Introduction
Extracellular signal-regulated kinase (ERK) is a
member of the mitogen-activated protein kinase family and has been
implicated in a number of biological functions, including learning
and memory in the hippocampus and pain-related negative affects in
the anterior cingulated cortex (1–3).
Several studies have identified that ERK in the spinal cord
contributes to the induction and development of pain. Firstly, it
was reported that spinal ERK is persistently activated in
inflammatory pain induced by the injection of complete Freund’s
adjuvant into the hind paw and the inhibition of activated spinal
ERK attenuated the development of mechanical hypersensitivity
(4). Subsequently, it was reported
that spinal ERK is sequentially activated in neurons, astrocytes
and microglia in rat models of neuropathic pain (5). Pretreatment or post-treatment with an
ERK inhibitor through intrathecal delivery markedly inhibited or
reversed the mechanical allodynia and thermal hyperalgesia
following neuropathy (5). These
observations indicate that the persistent activation of spinal ERK
regulates chronic pain. However, few studies have investigated
whether spinal ERK is involved in surgical pain processing.
Compared with chronic pain, surgical pain is more
transient and requires specific perioperative pain management
(6,7). Compared with inflammatory and
neuropathic pain, surgical pain is a unique acute pain state in
which there are various central sensitization mechanisms, in
particular in the spinal cord. The N-methyl-D-aspartate
(NMDA)-dependent mechanism regulates the development of neuropathic
and inflammatory pain. However, it has been reported that the
NMDA-independent but not the NMDA-dependent mechanism mediates the
mechanical pain hypersensitivity induced by incision, a surgical
pain mode (8). In addition, a
previous study revealed that administration of tumor necrosis
factor (TNF) IgG fusion protein did not attenuate the mechanical
hypersensitivity induced by a surgical incision (9). These observations indicate that TNF
does not contribute to incisional pain behavior, although TNF is
known to regulate other forms of persistent pain (10). The distinct neurochemical changes
in the spinal cord following the surgical incision indicate that
the role of ERK in surgical pain may be different from that in
other types of pain. Therefore, the present study aimed to explore
the role of ERK in the spinal cord in mechanical hypersensitivity
following surgical incision.
Materials and methods
Animals
Adult male Sprague-Dawley rats (150–250 g) obtained
from Central South University Animal Services (Changsha, China)
were used in this study. All rats were kept in an air-conditioned
(23–26°C, 60–70% relative humidity) vivarium with a 12 h dark/light
cycle (light on from 8:00 am to 8:00 pm). The experimental protocol
complied with the National Institutes of Health Guide for the Care
and Use of Laboratory Animals and was approved by the Animal Care
and Use Committee of Central South University. All efforts were
taken to minimize the suffering of the rats.
Surgical preparation and groups
The incisional pain model was established by
unilateral hind paw incision. A detailed description of this
surgical model in rats has been described previously (11). Our preliminary study observed that
inhaled anesthetics (isoflurane or sevoflurane) induced the
immediate expression of spinal ERK. Therefore, 10% chloral hydrate
(30mg/kg) was used as an anesthetic when examining ERK expression
in the rats shortly after the establishment of incisional pain (1,
2, 5 and 10 min). At later times (1, 3 and 6 h and 1 and 3 days),
1.5% sevoflurane was used to anesthetize the animals as described
previously (1). This was due to
the short-term anesthetic effect of sevoflurane, which leads to the
rats regaining consciousness within minutes. In brief, a 1-cm long
longitudinal incision was made into the planta skin and through to
the plantaris muscle. The muscle was then elevated and incised
longitudinally (0.5 cm). The shin was then closed by 4–0 nylon
sutures. A topical triple antibiotic ointment was applied to the
hind paw following surgery. Sham surgery was performed using the
same procedure but with no incision.
For immunohistochemistry experiments, experimental
rats were randomly divided and sacrificed at 1, 2, 5 and 10 min, 1
and 6 h and 1 and 3 days (n=8–10 for each group). In an independent
group, rats were implanted with an intrathecal catheter for
intrathecal delivery or intraperitoneal injection to enable the
effects of inhibitors on ERK expression and pain behaviors to be
investigated.
Intrathecal catheterization
In brief, a polyethylene-10 cathether was implanted
in the intrathecal space of the spinal cord at the lumbar
enlargement in a rat anesthetized with chloral hydrate (30 mg/100
g) (12). The mitogen-activated
protein kinase kinase (MEK) inhibitor, U0126, was purchased from
Sigma-Aldrich (1 μg dissolved in 10% DMSO; St. Louis, MO, USA).
U0126 was intrathecally administered at 20 min prior to or 20 min
following the incision and DMSO was injected as a vehicle
control.
Immunohistochemistry
The rats were deeply anesthetized with chloral
hydrate (80 mg/kg) and perfused transcardially with 100 ml
phosphate-buffered saline, followed by 4% paraformaldehyde in 0.1 M
phosphate buffer. L4–5 spinal cord segments were fixed for 4 h with
4% paraformaldehyde and then immersed in 20% sucrose in phosphate
buffer (pH 7.4) overnight. Transverse spinal cord sections (30 μm)
were cut and processed for immunohistochemistry using the ABC
method. In brief, sections were mounted on 3-aminopropyl
triethoxy-silane-coated slides and incubated with mouse anti-p-ERK
antibody (dilution 1:1,000; Cell Signaling Technology, Danvers, MA,
USA) at room temperature overnight. The secondary reagents used for
localization were biotinylated goat anti-mouse IgG and an ABC kit
(Vector Laboratories, Burlingame, CA, USA). Diaminobenzidine
tetrahydrochloride (Sigma-Aldrich) was used as a peroxidase
substrate.
Nociceptive testing
Mechanical allodynia was assayed by measuring the
paw withdrawal threshold (PWT) using nylon von Frey filaments
(13). In brief, the rats were
placed on wire mesh platforms in clear cylindrical plastic
enclosures and von Frey filaments (0.4–15.1 g) were applied to the
edge of the wound in the incised hind paw or to the center of the
plantar surface of the unincised paw. The up-down method was
performed. The test was consecutive; in the absence of paw
withdrawal response, a stronger stimulus was applied, otherwise a
weaker stimulus was selected. Testing proceeded in this manner
until four fibers had been applied after the first to cause a
withdrawal response, allowing the estimation of the mechanical
withdrawal threshold. Behavioral tests were performed prior to and
at 10 and 30 min and 1, 3 and 6 h until 5 days following
incision.
Statistical analysis
Eight non-adjacent sections from each specimen of
L4–5 lumbar spinal cord were randomly selected and p-ERK expression
was determined by counting the p-ERK-positive cells on the L4–5
spinal superficial dorsal horn (lamina I and II). Data collection
was performed by an individual who was blind to the treatments the
animals had received. SPSS v13.0 (SPSS Inc., Chicago, IL, USA) and
Prism 5.0 (Graphpad Software, San Diego, CA, USA) were used to
perform statistical analysis. Data are presented as the mean ± SEM.
Differences between groups were compared with one-way or two-way
ANOVA followed by post hoc Dunnett or Tukey post hoc multiple
comparison tests where appropriate. P<0.05 was considered to
indicate a statistically significant difference.
Results
ERK activation in the spinal cord dorsal
horn following hind paw incision
In sham-operated rats, low levels of p-ERK
expression were identified in the dorsal horn of the spinal cord
(Fig. 1A). However, 1 min
following hind-paw incision, increased p-ERK immunoreactivity (IR)
was detected within numerous dorsal horn neurons in the ipsilateral
side (Fig. 1B and I). The induced
p-ERK expression reached peak levels at 5 min post-incision
(Fig. 1C and I), returned to
baseline levels 10 min post-incision and remained at these levels
thereafter (Fig. D-G and I).
A more highly magnified version of Fig. 1D revealed that the elevated p-ERK
IR was localized in neurons in the cytoplasm of the soma and
nucleus as well as in the neurites in the superficial dorsal horn
(lamina I and II; Fig. 1H).
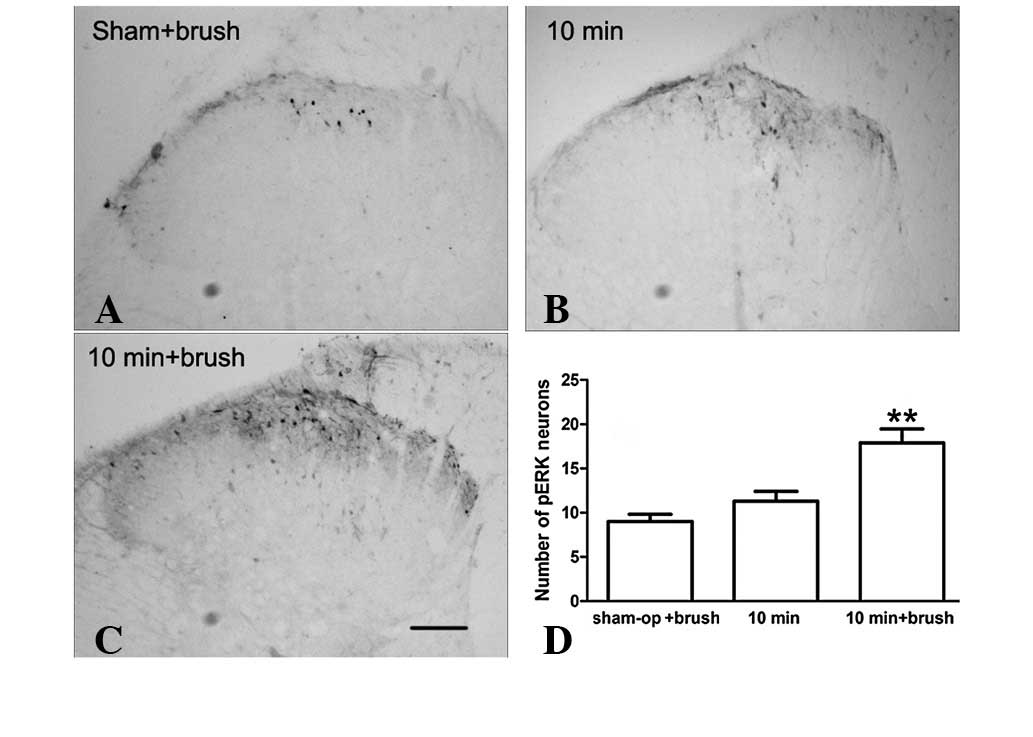
Effect of brushing the incised skin on
expression of ERK at later times following incision
The transient activation of spinal ERK may be
associated only with the initiation of incisional pain. However,
activated spinal ERK is also involved in the maintenance of
inflammatory and neuropathic pain (14). Our recent study demonstrated that
ERK, in the anterior cingulated cortex, is reactivated by innocuous
stimuli at the time when p-ERK expression has returned to basal
levels during incisional pain (1).
Since the mechanical allodynia induced by innocuous stimuli is
clinically similar to the incident pain induced by coughing or
moving and is a hallmark of postoperative pain, incised skin
brushing was performed, as described previously, and we examined
whether innocuous stimuli reactivated p-ERK expression in the
painful condition. As demonstrated in Fig. 2, p-ERK expression levels were
markedly increased in the superficial dorsal horn (lamina I and II)
with brushing compared with those in the incision+saline and
sham-surgery groups. These observations indicate that the
expression of p-ERK is activity-dependent at later times
post-incision.
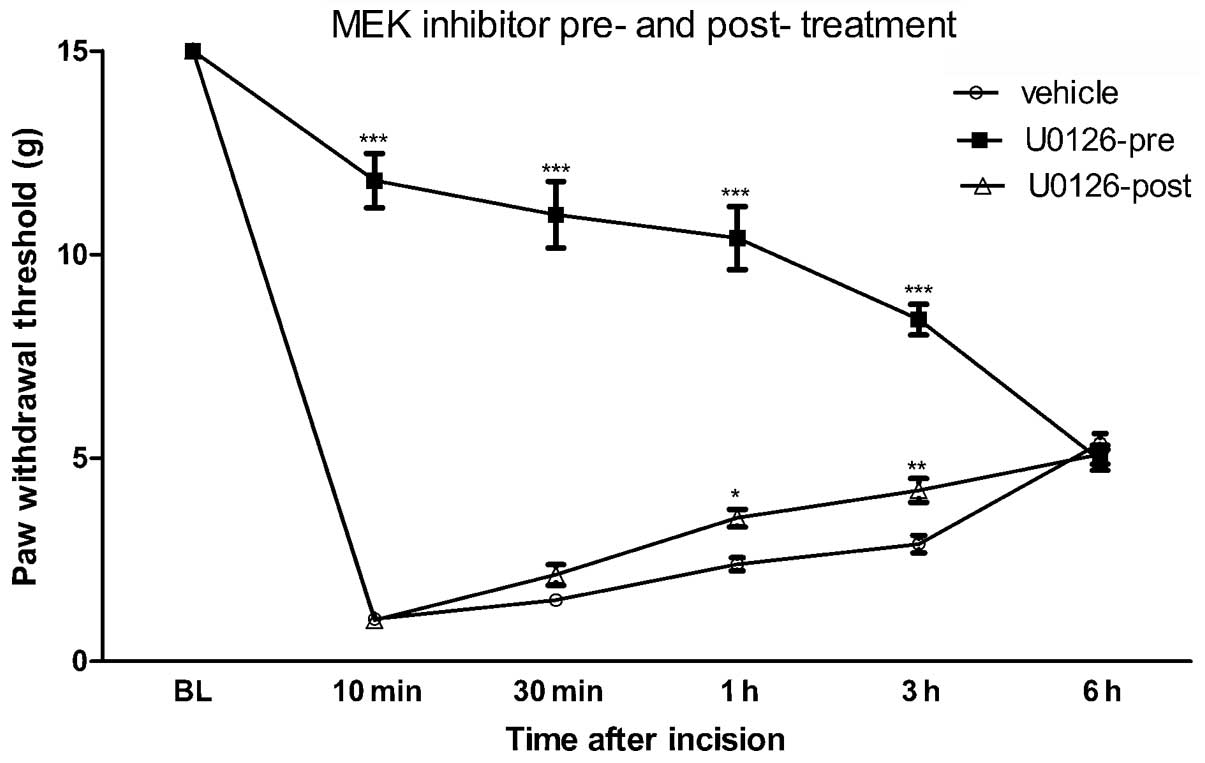
Effect of MEK inhibition on
post-incisional pain hypersensitivity
The transient activation of ERK in the spinal cord
following hind paw incision indicates that spinal ERK may be
involved in the development of post-surgical pain. To test this
hypothesis, a specific MEK inhibitor, U0126, was intrathecally
delivered 20 min prior to or following the hind paw incision and
the PWT was measured. Previous studies have revealed that 1 μg
U0126 is sufficient to block ERK activity and therefore 1 μg U0126
was administered intrathecally in the present study (1,4). As
revealed in Fig. 3, pretreatment
with U0126 attenuated the reduction in PWT from 10 min and up to 6
h post-incision.
By contrast, U0126 post-treatment had only a limited
effect on the reduced PWT at the indicated times following hind paw
incision (Fig. 3). In addition, a
significant difference between U0126 pre- and post-treatment in the
pain behavioral response to hind-paw incision was observed
(P<0.001). These results indicate that the transient activation
of ERK in the spinal dorsal horn contributes to the induction but
not the maintenance of incision-evoked pain hypersensitivity.
Discussion
It is well known that spinal ERK is persistently
activated and contributes to pain processing in chronic
inflammatory or neuropathic pain (14). The present study reveals that
spinal ERK is also activated in response to surgical incision,
indicating that ERK may also be involved in the development of
surgical pain. However, unlike inflammatory and neuropathic pain,
the activation of spinal ERK in incisional pain is transient and
returns to baseline levels rapidly (10 min post-incision). This
indicates that there are distinct mechanisms of central
sensitization for incisional pain and other types of pain. However,
the immediate increase in p-ERK expression levels in neurons is
similar to the early activation of p-ERK in neuropathic and
inflammatory pain. In the neuropathic pain model, p-ERK expression
in the spinal cord dorsal horns is sequentially activated in the
neurons, microglia and astrocytes (5). The activation of neuronal p-ERK is
also extremely transient and is sustained for <6 h (5). The rapid activation of neuronal ERK
in various types of pain may be due to direct burst firing induced
by noxious stimuli. Consistent with this hypothesis,
electrophysiological studies have identified that sustained C-fiber
stimulation is able to induce the activation of p-ERK in the spinal
cord (15).
Of note, in the later phases of neuropathic pain,
increased p-ERK expression largely occurs in activated glial cells
(microglia and then astrocytes). Although our previous study also
demonstrated that surgical incision induces the activation of
microglia and astrocytes in the spinal cord, increased p-ERK
expression was observed in microglia or astrocytes (16). One hypothesis is that surgical
incision may not result in the robust activation of cytokines, in
particular TNF-α. With respect to this hypothesis, exogenous TNF-α
may markedly induce p-ERK expression in neurons or glial cells. In
addition, peripheral inflammation and spinal nerve injury lead to
the robust activation of cytokines, including TNF-α and
interleukin-1β (IL-1β), in the glial cells (17–19).
Inhibition of TNF-α may inhibit the activation of p-ERK in the
spinal cord in these types of pain model. However, TNF-α in the
spinal cord is not activated in glial cells following hind-paw
incision. In addition, blocking TNF-α does not attenuate the
mechanical hypersensitivity in post-incisional pain. Together with
our previous studies, the results of the present study indicate
that surgical incision may induce milder neuroimmune responses in
the spinal cord.
The transient activation of ERK in the spinal cord
indicates that spinal ERK mainly contributes to the initiation, but
not the maintenance of surgical pain. Indeed, the intrathecal
administration of a MEK inhibitor, U0126, prior to spinal ERK
activation (pretreatment) markedly attenuated the pain
hypersensitivity following the incision. By contrast, when U0126
was administered after the return of p-ERK expression to baseline,
a marginal analgesic effect on incision-evoked pain
hypersensitivity was observed. However, U0126 pretreatment exerts
an analgesic effect >6 h after incision, indicating that
blocking the induction of pain may also affect the subsequent
maintenance of pain. This may be through the inhibition of noxious
stimulus-evoked spinal synaptic long-term potentiation during
central sensitization. Consistent with this hypothesis, a recent
study revealed that the brief application of high doses of opioids
not only reduces pain but also erases the spinal memory trace of
pain (20).
In the present study, innocuous stimulation by
brushing was found to reactivate the spinal ERK expression when it
had returned to the baseline following incision. Pain evoked by
innocuous mechanical stimuli in the incisional pain model mimics
clinically incident pain (pain evoked by body movement or
coughing), a hallmark of postoperative pain. Although pain during
rest is generally easy to treat, incident pain remains a major
challenge for postoperative pain control. The reactivation of p-ERK
in response to brushing the incised skin indicates that p-ERK
expression in the later phases of incisional pain is
activity-dependent and associated with incident pain. Of note, our
previous study demonstrated that surgical incision induced the
upregulation of brain-derived neurotrophic factor (BDNF) in the
spinal cord (21). Previous
studies have reported that U0126 inhibits the upregulation of BDNF
in inflammatory pain induced by Freund’s complete adjuvant and
neuropathic pain (22,23). These observations indicate that
BDNF-ERK signaling may contribute to incident pain in postoperative
pain.
In conclusion, the present study reveals that
surgical incision immediately induces the transient activation of
ERK in the spinal cord. Transient activation of spinal ERK appears
to mainly regulate the initiation, but not the maintenance of
incisional pain as pretreatment but not post-treatment with the MEK
inhibitor markedly attenuated incision-evoked pain
hypersensitivity. The inhibitor was found to suppress the transient
activation of spinal ERK and reactivation of ERK in response to
brushing post-incision, indicating that MEK may regulate, at least
in part, incisional pain through MEK/ERK pathways.
Acknowledgements
The present study was supported by a grant from the
National Natural Science Foundation of China (no. 81070897).
References
|
1
|
Dai RP, Li CQ, Zhang JW, Li F, Shi XD,
Zhang JY, et al: Biphasic activation of extracellular
signal-regulated kinase in anterior cingulate cortex distinctly
regulates the development of pain-related anxiety and mechanical
hypersensitivity in rats after incision. Anesthesiology.
115:604–613. 2011. View Article : Google Scholar
|
|
2
|
Cao H, Gao YJ, Ren WH, Li TT, Duan KZ, Cui
YH, et al: Activation of extracellular signal-regulated kinase in
the anterior cingulate cortex contributes to the induction and
expression of affective pain. J Neurosci. 29:3307–3321. 2009.
View Article : Google Scholar
|
|
3
|
Igaz LM, Winograd M, Cammarota M,
Izquierdo LA, Alonso M, Izquierdo I, et al: Early activation of
extracellular signal-regulated kinase signaling pathway in the
hippocampus is required for short-term memory formation of a
fear-motivated learning. Cell Mol Neurobiol. 26:989–1002. 2006.
|
|
4
|
Ji RR, Befort K, Brenner GJ and Woolf CJ:
ERK MAP kinase activation in superficial spinal cord neurons
induces prodynorphin and NK-1 upregulation and contributes to
persistent inflammatory pain hypersensitivity. J Neurosci.
22:478–485. 2002.PubMed/NCBI
|
|
5
|
Zhuang ZY, Gerner P, Woolf CJ and Ji RR:
ERK is sequentially activated in neurons, microglia and astrocytes
by spinal nerve ligation and contributes to mechanical allodynia in
this neuropathic pain model. Pain. 114:149–159. 2005. View Article : Google Scholar
|
|
6
|
Costantini R, Affaitati G, Fabrizio A and
Giamberardino MA: Controlling pain in the post-operative setting.
Int J Clin Pharmacol Ther. 49:116–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CL and Raja SN: Treatment of acute
postoperative pain. Lancet. 377:2215–2225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zahn PK, Pogatzki-Zahn EM and Brennan TJ:
Spinal administration of MK-801 and NBQX demonstrates
NMDA-independent dorsal horn sensitization in incisional pain.
Pain. 114:499–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zahn PK, Subieta A, Park SS and Brennan
TJ: Effect of blockade of nerve growth factor and tumor necrosis
factor on pain behaviors after plantar incision. J Pain. 5:157–163.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Berta T, Xu ZZ, Liu T, Park JY
and Ji RR: TNF-alpha contributes to spinal cord synaptic plasticity
and inflammatory pain: distinct role of TNF receptor subtypes 1 and
2. Pain. 152:419–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brennan TJ, Vandermeulen EP and Gebhart
GF: Characterization of a rat model of incisional pain. Pain.
64:493–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LoPachin RM, Rudy TA and Yaksh TL: An
improved method for chronic catheterization of the rat spinal
subarachnoid space. Physiol Behav. 27:559–561. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaplan SR, Bach FW, Pogrel JW, Chung JM
and Yaksh TL: Quantitative assessment of tactile allodynia in the
rat paw. J Neurosci Methods. 53:55–63. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji RR, Gereau RW 4th, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–148.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ji RR, Baba H, Brenner GJ and Woolf CJ:
Nociceptive-specific activation of ERK in spinal neurons
contributes to pain hypersensitivity. Nat Neurosci. 2:1114–1119.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fu D, Guo Q, Ai Y, Cai H, Yan J and Dai R:
Glial activation and segmental upregulation of interleukin-1beta
(IL-1beta) in the rat spinal cord after surgical incision.
Neurochem Res. 31:333–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Samad TA, Moore KA, Sapirstein A, Billet
S, Allchorne A, Poole S, et al: Interleukin-1beta-mediated
induction of Cox-2 in the CNS contributes to inflammatory pain
hypersensitivity. Nature. 410:471–475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng W, Ouyang H, Zheng X, Liu S, Mata M,
Fink DJ, et al: Glial TNFalpha in the spinal cord regulates
neuropathic pain induced by HIV gp120 application in rats. Mol
Pain. 7:402011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andrade P, Visser-Vandewalle V, Hoffmann
C, Steinbusch HW, Daemen MA and Hoogland G: Role of TNF-alpha
during central sensitization in preclinical studies. Neurol Sci.
32:757–771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Drdla-Schutting R, Benrath J,
Wunderbaldinger G and Sandkuhler J: Erasure of a spinal memory
trace of pain by a brief, high-dose opioid administration. Science.
335:235–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CQ, Xu JM, Liu D, Zhang JY and Dai RP:
Brain derived neurotrophic factor (BDNF) contributes to the pain
hypersensitivity following surgical incision in the rats. Mol Pain.
4:272008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cruz Duarte P, St-Jacques B and Ma W:
Prostaglandin E2 contributes to the synthesis of brain-derived
neurotrophic factor in primary sensory neuron in ganglion explant
cultures and in a neuropathic pain model. Exp Neurol. 234:466–481.
2012.PubMed/NCBI
|
|
23
|
Obata K, Yamanaka H, Dai Y, Mizushima T,
Fukuoka T, Tokunaga A, et al: Activation of extracellular
signal-regulated protein kinase in the dorsal root ganglion
following inflammation near the nerve cell body. Neuroscience.
126:1011–1021. 2004. View Article : Google Scholar : PubMed/NCBI
|