% new-born calf serum
and cell culture medium (RPMl-1640 nutrient solution containing 100
IU/ml penicillin and 100 μg/ml streptomycin), then cultivated with
5% CO2 at 37°C. The cells with adherent growth were
removed following mechanical blowing and prepared to the desired
cell suspension concentration with an appropriate amount of
physiological saline. A 0.2 ml suspension of 2×106
cancer cells was subcutaneously inoculated into the right axillary
of the ICR mice. The cells were passaged twice in the mice and the
tumor body was peeled off in 1 mm3 sections. These cells
were then subcutaneously inoculated with a surgical trocar into the
right axillary of the mice in groups C and D. After 7 days, the
mice with a tumor diameter of ~0.5 cm were selected to establish
the transplanted tumor model. Following the successful transplant
of the tumors into groups C and D, the mice in groups B and D were
administered an intraperitoneal injection of 100 mg/kg
streptozotocin (STZ) sodium citrate buffer (Sigma, St. Louis, MO,
USA) and the normal control group was administered an
intraperitoneal injection of pH 4.4 sodium citrate buffer. A T2DM
model was established when the blood sugar measured >11.0 mmol/l
following two consecutive tail amputations. Groups B and D
continued to be provided with high-fat and high-sugar diets and the
other groups were provided with normal diets.
Experiment detection
On the 3rd day subsequent to the establishment of
the transferred tumor model, the long and short diameters of the
subcutaneous tumor were measured with a vernier caliper to
calculate the tumor volume and draw the tumor growth curves. The
animals were sacrificed following 28 days of tumor growth and the
subcutaneous tumor was peeled off and weighed with an electronic
scale. The serum IGF-1 level was measured using a kit (Boster
Biological Engineering Company, Wuhan, China), following the
manufacturer’s instructions. The values in each well were read at
the 450 nm wavelength following all the surgical procedures. The
standard curve was drawn on logarithmic coordinate paper according
to the measured A-values of each standard substance and the test
results of the serum specimens in all the laboratory mice were
compared with the standard curve to obtain their serum IGF-1
levels. The VEGF expression in the tumor tissues was detected by
immunohistochemistry (SP method). Claybank particles that appeared
in the normal mucosa epithelial cells or in the cytoplasm of tumor
cells were considered as evidence of positive expression. Positive
expression was judged according to the standard of Volm: i) The
uniformly colored tumor area was selected and the colored cells
were counted using ×400 magnification. The percentage of colored
cells was calculated and represented the total number of cells in
the respective field of vision. The means were taken and graded
from 0–3 according to the percentage of the colored cells
accounting for the total number of cells in the field of vision.
Grade 0, 0%; grade 1, <25%; grade 2, 25–56%; and grade 3,
>56%. ii) The cells were graded according to the degree of
coloration: Grade 0, no color; grade 1, weakly colored; grade 2,
moderately colored; and grade 3, strongly colored. The results were
judged according to the sum of the i) and ii) values: negative, 0;
weakly positive, 1–2; moderately positive, 3–4; and strongly
positive, 5–6.
Statistical analysis
The measurement data were presented as the mean ± SD
and all the indicator analyses were performed by SPSS 13.0
software. The inter-group comparison used LSD (least significant
difference; homogeneity of variance) or Tamhane (heterogeneity of
variance) tests for statistical analysis. P<0.05 was considered
to indicate a statistically significant difference.
Results
General observation
The mice in groups B and D developed a significant
increase in weight subsequent to being fed with a high-fat and
high-sugar diet for 4 weeks. The mice in groups C and D developed
tumors, mostly elliptical in shape, following the inoculation with
CRC cells and tumors were left to grow for 1 week, and two weeks
later, an irregular and uneven surface gradually appeared. The
tumors of the two groups continued to grow, but were more evident
in the CRC-DM group. There were no significant differences in diet
or activity between each group. No deaths occurred as a result of
the experiment, with the exception of two mice that escaped from
the blank control group.
Comparison of the change in tumor volume
and weight between the CRC and CRC-DM groups
On the 7th day subsequent to the inoculation of the
tumor cells in the CRC and the CRC-DM groups, the tumor tissues had
grown and the tumor volumes had increased, although this was more
evident in the CRC-DM group (Fig.
4). Subsequent to the experiment, the average weight of the
transplanted tumors in the CRC-DM group was 2.30±0.43 g, which was
higher than that in the CRC group (1.19±0.25 g), with a
statistically significant difference between the two groups. This
indicated that the tumors based on type 2 diabetes were able to
grow larger and more rapidly (Fig.
1 and Table I).
 | Table IComparison of transplanted tumor
weight in mice between the two groups. |
Table I
Comparison of transplanted tumor
weight in mice between the two groups.
| Groups | Number of mice | Tumor weight (g) |
|---|
| CRC | 12 | 1.19±0.25 |
| DM-CRC | 12 | 2.30±0.43a |
Comparison of serum IGF-1 values in each
group
Compared with the control group, the serum IGF-1
levels in the CRC group were slightly higher, but with no
statistically significant difference, while the serum IGF-1 levels
in the DM and CRC-DM groups were increased with a statistically
significant difference (P<0.05). Compared with the CRC group,
the serum IGF-1 levels in the DM-CRC group were increased, with a
statistically significant difference between the two groups
(P<0.05). This indicated that the serum IGF-1 levels in the DM
group were higher than those in the non-DM groups and that there
were no significant changes in the serum IGF-1 levels subsequent to
the transplantation of tumor cells when based on DM (Table II).
 | Table IISerum levels of IGF-1. |
Table II
Serum levels of IGF-1.
| Groups | Number of mice | IGF-1 value
(ng/ml) |
|---|
| Blank control | 6 | 69.83±25.57 |
| CRC | 12 | 70.17±25.27 |
| DM | 8 | 103.63±31.67a |
| DM-CRC | 12 | 105.33±32.32a,b |
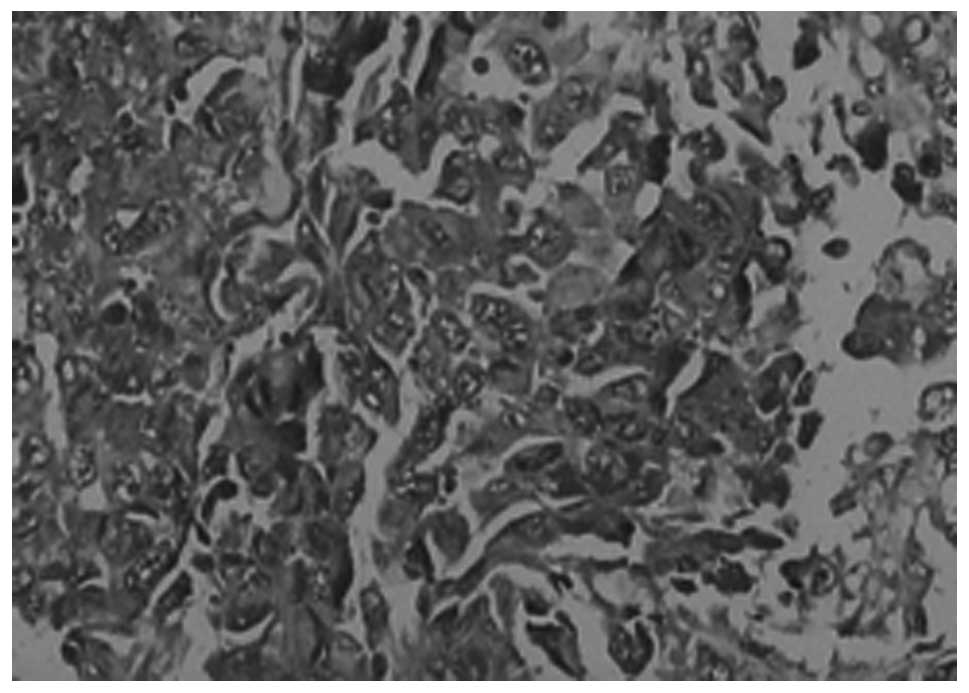
Comparison of VEGF expression in the
tumor tissues between the CRC and CRC-DM groups
As shown in Table
III, VEGF was primarily expressed in the cytoplasm of the tumor
cells. There were varying levels of expression in the two groups,
but a more evident expression was present in the CRC-DM group than
in the CRC group (P<0.05; Table
III, Figs. 2 and 3).
 | Table IIIComparison of VEGF expression in the
transplanted mouse tumors. |
Table III
Comparison of VEGF expression in the
transplanted mouse tumors.
| Groups | Number of mice | VEGF expression
(%) |
|---|
| CRC | 12 | 42.9±7.5 |
| DM-CRC | 12 | 70.0±11.5a |
Discussion
In previous studies, the T2DM mouse models were
established by the transplantation of tumor cells into normal and
nude mice or by inducing insulin resistance with a high-fat diet
(6), however, there were few
studies on the concurrent establishment of a T2DM and colorectal
transplanted tumor model. In the present study, the results of the
concurrent establishment of the two models was shown. The
colorectal transplanted tumors were passaged for only two
generations, in line with the requirement of <15 generations for
passaging in vivo, effectively preventing the repeated
passages in the tumor body from causing a reduction in dissimilar
types of cell phenotypes and changes in biological characteristics.
Inoculation and passaging should be strictly performed under
aseptic conditions. In the CRC-DM group, the tumor cells were
transplanted after the mice were fattened. Subsequent to the growth
of the tumor cells into a group of a certain size, STZ was injected
again and the blood sugar level was measured until it was >11.0
mmol/l. At the same time, the transplanted tumor continued to grow;
the tumor exhibited rapid growth during the overall study, with no
growth failures or extinction phenomena, indicating that the mouse
colorectal transplanted tumor had not been excluded for
histoincompatibility, therefore providing a tumor specimen for the
study.
IGF-1, also known as somatomedin C, is mainly
synthesized and secreted by the liver. In the blood, IGF-1 and
IGF-binding protein (IGFBP) exist in a complex form, but have no
activity, and only combined with its specific receptors, IGF-1 was
able to cause a series of biochemical reactions. Subsequent to
IGF-1 combining with its receptors, the receptor is phosphorylated
itself and causes various types of signal activation reactions,
including those of the tyrosine kinase and phosphatidylinositol
(IP)-3 kinase/protein kinase B (PKB) pathways. This may also
activate the insulin receptor substrate (IRS) family proteins and
cause certain biological effects using these pathways. It has been
observed in previous studies that in the majority of patients with
diabetes, the IGF-1 level was higher than in healthy individuals,
while the IGFBP was lower (7).
However, there have also been other studies that observed the blood
IGF-1 and IGFBP levels in patients with T2DM and identified that
the IGF-1 levels were increased only following the development
complications. The present study observed that the mouse serum
IGF-1 levels in the T2DM model were increased compared with the
control group, which proved our previous assumptions that the IGF-1
level in T2DM patients was higher than in healthy individuals.
Considering that the plasma insulin levels in T2DM show a delayed
release with sugar stimulation, a previous study showed that the
plasma insulin levels with hollow and sugar stimulation were often
higher than the normal levels and that the plasma insulin levels
were often able to show mitogenic characteristics and improve the
IGF-1 level to achieve biological characteristics (7). Since IGFBP may be combined with IGF-1
to inhibit the physiological role of IGF-1, insulin may reduce the
secretion of hepatogenic IGFBP and indirectly cause the increase in
the IGF-1 level (8). IGF-1 may
promote growth, development and metabolism, as well as the
immunomodulatory effect. It has been demonstrated that IGF-1 is
closely correlated with the growth and transfer of tumors. IGF-1
was observed with strong mitosis immunogenicity and anti-apoptotic
activity and was able to be produced by the autocrine and paracrine
function of tumor cells to promote the differentiation and growth
of the cells (9). Exogenous IGF-1
as a growth factor may also promote the growth of the tumor cells.
IGF-1 combines with its receptors to activate the PI3K/Akt and MAPK
signaling pathways (10),
inhibiting tumor cell apoptosis and promoting cell proliferation,
respectively (11–14). In the present study, the results
indicated that the growth of the transplanted colorectal tumor
tissues from the mouse T2DM model was more rapid than that from the
normal mice. The tumor volume and weight were also higher than in
the normal mice, indicating that the high level of IGF-1 caused by
T2DM was a significant factor for CRC occurrence and
development.
VEGF, also termed vascular permeable factor, is a
highly specific vascular endothelial cell mitogen. Its main
biological functions include the ability to i) selectively enhance
vascular endothelial cell mitosis to stimulate the endothelial
cells proliferation and promote angiogenesis; and ii) strengthen
the permeability of blood vessels, particularly small blood
vessels, to conduct the extravasation and deposition of plasma
proteins and other macromolecules into the extravascular matrix,
providing nutrition for the establishment of a new capillary
network of blood vessels. The biological activity of VEGF is mainly
mediated by the tyrosine kinase receptor, with high affinity, and
combines with its specific VEGF receptor (VEGFR) to cause a series
of signal transductions, release a variety of cytokines and growth
factors, stimulate vascular endothelial cell proliferation and
migration and promote angiogenesis. This is important in the growth
and metastasis of tumors (15).
Studies (16,17) have shown that VEGF expression is
positively correlated with the infiltration depth, lymph node
metastasis, distant metastasis and Dukes’ staging of CRC, thereby
affecting the growth and metastasis of tumors. The present study
has shown that the growth of colorectal transplanted tumors from
the mouse T2DM model was more evident than that from normal mice;
the tumors volumes were larger and the VEGF expression of the tumor
tissues was stronger, as observed by immunohistochemistry
(P<0.05). Diabetes causes an increase in blood sugars and free
fatty acids, providing energy for tumor growth. Long-term
hyperglycemia would also cause the basement membrane of the
capillary blood vessels to thicken and the permeability of the
capillary blood vessels to decline. This would cause the enzyme
system participating in the aerobic metabolism of cells to be
damaged and also cause disorder in the aerobic metabolism process,
with enhanced anaerobic glycolysis. It has been shown that the
ability for glycolysis in normal cells is weaker than in tumor
cells, therefore, the hyperglycemia state is more conducive to the
growth of the tumor cells (18).
This may be a reason for the more rapid growth of tumors based on
diabetes. The present study has also indicated that the serum IGF-1
levels in the DM-CRC group were higher than those in the CRC group,
while at the same time, the VEGF-positive expression rate in the
tumor tissues was also stronger than that in the CRC group,
indicating that they had a certain synergy to CRC growth. IGF-1 is
able to stimulate endothelial cell migration and morphological
differentiation to induce angiogenesis and may also be combined
with the subendothelial extracellular matrix across vascular
endothelial cells using the paracellular pathway. This may
therefore play a role in endothelial cell subsistence and
stabilization (19) and enhance
the VEGF expression in the CRC cells, potentially resulting in the
promotion of tumor angiogenesis and tumor growth and transfer,
indicating that diabetes may promote tumor growth and that there is
a link between them.
However, the present study was only aimed to
actively utilize tumor cells based on the mouse T2DM model in order
to observe tumor growth. The study confirmed that diabetes was able
to promote tumor growth, but did not prove that diabetes was able
to induce tumor generation. VEGF was one of the cell signaling
molecules used to promote the growth of the tumors. Additionally,
the mechanism of T2DM in the induction of intestinal tumor
generation was investigated to discuss the numerous types of cell
signaling pathways that cause tumor growth.
References
|
1
|
Larsson SC, Orsini N and Wolk A: Diabetes
mellitus and risk of colorectal cancer: a meta-analysis. J Natl
Cancer Inst. 97:1679–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meyerhardt JA, Catalano PJ, Haller DG,
Mayer RJ, Macdonald JS, Benson AB III and Fuchs CS: Impact of
diabetes mellitus on outcomes in patients with colon cancer. J Clin
Oncol. 21:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levi F, Pasche C, Lucchini F and La
Vecchia C: Diabetes mellitus, family history, and colorectal
cancer. J Epidemiol Community Health. 56:479–480. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giouleme O, Diamantidis MD and Katsaros
MG: Is diabetes a causal agent for colorectal cancer?
Pathophysiological and molecular mechanisms. World J Gastroenterol.
17:444–448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pechlivanis S, Bermejo JL, Pardini B, et
al: Genetic variation in adipokine genes and risk of colorectal
cancer. Eur J Endocrinol. 160:933–940. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kobayashi M, Ohno T, Tsuchiya T and Horio
F: Characterization of diabetes-related traits in MSM and JF1 mice
on high-fat diet. J Nutr Biochem. 15:614–621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ezzat VA, Duncan ER, Wheatcroft SB and
Kearney MT: The role of IGF-I and its binding proteins in the
development of type 2 diabetes and cardiovascular disease. Diabetes
Obes Metab. 10:198–211. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ooi GT, Tseng LY, Tran MQ and Rechler MM:
Insulin rapidly decreases insulin-like growth factor-binding
protein-1 gene transcription in streptozotocin-diabetic rats. Mol
Endocrinol. 6:2219–2228. 1992.PubMed/NCBI
|
|
9
|
Guastamacchia E, Resta F, Triggiani V, et
al: Evidence for a putative relationship between type 2 diabetes
and neoplasia with particular reference to breast cancer: role of
hormones, growth factors and specific receptors. Curr Drug Targets
Immune Endocr Metabol Disord. 4:59–66. 2004. View Article : Google Scholar
|
|
10
|
Lee CT, Park KH, Adachi Y, et al:
Recombinant adenoviruses expressing dominant negative insulin-like
growth factor-I receptor demonstrate antitumor effects on lung
cancer. Cancer Gene Ther. 10:57–63. 2003. View Article : Google Scholar
|
|
11
|
Bai HZ, Pollman MJ, Inishi Y and Gibbons
GH: Regulation of vascular smooth muscle cell apoptosis: Modulation
of bad by a phosphatidylinositol 3-kinase-dependent pathway. Circ
Res. 85:229–237. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui T, Li L, del Monte F, et al:
Adenoviral gene transfer of activated phosphatidylinositol
3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in
vitro. Circulation. 100:2373–2379. 1999.
|
|
13
|
Ibrahim YH and Yee D: Insulin-like growth
factor-I and cancer risk. Growth Horm IGF Res. 14:261–269. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kohda M, Hoshiya H, Katoh M, et al:
Frequent loss of imprinting of IGF2 and MEST in lung
adenocarcinoma. Mol Carcinog. 31:184–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin LJ, Zheng CQ, Jin Y, Ma Y, Jiang WG
and Ma T: Expression of survivin protein in human colorectal
carcinogenesis. World J Gastroenterol. 9:974–977. 2003.PubMed/NCBI
|
|
16
|
Minagawa N, Nakayama Y, Hirata K, et al:
Correlation of plasma level and immunohistochemical expression of
vascular endothelial growth factor in patients with advanced
colorectal cancer. Anticancer Res. 22:2957–2963. 2002.PubMed/NCBI
|
|
17
|
Hashim AF, Al-Janabi AA, Mahdi LH,
Al-Toriahi KM and Yasseen AA: Vascular endothelial growth factor
(VEGF) receptor expression correlates with histologic grade and
stage of colorectal cancer. Libyan J Med. 5:2010. View Article : Google Scholar
|
|
18
|
Chang CK and Ulrich CM: Hyperinsulinaemia
and hyperglycaemia: possible risk factors of colorectal cancer
among diabetic patients. Diabetologia. 46:595–607. 2003.PubMed/NCBI
|
|
19
|
Grulich-Henn J, Ritter J, Mesewinkel S,
Heinrich U, Bettendorf M and Preissner KT: Transport of
insulin-like growth factor-1 across endothelial cell monolayers and
its binding to the subendothelial matrix. Exp Clin Endocrinol
Diabetes. 110:67–73. 2002. View Article : Google Scholar : PubMed/NCBI
|