Introduction
Gliomas are the most common type of intracranial
tumor and have the highest rate of mortality (1). Despite advanced diagnostics and
treatments, the average survival time does not exceed 15 months.
Matsukado et al observed that more than half of untreated
gliomas reached the contralateral hemisphere (2). Malignant gliomas display a prominent
degree of invasiveness into the surrounding normal brain tissue
(3), which compromises therapeutic
efforts and ultimately explains the high rates of recurrence and
the poor prognosis for patients. The implantation of a C6 glioma in
the rat brain and subsequent study of its biological behavior,
including the extent of tumor development, spontaneous regression
of the glioma, the best experimental time window, cell invasion
pathological characteristics and neoangiogenesis, have been
beneficial to the clinical treatment of gliomas.
A number of in vivo imaging modalities are
used to assess tumor development in preclinical animal models. MRI
is considered to be the method of choice for studying the
biological behavior of a tumor, as it enables the imaging of an
entire organ or animal longitudinally over time. MRI has the best
soft-tissue contrast, excellent sensitivity and a spatial
resolution approaching the cellular level with a voxel size of 10
μm in vitro and 50 μm in vivo. Furthermore, MRI
provides information regarding tumor location and size, the extent
of edema, relative blood volume fraction and blood-brain barrier
status without ionizing radiation (4). In addition, MRI is thought to be the
most promising technique for non-invasive cell tracking in
vivo. MRI negative contrast agents, such as superparamagnetic
iron oxide (SPIO) (5) and
micron-sized iron oxide particles (6,7), are
the most frequently used, and have been used to magnetically label
cells ex vivo, providing researchers with the ability to
monitor the distribution and migration of these cells in animals
and humans in vivo(8–10).
Intracellular SPIOs placed in a magnetic field cause signal
dephasing due to B0 inhomogeneities induced close to the
cells. The disruption of the magnetic field extends to a much
larger distance than the actual size of the SPIO nanoparticles,
making it possible to detect low numbers of cells, even single
cells (11), in vivo.
In designing this study, we had two objectives: i)
to investigate the long-term course and biological behavior of
orthotopically implanted C6 gliomas; and ii) to dynamically monitor
the distribution of SPIO-labeled C6 cells in rats with MRI.
Materials and methods
Cell culture and labeling
Rat C6 glioma cell lines were provided by the State
Key Laboratory of Biotherapy (Sichuan, China) and cultured at 37°C
in a humidified atmosphere with 5% CO2 in Dulbecco’s
modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA)
supplemented with 10% heat-inactivated foetal bovine serum, 100
kU/l penicillin and 100 mg/l streptomycin. Superparamagnetic
nanocrystals for labeling were amphiphilic polyethyleneimine
(PEI)/SPIO nanocomposites (mean diameter <100 nm), provided by
the National Engineering Research Center for Biomaterials, Sichuan
University. The SPIO nanoparticles were added to DMEM (5 μg Fe/ml)
(12). The C6 cells were incubated
in the culture medium for 12 h at 37°C in a 5% CO2
atmosphere. The cells were washed thoroughly with
phosphate-buffered saline (PBS) to remove unincorporated SPIOs and
collected. In the control group, C6 cells were cultured without
SPIOs.
Trypan blue viability assay
Labeled and unlabeled cells were suspended in PBS at
a concentration of 1×106/ml and mixed with 0.4% trypan
blue dye in a 1:1 ratio. The mixture (10 μl) was loaded onto a
hemocytometer and the cells were counted. Cells with an intact
membrane excluded the dye and were considered to be live cells. The
percentages of live and dead cells were determined.
Animal model
The local Institutional Animal Care and Use
Committee approved all animal procedures. The hosts were 12 male
Wistar rats weighing 300–350 g, obtained from the West China
Experimental Animal Center. They were kept on a regular daylight
schedule with rodent animal chow and water ad libitum. For
implantation surgery, each animal was anesthetized with 10% chloral
hydrate (4 ml/kg i.p.) prior to surgery. The head was placed on a
stereotactic head holder and a scalp incision was made along the
median line. A burr hole 1 mm in diameter was drilled into the
skull, 3 mm lateral to the bregma. A 25 μl cell suspension
(1×106 C6 cells in DMEM-free serum) was injected into
the right caudate nucleus, at a depth of 6 mm beneath the skull,
using a Hamilton syringe. The needle was slowly removed 5 min after
injection and the burr hole was plugged with vegetal wax.
MRI
All experiments were performed under anesthesia with
the following parameters: 5% isoflurane for induction, and 2%
isoflurane for maintenance in 60% air and 40% oxygen. The rectal
temperature was maintained at 37.0±0.5°C throughout the experiments
using a warming pad with hot water circulation, which was placed on
the back of the animal. All measurements were performed on a Bruker
Biospec 7T/30 cm horizontal bore magnet (Bruker BioSpin MRI,
Ettlingen, Germany) using a volume coil for the rat head (inner
diameter, 40 mm; outer diameter, 75 mm; length, 100 mm) at multiple
time-points (Table I). The
parameters were as follows: matrix (MTX), 256×256; FOV, 3 cm; slice
thickness, 1 mm; interslice, 1 mm. MSME T1 TR/TE, 561/14 msec; FA,
180°; NEX, 4; total scanning time, 9 min, 34 sec, 502 msec; RARE T2
TR/TE, 3000/45 msec; FA, 180°; NEX, 4; total scanning time, 6 min,
24 sec, 0 msec. Post-contrast T1-weighted images (T1WIs) were
obtained 5 min after the intravenous injection of 0.1 mmol/kg
gadopentetate dimeglumine contrast agent (Bayer Schering Pharma AG,
Berlin, Germany).
 | Table IScanning time points of every rat in
the experimental group and control group. |
Table I
Scanning time points of every rat in
the experimental group and control group.
| A, Experimental
group |
|---|
|
|---|
| Rat | Scanning
time-point |
|---|
| No. 1 | 2 h | 1 day | 11 days | 20 days | |
| No. 2 | 1 day | 3 days | 9 days | 20 days | |
| No. 3 | 1 day | 2 days | 3 days | 7 days | |
| No. 4 | 2 h | 2 days | 3 days | 9 days | 27 days |
| No. 5 | 2 days | 7 days | | | |
| No. 6 | 2 days | 11 days | 20 days | 27 days | |
| No. 7 | 11 days | 20 days | | | |
| No. 8 | 2 days | 3 days | 9 days | 20 days | 27 days |
|
| B, Control group |
|
| Rat | Scanning
time-point |
|
| No. 1 | 10 days | 20 days | 27 days |
| No. 2 | 10 days | 20 days | |
| No. 3 | 10 days | 20 days | 27 days |
| No. 4 | 10 days | 20 days | |
Histological examination
All animals were sacrificed after MRI study for
histological examination. Brains were immersed in 4%
paraformaldehyde and embedded in paraffin; 10 μm coronal sections
were then cut through the cortical hemispheres and stained with
hematoxylin and eosin (HE) and Perl’s solution, the latter of which
was used for iron detection.
Data analysis
All images were processed using ParaVision software
(v.5.0; Bruker Biospin MRI). Regions of interest (ROIs) were drawn
manually at four regions in the tumor on the biggest slice of the
tumors for each model. In each case, contralateral ROIs of
equivalent surface area were also drawn. Data are presented as the
means ± SD. Statistically significant differences between the
tumors and the contralateral areas and between the labeled
experimental and unlabeled control groups were analyzed by an
unpaired, two-tailed Student’s t-test using statistical software
(SPSS 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
The viabilities of the labeled and unlabeled cells
were analyzed using a trypan blue assay. We observed no significant
difference between the viabilities of the labeled (93.5%) and
unlabeled cells (94.2%).
All 12 rats tolerated infusion of the SPIO-labeled
and unlabeled C6 cells and no difference in clinical course was
observed between the animals with implanted tumors who received
labeled cells and the controls who received unlabeled cells. The C6
cells did not form a tumor in one rat; therefore, the tumor
formation rate was 92%. In the experimental group, we observed
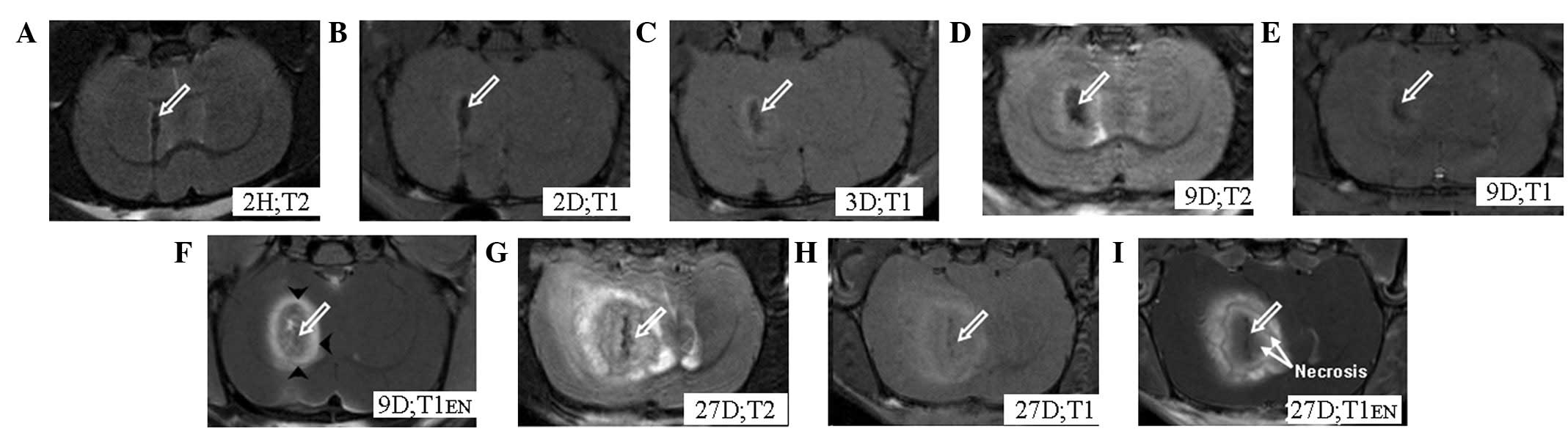
pronounced hypointense signal bands, which gradually faded over
time, but remained visible on the MRI 27 days after implantation
(Fig. 1). All animals were
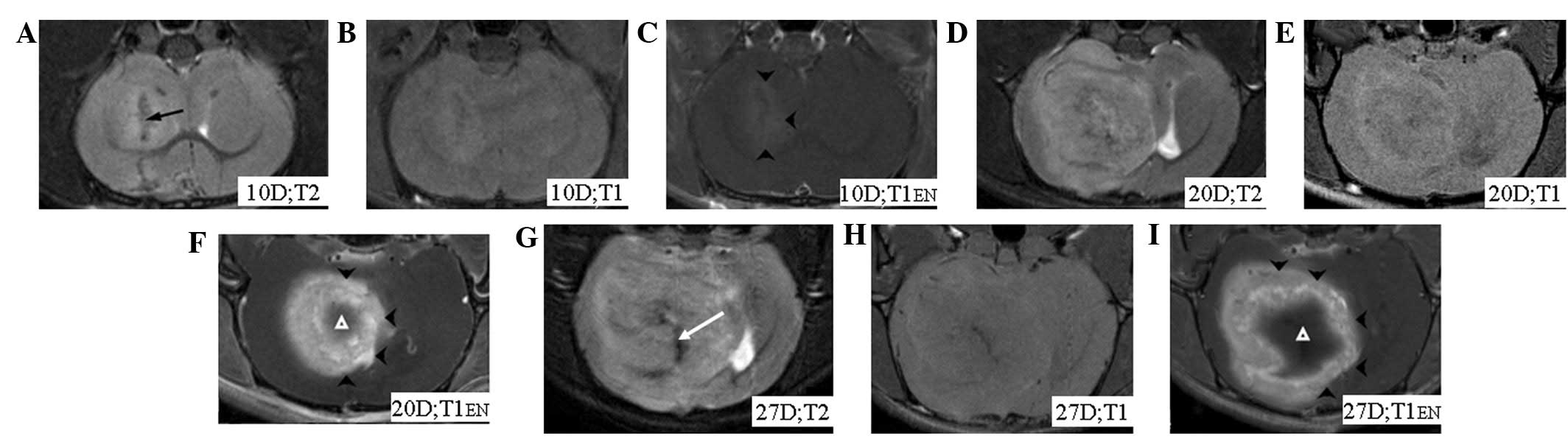
euthanized after day 27. In the control group, no hypointense
signal band was observed (Fig. 2).
MRI images from the experimental group revealed tumors from the 2nd
day after implantation; these presented as slightly hyperintense
regions with indefinite boundaries surrounding focal
hypointensities on the T1WIs. The tumors were enhanced on the
post-contrast T1WIs, with the exception of the necrotic areas
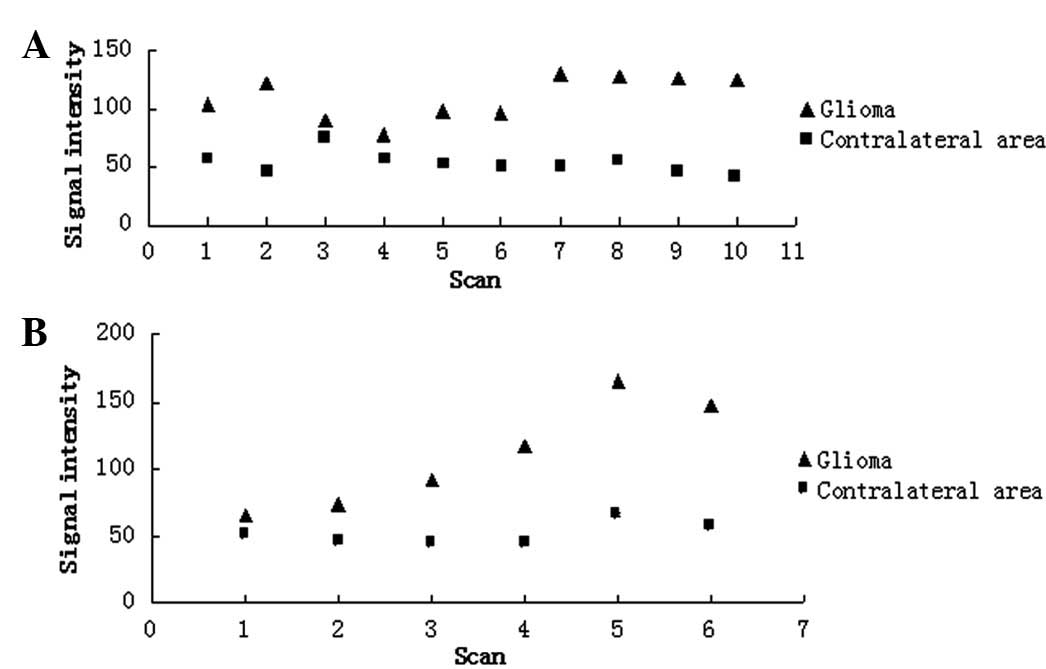
(Fig. 1). Following the injection
of contrast media, the signal intensity was found to increase
(P<0.05) in the tumor regions of the labeled experimental and
unlabeled control group animals when compared with the
contralateral tissue values of the same animals. There was no
statistically significant difference (P>0.05) in the signal
intensity of the tumor regions between the labeled experimental and
unlabeled control groups (Fig.
3).
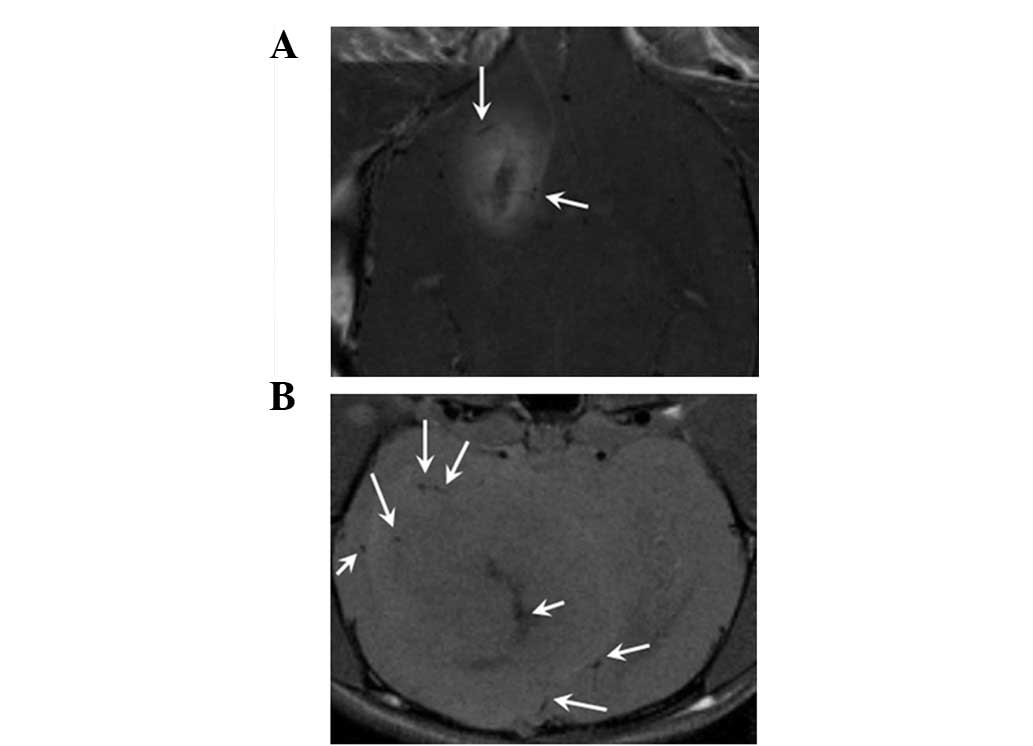
On the 9th day after implantation, thick tumor
feeder vessels ~0.2 mm in diameter were observed and these
increased rapidly over time. On day 27, diffuse, thick tumor feeder
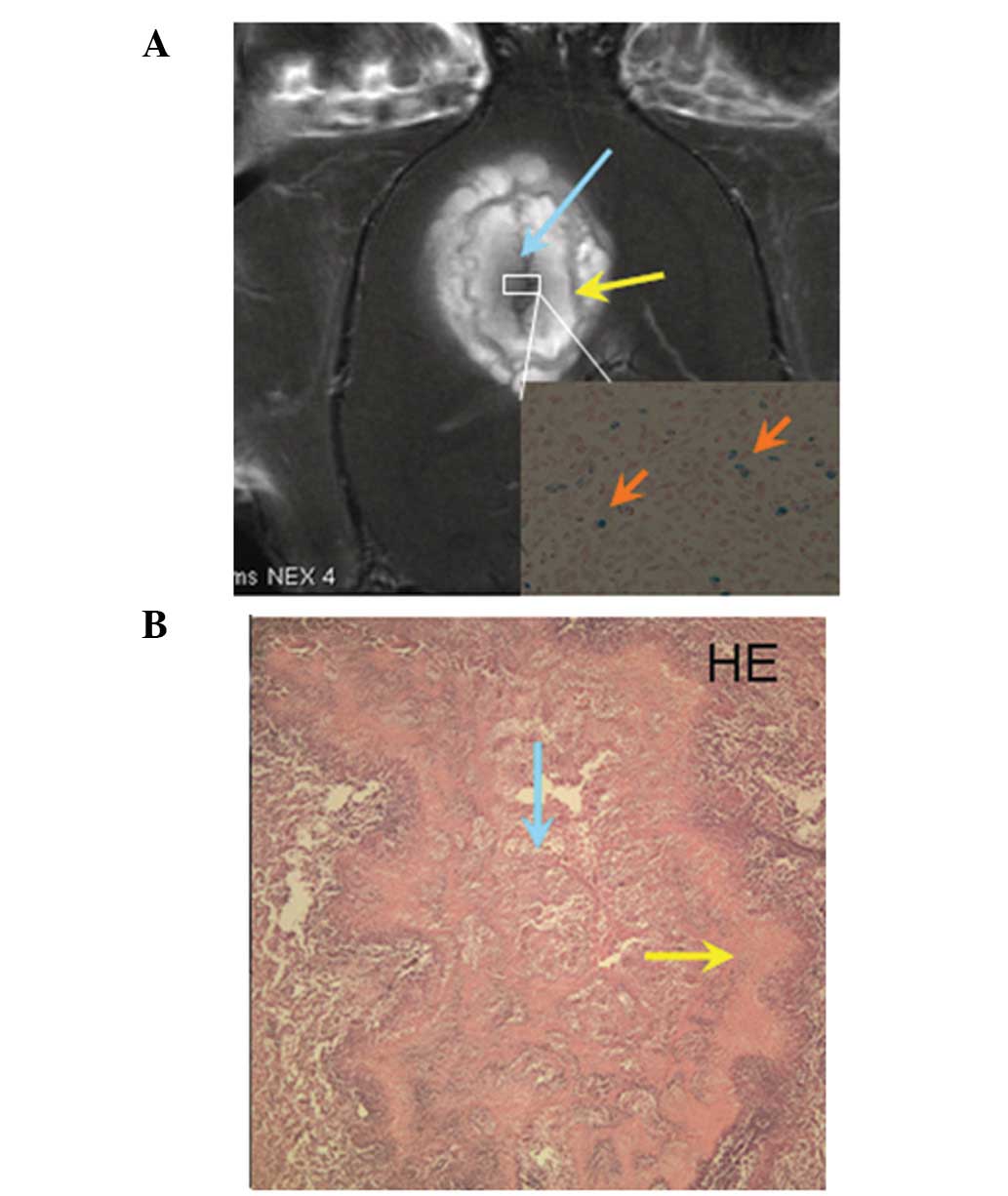
vessels were observed around as well as within the tumors (Fig. 4). Edema was observed in both
groups, and presented as hyperintense regions around the tumors on
the T2WIs. Besides the central areas of the tumors (around the
pronounced hypointense signal bands), cogwheel-shaped bands were
observed surrounding these central areas and neither were enhanced
in the post-contrast T1WIs (Fig.
5A) in accordance with the necrosis observed in the
photomicrographs following HE staining (Fig. 5B). The layers of necrosis were so
clear in the MRI that these images were very similar to the
photomicrographs of the HE-stained specimens. The blue dots in the
Perl’s-stained photomicrograph represent nanoparticles in the tumor
cells (Fig. 5A, insert). No blue
dots were observed in the control group.
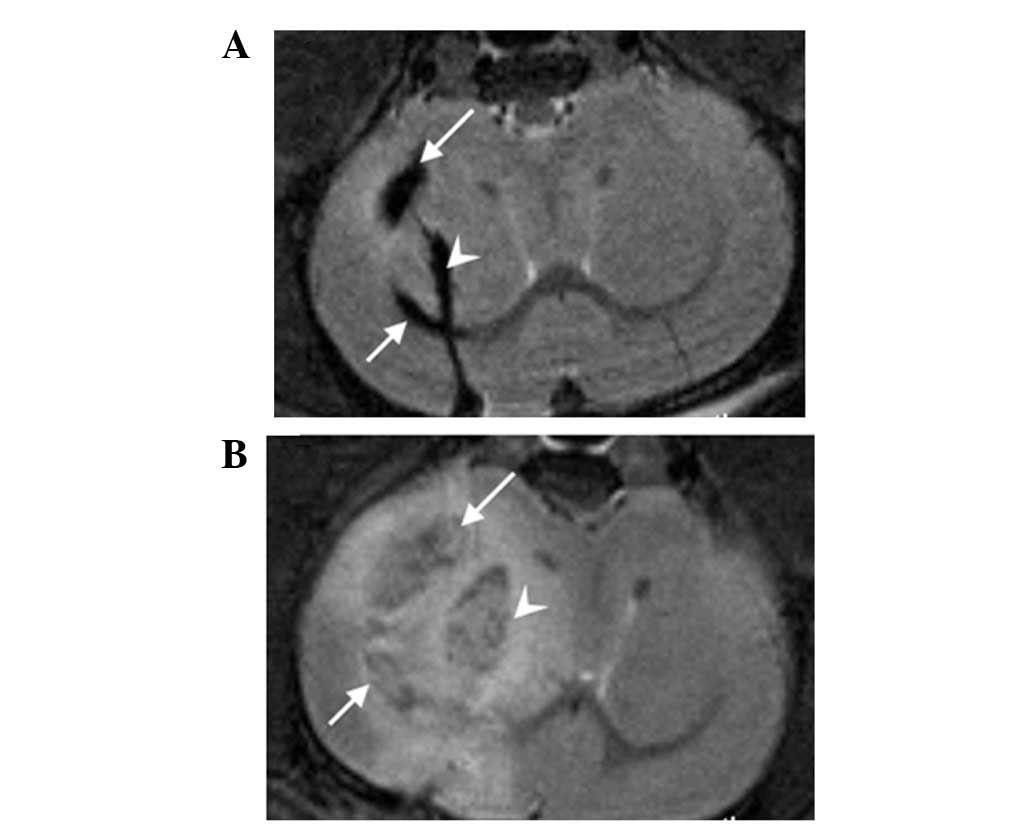
In the experimental group, hypointense regions were
observed not only in the right caudate nucleus but also in the
right lateral ventricle in 4/8 model animals; these were caused by
some of the labeled tumor cells flowing into the lateral ventricle.
In later MRI images, the tumor cells in the right caudate nucleus,
as well as those in the lateral ventricle, had grown into tumors.
The implanted tumor was generally irregular (Fig. 6), but in the control group it was
not possible to discern whether the implanted tumor cells were only
in the right caudate nucleus or if they had flowed into the lateral
ventricle in the early stages, as the signal from the unlabeled
cells was similar to that from normal brain tissue.
Discussion
C6 cells have been implanted in Sprague-Dawley, BDX,
BDIX and Wistar rat strains for glioma modeling. It is currently
assumed that C6 cells were first produced in Wistar rats exposed to
N-nitrosomethylurea (13). The C6
glioma model is simple, reliable and easily reproducible; it also
has a high tumor formation rate. In this study, tumors were formed
in 92% (11/12) of the model animals. A study by Vince et
al(14) confirmed that the C6
glioma model best mimics the cellular biological behavior of early
glioma progression in humans; C6 cells are very similar to human
glioma cells in terms of the gene expression involved in tumor
progression (15). The present
study monitored the tumors from 1 h to 27 days after implantation
using 7.0T MRI and it was discovered that it was possible to detect
tumors as early as 2 days after implantation. By contrast, Doblas
et al recently reported a latency time in C6 gliomas of
11±1.93 days (16). However, they
did not take MRI images of the rats until 7 days after
implantation, whereas we took images as early as 1 h after
implantation to enable monitoring of the early biological behavior
of C6 gliomas. To the best of our knowledge, this is the first
report demonstrating that C6 gliomas may be detected as early as 2
days after implantation.
MRI provides a more rapid and complete account of
the cell and tumor burden than is possible by histological
analysis, and permits repeated sampling of individual experimental
animals over long periods of time. In our study, the central
hypointense signal area and the peripheral cogwheel-shaped
hypointense signal band in the tumor were observed on the
post-contrast T1WIs, in accordance with the necrosis observed in
the photomicrographs of HE-stained specimens and the layers of
necrosis were so clear that the MRI images were very similar to the
photomicrographs of the HE-stained specimens. There has been no
other report of these manifestations of the C6 glioma model using
MRI. With MRI, we were able to image entire brain volumes in
vivo with scan times <10 min/animal, in contrast to the many
hours required for histological assessment of cell and tumor
distribution of just a portion of a rat brain at a single
time-point. Labeling tumor cells with SPIO and performing an MRI
scan dynamically monitors the development and biological behavior
of glioma at a very early stage.
SPIOs have been used as ex vivo cell labeling
agents for various types of mammalian cells and for in vivo
cellular MRI (17), including in
tumor cells (5), dendritic cells
(18), stem cells (19) and microglia (20), and have recently been used in a
clinical environment (18).
Techniques for labeling cells have been extensively studied. As
previously reported, SPIO labeling procedures have been established
that do not affect cell viability, proliferation or differentiation
potential in vitro(21,22).
However, Schäfer et al(23)
revealed that SPIO or ultrasmall SPIO (USPIO) labeling without a
transfection reagent has a biological impact on mesenchymal stem
cells (MSCs) by upregulating the transferrin receptor and the
authors suggest caution when using this labeling procedure in
clinical applications. Furthermore, SPIO labeling with a
transfection reagent coats the cellular surface. In this study, we
labeled tumor cells without a transfection reagent. We used
self-assembly amphiphilic PEI/SPIO nanocomposites, which label
cells at low dosages, have good biocompatibility and high labeling
rates and no detrimental effects on cell proliferation (12). It remains unknown as to whether the
transferrin receptor in tumor cells is upregulated as it is in the
MSCs. Further experiments are required to elucidate the mechanisms
and consequences of these effects.
We labeled C6 cells with SPIOs, ensuring that they
appeared hypointense on T2WIs, enabling easy differentiation of
these cells from normal brain tissue and thus also the localization
of the implanted cells. In 4/8 experimental animals, the labeled
tumor cells flowed into the lateral ventricle and later grew into
particularly irregular tumors. Thus, labeling cells with SPIOs
prior to implantation facilitates early MRI scanning and detection
of the localization of implanted cells, allowing confirmation that
the cells are in the expected location and the ability to remove
unfavourable models at an early stage.
In the experimental group, we observed pronounced
hypointense signal bands that faded over time but remained visible
on day 27 after implantation, our study endpoint, indicating that
the SPIO-labeled cells create hypointense signals that last for at
least 27 days. One of the drawbacks of labeling cells with
exogenous nanoparticles is the loss of signal over time. This loss
may be attributed to either biodegradation or dilution of the
contrast agent in asymmetric cell division (24). Arbab et al observed that the
ability to detect Feridex-labeled cells with Perl’s stain was lost
after 5–8 cell divisions (22).
One approach for overcoming such limitations is to use MRI reporter
genes where the daughter cells retain a constant amount of the
contrast-enhancing agent after each cell division (25,26),
however, these labels are less sensitive than SPIOs and the
procedure is more complicated. In many cases, it is difficult to
distinguish labeled cells from other hypointense regions in
T2/T2*-weighted MRI images. Hypointensities may have a
physiological origin, such as hemoglobin in blood, a pathological
origin, such as blood clots, or an experimental origin from
traumatic procedures, such as cell injections (Fig. 2A). Hypointensities on MRI images
remain a major obstacle in the attempt to increase the specificity
of cell tracking, which prevents this method from being used in
certain applications, particularly those that involve trauma and
hemorrhage. Using positive contrast media solves this problem,
however, the sensibility of positive contrast media is not as high
as that of negative contrast media.
Acknowledgements
This study was supported by a grant from the Major
State Basic Research Development of China (973 Program, No.
2011CB935800) and the National Natural Science Foundation of China
(No. 81071204). The authors are grateful to the State Key
Laboratory of Biotherapy for providing the C6 glioma cell line.
References
|
1
|
Wrensch M, Minn Y, Chew T, Bondy M and
Berger MS: Epidemiology of primary brain tumors: current concepts
and review of the literature. Neuro Oncol. 4:278–299.
2002.PubMed/NCBI
|
|
2
|
Matsukado Y, Maccarty CS and Kernohan JW:
The growth of glioblastoma multiforme (astrocytomas, grades 3 and
4) in neurosurgical practice. J Neurosurg. 18:636–644. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demuth T and Berens ME: Molecular
mechanisms of glioma cell migration and invasion. J Neurooncol.
70:217–228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weissleder R: Scaling down imaging:
molecular mapping of cancer in mice. Nat Rev Cancer. 2:11–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang F, Xie J, Liu G, He Y, Lu G and Chen
X: In vivo MRI tracking of cell invasion and migration in a rat
glioma model. Mol Imaging Biol. 13:695–701. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shapiro EM, Skrtic S, Sharer K, Hill JM,
Dunbar CE and Koretsky AP: MRI detection of single particles for
cellular imaging. Proc Natl Acad Sci USA. 101:10901–10906. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walton RM, Magnitsky SG, Seiler GS,
Poptani H and Wolfe JH: Transplantation and magnetic resonance
imaging of canine neural progenitor cell grafts in the postnatal
dog brain. J Neuropathol Exp Neurol. 67:954–962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bulte JW and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Valable S, Barbier EL, Bernaudin M,
Roussel S, Segebarth C, Petit E and Rémy C: In vivo MRI tracking of
exogenous monocytes/macrophages targeting brain tumors in a rat
model of glioma. Neuroimage. 40:973–983. 2008. View Article : Google Scholar
|
|
10
|
Reddy AM, Kwak BK, Shim HJ, Ahn C, Lee HS,
Suh YJ and Park ES: In vivo tracking of mesenchymal stem cells
labeled with a novel chitosan-coated superparamagnetic iron oxide
nanoparticles using 3.0T MRI. J Korean Med Sci. 25:211–219. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Heyn C, Ronald JA, Ramadan SS, et al: In
vivo MRI of cancer cell fate at the single-cell level in a mouse
model of breast cancer metastasis to the brain. Magn Reson Med.
56:1001–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu G, Xia C, Wang Z, Lv F, Gao F, Gong Q,
Song B, Ai H and Gu Z: Magnetic resonance imaging probes for
labeling of chondrocyte cells. J Mater Sci Mater Med. 22:601–606.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benda P, Lightbody J, Sato G, Levine L and
Sweet W: Differentiated rat glial cell strain in tissue culture.
Science. 161:370–371. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vince GH, Bendszus M, Schweitzer T,
Goldbrunner RH, Hildebrandt S, Tilgner J, Klein R, Solymosi L,
Christian Tonn J and Roosen K: Spontaneous regression of
experimental gliomas--an immunohistochemical and MRI study of the
C6 glioma spheroid implantation model. Exp Neurol. 190:478–485.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sibenaller ZA, Etame AB, Ali MM, Barua M,
Braun TA, Casavant TL and Ryken TC: Genetic characterization of
commonly used glioma cell lines in the rat animal model system.
Neurosurg Focus. 19:E12005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doblas S, He T, Saunders D, Pearson J,
Hoyle J, Smith N, Lerner M and Towner RA: Glioma morphology and
tumor-induced vascular alterations revealed in seven rodent glioma
models by in vivo magnetic resonance imaging and angiography. J
Magn Reson Imaging. 32:267–275. 2010. View Article : Google Scholar
|
|
17
|
Corot C, Robert P, Idée JM and Port M:
Recent advances in iron oxide nanocrystal technology for medical
imaging. Adv Drug Deliv Rev. 58:1471–1504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Vries IJ, Lesterhuis WJ, Barentsz JO,
et al: Magnetic resonance tracking of dendritic cells in melanoma
patients for monitoring of cellular therapy. Nat Biotechnol.
23:1407–1413. 2005.PubMed/NCBI
|
|
19
|
Rice HE, Hsu EW, Sheng H, Evenson DA,
Freemerman AJ, et al: Superparamagnetic iron oxide labeling and
transplantation of adipose-derived stem cells in middle cerebral
artery occlusion-injured mice. AJR Am J Roentgenol. 188:1101–1108.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleige G, Nolte C, Synowitz M, Seeberger
F, Kettenmann H and Zimmer C: Magnetic labeling of activated
microglia in experimental gliomas. Neoplasia. 3:489–499. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng XX, Wan JQ, Jing M, Zhao SG, Cai W
and Liu EZ: Specific targeting of gliomas with multifunctional
superparamagnetic iron oxide nanoparticle optical and magnetic
resonance imaging contrast agents. Acta Pharmacol Sin.
28:2019–2026. 2007. View Article : Google Scholar
|
|
22
|
Arbab AS, Bashaw LA, Miller BR, Jordan EK,
Lewis BK, Kalish H and Frank JA: Characterization of biophysical
and metabolic properties of cells labeled with superparamagnetic
iron oxide nanoparticles and transfection agent for cellular MR
imaging. Radiology. 229:838–846. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schäfer R, Kehlbach R, Wiskirchen J,
Bantleon R, Pintaske J, Brehm BR, Gerber A, Wolburg H, Claussen CD
and Northoff H: Transferrin receptor upregulation: in vitro
labeling of rat mesenchymal stem cells with superparamagnetic iron
oxide. Radiology. 244:514–523. 2007.PubMed/NCBI
|
|
24
|
Walczak P, Kedziorek DA, Gilad AA, Barnett
BP and Bulte JW: Applicability and limitations of MR tracking of
neural stem cells with asymmetric cell division and rapid turnover:
the case of the shiverer dysmyelinated mouse brain. Magn Reson Med.
58:261–269. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gilad AA, Winnard PT Jr, van Zijl PC and
Bulte JW: Developing MR reporter genes: promises and pitfalls. NMR
Biomed. 20:275–290. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gilad AA, McMahon MT, Walczak P, Winnard
PT Jr, Raman V, van Laarhoven HW, Skoglund CM, Bulte JW and van
Zijl PC: Artificial reporter gene providing MRI contrast based on
proton exchange. Nat Biotechnol. 25:217–219. 2007. View Article : Google Scholar : PubMed/NCBI
|