Introduction
Human corneal endothelial cells (HCECs) are composed
of a monolayer of hexagonal endothelial cells, which have limited
regenerative capacity after birth. A normal density of HCECs and
several functions are essential for maintaining corneal
transparency (1,2). Corneal edema and blindness occur when
a large number of endothelial cells are destroyed by disease or
trauma (3–5). This type of blindness, also known as
secondary corneal endothelial decompensation, can be cured by
corneal transplantation with healthy donor corneas (6,7).
However, the majority of individuals cannot be treated by corneal
transplantation due to a shortage of cornea donors (8). Transplanting cultured HCECs has long
been considered a promising method of expanding the donor pool for
endothelial decompensation cases (9). Direct cell seeding into Descemet’s
membrane has been attempted in human and rabbit models assisted by
superparamagnetic iron oxide nanoparticles (SPIONs) (10,11).
Under an external magnetic field, the labeled CECs homogeneously
adhered to the posterior surface of the corneal stromal bed
(10,11). However, following SPION labeling,
the dehydrating function required to maintain optical transparency
by preventing the stromal layer from becoming excessively hydrated,
has not been determined. In this study, we examined electro-osmosis
across the endothelial layer and ion channels located in the
basolateral and apical membranes. The expression patterns of marker
proteins and the adhesive and proliferative ability of the ex
vivo cultured SPION-labeled versus unlabeled cells were also
determined.
Materials and methods
Cell culture
Rabbit corneal endothelial cells (RCECs) were
isolated from freshly peeled corneal Descemet’s membrane of young
(8 weeks) New Zealand white rabbit eyes. Peeled endothelia were
incubated in a disaggregating solution (300 units type I
collagenase, 100 units hyalronidase and 1% antibiotic/antimycotic
solution) in DMEM (Invitrogen, Carlsbad, CA, USA) for ~2 h at 37°C.
RCECs were collected by centrifugation at 450 × g for 5 min. The
cells from two corneas were suspended in a 35-mm petri dish with 2
ml DMEM/F12 supplemented with 10% fetal bovine serum (Wisent,
Montreal, QC, Canada) and 1% penicillin and streptomycin
(Sigma-Aldrich, St. Louis, MO, USA). Cells were incubated at 37°C
with 5% CO2 and passaged 3 days later when they reached
90% confluency.
All animal protocols were approved by the Tongji
University Experimental Animal Center, in accordance with the ARVO
Statement for the Use of Animals in Ophthalmic and Vision
Research.
Synthesis of dextran-coated SPIONs
Ferric chloride, ferrous sulfate and dextran were
purchased from Sigma-Aldrich. To prepare an iron salts solution,
two equivalents of ferric chloride and one equivalent of ferrous
sulfate were mixed in an aqueous solution. Upon saturation with
nitrogen gas, equal volumes of 10% dextran and 20% mixed iron salt
solution were combined by stirring. Precipitation was achieved by
adjusting the pH to 10.0 with 25% ammonia solution. The reaction
proceeded for 3 h at 60°C, with continuous stirring. The SPIONs
produced were purified using magnetic separation and
ultrafiltration. The hysteresis curves of particles were determined
using a vibrating magnetometer (Molspin, Newcastle, UK).
Transmission electron microscopy (TEM, JEM-2010, JEOL Ltd., Tokyo,
Japan) micrographs were obtained at an accelerating voltage of 200
kV. The prepared liposomes were diluted with deionized water and
the mean hydrodynamic particle size and ζ-potential were determined
at 25°C using a Nano S Zetasizer (Malvern Instruments, Malvern,
UK). Each experiment was repeated three times.
Labeling of RCECs with SPIONs
Dextran-coated SPIONs, ~50 nm in size, at a
concentration of 5 mg/ml, were added to a 50 ml conical tube
containing serum-free RPMI-1640, with 25 mM HEPES, MEM
non-essential amino acids, sodium pyruvate and L-glutamine
(Biosource, Camarillo, CA, USA). Protamine sulfate (Pro,
Sigma-Aldrich), supplied at 10 mg/ml, was prepared as a fresh stock
solution of 1 mg/ml in sterile deionized water immediately prior to
labeling. The culture medium was aspirated from the flasks
containing RCECs and replaced with media containing SPIONs.
Following 2 h of incubation at 37°C, an equal amount of complete
medium was added to achieve a final SPION concentration of 4–46
μg/ml, respectively. Cells were incubated overnight (16 h) and
washed three times with sterile PBS containing 10 U/ml heparin
sulfate (American Pharmaceuticals, Schaumburg, IL, USA).
MTT assay
The cytotoxicity of SPIONs on RCECs was measured by
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl2H-tetrazolium bromide
(MTT) (Sigma-Aldrich) assay. Subconfluent cells in a 96-well plate
were incubated with SPIONs for 3 and 6 h. MTT (10 μl, 5 mg/ml) in
PBS was added to each well and incubated for 5 h. Following the
addition of a solution consisting of 10% sodium dodecyl sulfate
(SDS) (Sigma-Aldrich), 5% isopropyl alcohol and 0.012 mol/l HCl,
the 96-well plate was incubated at 37°C overnight. Absorbance
values of the 96-well plate were measured at 570 nm with a
reference wavelength of 650 nm using a SpectraMax reader (Molecular
Devices, Sunnyvale, CA, USA).
TEM
RCECs were grown in a 10 cm petri dish (Nunc, Thermo
Fisher Scientific Inc., Waltham, MA, USA) until they achieved 75%
confluency, then were exposed to SPIONs at the concentrations and
times indicated. At the end of the incubation period the cells were
washed twice with PBS, detached from the petri dishes with
trypsin/EDTA, centrifuged and fixed in 2.3% cacodylate-buffered
glutaraldehyde (Sigma-Aldrich) for 24 h. Samples were postfixed in
1.3% osmium tetroxide in a 0.2 M cacodylate buffer (pH 7.4) for 1
h, dehydrated in graded ethanol solutions, then in propylene oxide,
and embedded in 50% (w/w) epoxy embedding medium, 26% (w/w)
dodecenyl succinic anhydride (DDSA), 23% (w/w) methyl nadic
anhydride and 1% (w/w) 2,4,6-tris(dimethylaminomethyl)phenol
(Sigma-Aldrich). Blocks were cured for 48 h at 60°C. Thin sections
were cut using an ultramicrotome (Ultracut E, Reichert-Jung
Optische Werke AG, Wien, Austria) and mounted on 3 mm 200-mesh
copper grids. Grids were stained for 75 min in saturated uranyl
acetate solution (Fluka, St. Louis, MO, USA), then for 100 sec in
lead citrate (Ultrostain 2, Laurylab, St. Fons, France). Grids were
examined and photographed using a combined Philips CM10
transmission electron microscope and a MegaView III Soft Imaging
software system documented the results.
Homotypic adhesion assay
The homotypic adhesion assay was performed as
previously reported (8). Monolayer
RCECs incubated as described previously, on 24-well plates, were
washed gently with PBS three times. Cells (1×105 cells),
in 1 ml of medium with or without SPION labeling at 16 μg/ml, were
seeded into each well. The 24-well plate was placed in a horizontal
shaker and agitated at 0.55 × g at 37°C. Unattached cells were
removed prior to calculating the cell number under a microscope,
following incubation for 10, 30 and 60 min. The attached cell
numbers were calculated using the formula: number of adherent cells
= 1×105 - the number of unattached cells.
Immunocytochemistry
For immunocytochemical staining, cells were fixed
with 4% paraformaldehyde (PFA) followed by ice-cold methanol. After
blocking with 5% normal goat serum, the samples were incubated with
primary antibodies, including mouse anti-Nestin, rabbit anti-zonula
occluden-1 (ZO-1) and anti-Ki67, overnight at 4°C. Following
washing with PBS, the cells were incubated with
fluorescein-conjugated secondary antibodies and counterstained with
DAPI. Cell staining was examined under a fluorescence microscope
(AxioCamMR3, Carl Zeiss, Jena, Germany). Rabbit corneal fibroblasts
and epithelial cells were used for negative control staining to
exclude contamination from these cells.
Flow cytometric analyses
For Ki67 studies, RCECs prepared with or without
SPION labeling were passaged in 1:4 dilutions and dissociated into
single cells by 0.25% trypsin digestion. Cells were fixed in 70%
(w/v) ethanol, washed with PBS and incubated for 20 min with 1%
BSA. RCECs were incubated with a 1:20 dilution of anti-mouse Ki67,
washed and incubated with 1:1000 diluted Alexa Fluor 488 conjugated
goat anti-mouse IgG (Invitrogen), according to the manufacturer’s
instructions. Flow cytometric analyses were performed using a
FACSCalibur instrument (BD Biosciences, San Jose, CA, USA).
Measurement of corneal endothelial cell
pump function
The pump function of confluent monolayers of RCECs
was measured using an Ussing chamber as described previously
(12). Cells cultured on Snapwell
inserts coated with Type IV collagen were placed into the Ussing
chamber with the endothelial cell surface side in contact with one
chamber and the Snapwell membrane side in contact with another
chamber. The chambers were carefully filled with Krebs-Ringer
bicarbonate and maintained at 37°C using an attached heater. The
short circuit current was measured with narrow polyethylene tubes
positioned close to either side of the Snapwell insert and filled
with 3 M KCl and 4% agar gel connected to silver electrodes. These
electrodes were connected to the computer through the Ussing system
VCC-MC2 (Physiologic Instruments, San Diego, CA, USA) and an iWorx
118 Research Grade Recorder (iWorx Systems, Dover, NH, USA). When
the short circuit current had achieved a steady state for 10 min,
ouabain (1 mM) was added to the chamber and the short circuit
current was measured again.
Statistical analysis
Experimental results were analyzed by one-way
analysis of variance using SPSS version 12.0 software (SPSS, IBM,
Armonk, NY, USA). Summary statistics are expressed as the means ±
SD. In all statistical analyses, P<0.01 was considered to
indicate a statistically significant difference and all P values
were two-sided.
Results
Characterization of SPIONs
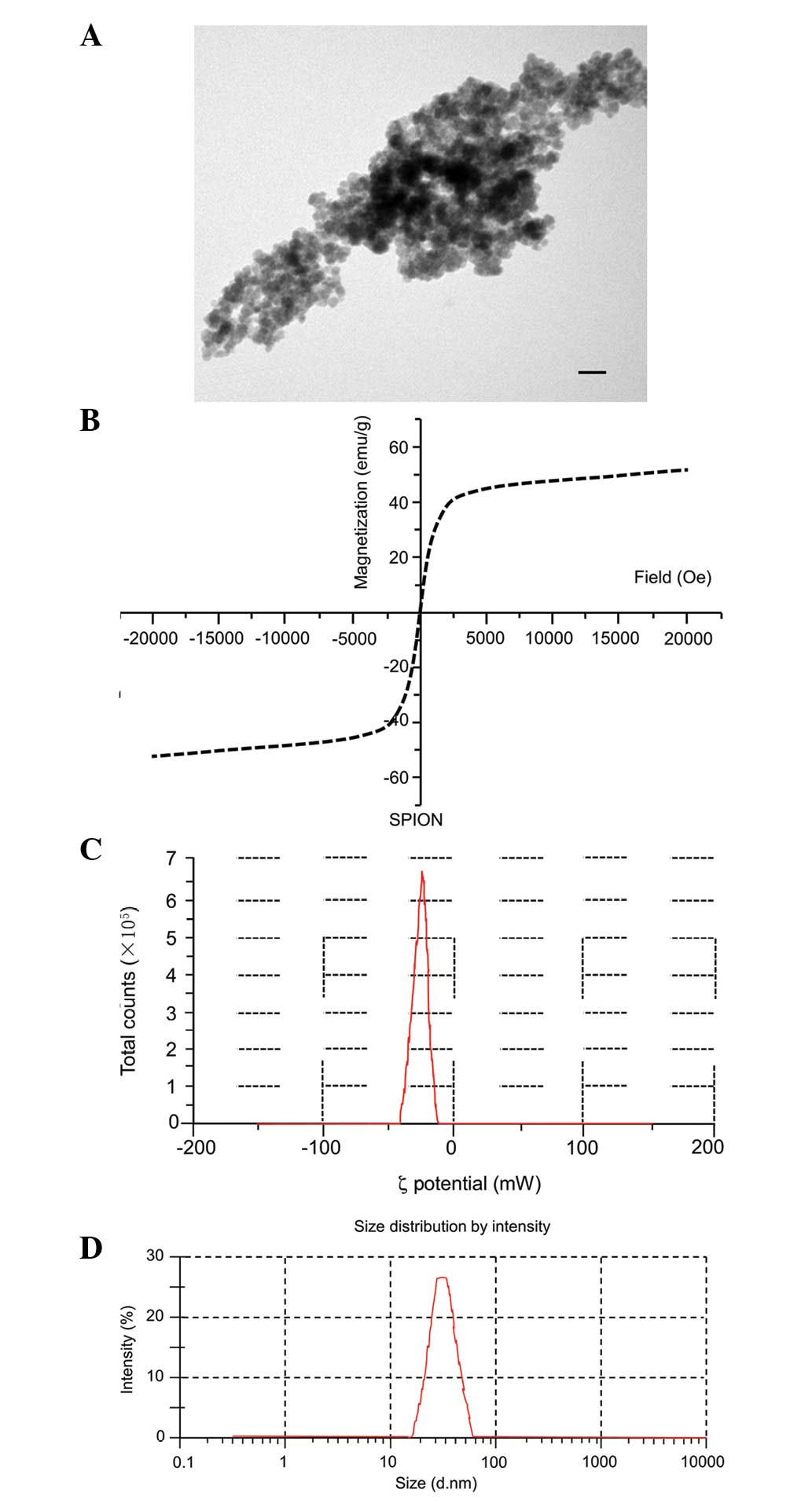
Morphology, size distribution and ζ-potential of the
SPIONs are shown in Fig. 1. SPIONs
appeared as spherical, well-dispersed particles (Fig. 1A). Hysteresis curves are shown in
Fig. 1B. The SPIONs exhibited
superparamagnetism at room temperature with saturation
magnetization of 55.4 emu/g and negligible remanence or coercivity.
The superparamagnetic character of these particles is demonstrated
by the absence of hysteresis. Measurements were conducted by using
ultrasonic vibrations to disperse the SPIONs in double-distilled
water. The SPION size distribution was almost homogeneous, with
ζ-potentials of −24.5 mV (Fig. 1C)
and diameters of 50.2±18.1 nm [polydispersity index (PDI) = 0.202]
(Fig. 1D).
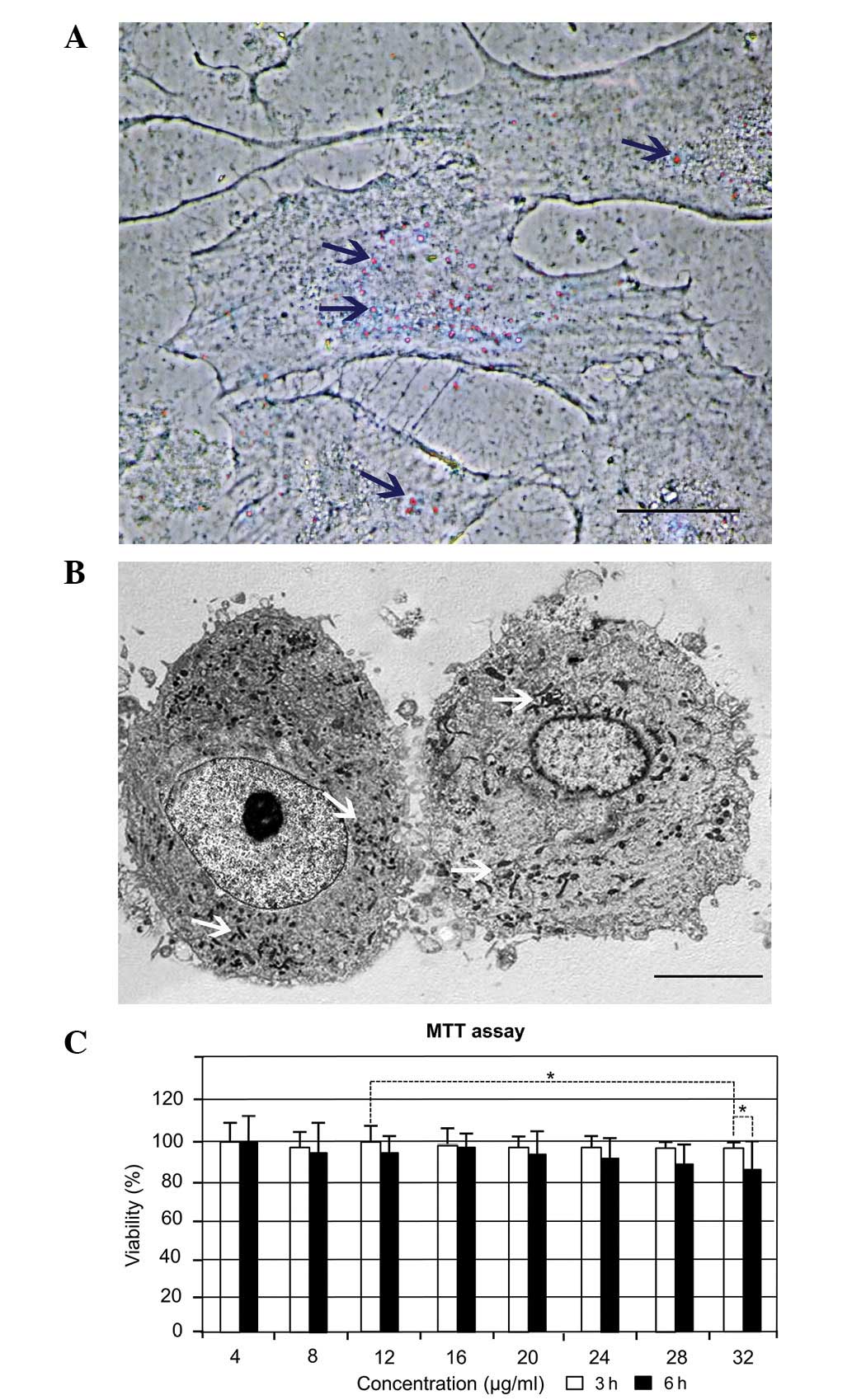
SPION cell labeling and cytotoxicity
results
Second passage RCECs were treated with SPIONs at
concentrations ranging from 4 to 32 μg/ml. Nanoparticles were shown
to be located within the cytoplasm, following 3–6 h incubation,
using Prussian blue staining (Fig.
2A). TEM was used to locate the SPIONs in the cells (Fig. 2B). Following internalization, the
SPIONs were located in vesicular structures within the cell
cytoplasm (Fig. 2B) and maintained
their initial size inside the intracellular vesicles. SPIONs were
seldom present in cell vesicles 5 days after the cell culture
medium had been replaced. The results of the MTT assay are shown in
Fig. 2C and demonstrate that cells
exposed to SPIONs at a mean size of 50 nm for 3 and 6 h resulted in
time- and concentration-dependent cytotoxicity at concentrations
>28 μg/ml. At 16 μg/ml, SPIONs had no significant cytotoxicity
to the RCECs and the viability of cells at 3 and 6 h was 98.1 and
97.5%, respectively (P>0.05). With increasing SPION
concentration up to 32 μg/ml, the percentage of viable cells
decreased to ~96.2% in 3 h. When the cells were incubated with the
same concentration of SPIONs for 6 h, cell viability decreased to
87.5%.
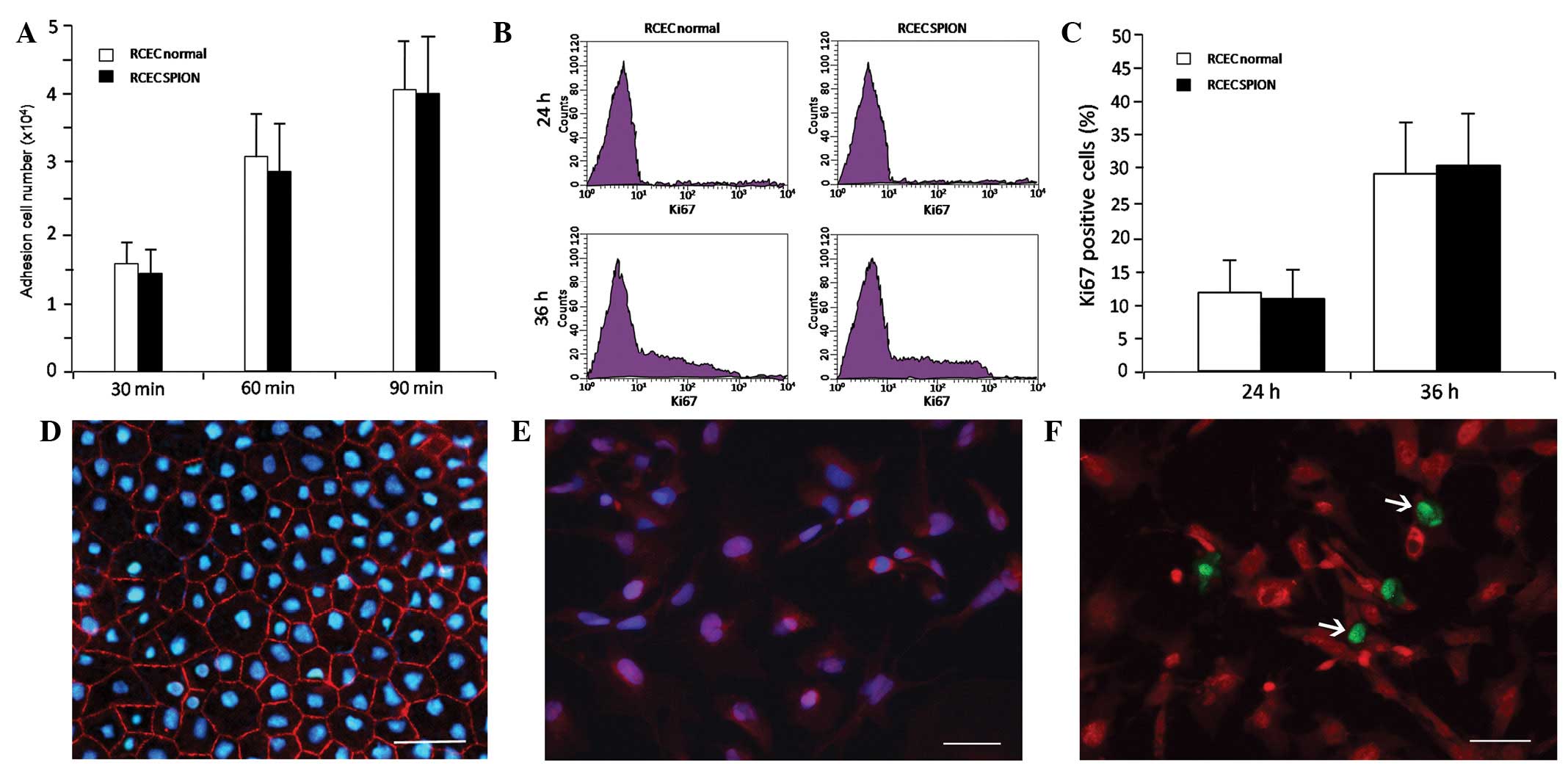
Effects of SPIONs on adhesion,
proliferation and markers of RCECs
To investigate the effect of the SPIONs on the
adhesion of RCECs, a homotypic adhesion assay was performed.
Following 30, 60 and 90 min incubations, statistical time-dependent
differences were observed between the unlabeled and labeled cells
with 16 μg/ml SPIONs (P<0.05, for all values), but no
significant differences in cell adhesion were observed between
RCECs (normal) and RCECs (SPION) at these time points (P>0.05)
(Fig. 3A). Quantitative flow
cytometric analysis revealed a time-dependent increase in
Ki-67-positive cells in RCECs incubated with or without 16 μg/ml
SPIONs, but no statistically significant differences were observed
between the two cell lines after 24 or 36 h incubation (P>0.05).
This demonstrates that 16 μg/ml SPION labeling did not alter the
proliferation of RCECs (Fig. 3B and
C). Cells labeled with 16 μg/ml SPIONs were immunostained for
ZO-1 (a marker of cell tight junctions, Fig. 3D), nestin (a marker of immature
cells, Fig. 3E) and for the cell
proliferation marker Ki-67 (Fig.
3F).
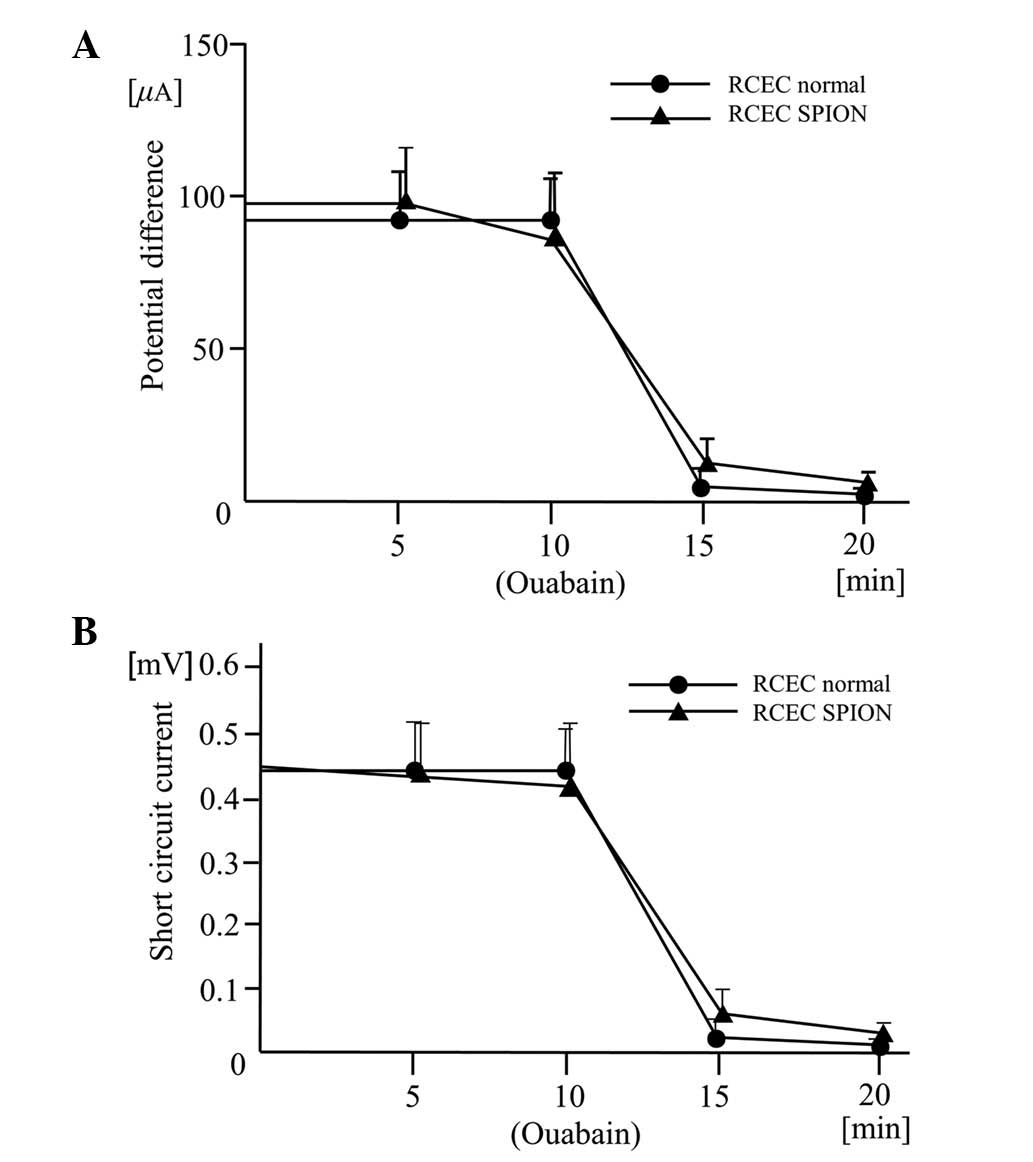
Effects of SPIONs on potential
differences and short circuit currents driven by
Na+/K+-ATPase
The traces of potential difference and short circuit
current driven by the Na+/K+-ATPase were
similar shapes in the two cell lines after 24 h with 16 μg/ml SPION
incubation. The potential differences and short circuit currents
maintained corneal transparency and were reduced in the two cell
lines by the presence of the Na+/K+-ATPase
inhibitor ouabain (Fig. 4). This
confirmed that the origin of the current is
Na+/K+-ATPase.
Discussion
The development of new diagnostic and therapeutic
technologies in nanomedicine includes nanotechnologies to improve
the early detection and treatment of human diseases (13,14).
There is an urgent need to understand the mechanisms of interaction
of nanomaterials, including nanoparticles, with living tissues and
to define the consequences of these interactions (15–17).
In this study, we used SPIONs to label RCECs and to observe several
functions crucial for maintaining the dehydration and transparency
of the cornea. TEM images (Fig. 2)
show the spherical shape and confirm the size of the particles to
be similar to ζ-size. The results of the MTT assay demonstrated
that cells exposed to 16 μg/ml SPIONs at a mean size of 50 nm for 6
h resulted in no marked cytotoxicity. The morphology of the RCECs
labeled by the SPIONs was similar to that of the unlabeled cells.
Morphological observations and immunocytochemical staining
confirmed that the cultured corneal endothelial cells were not
contaminated by corneal fibroblasts or epithelial cells. Although
the RCECs were directly cultured from the peeled corneal Descemet’s
membrane to which only endothelial cells are attached (1,18),
we were not able to demonstrate directly that the isolated cells
led to RCECs due to the lack of specific markers (2,20).
However, the characteristic hexagonal morphology and several
particular properties suggested that the cultures gave rise to
cells with features of CECs (2,19–21).
CECs accumulate Na+/K+-ATPase
at intercellular contacts along the lateral cell membranes in order
to maintain a bicarbonate gradient across the cell and sustain a
constant flow of water out of the stroma (22,23).
We used the Ussing chamber assay to detect cell pump function as
evaluated by cell electrophysiological measurements. The presence
of this protein in our RCEC populations indicated that SPION
labeling with 16 μg/ml for 24 h did not alter pump function. Prior
to the addition of ouabain, the potential difference and short
circuit current was detected in the RCEC (normal) and RCEC (SPION)
cell lines. Compared with the values of the RCEC (normal), RCEC
(SPION) had a similar potential difference and short circuit
current, indicating that a similar
Na+/K+-ATPase activity exists in the two cell
lines (22,23).
We also demonstrated cell-cell adhesion with the
homotypic adhesion assay (24),
and the adhesion ability of RCECs was similar between the two cell
lines during 90 min of cell culture. Quantitative flow cytometric
analyses revealed the presence of Ki-67-positive cells in RCECs
cultured with SPIONs for 36 h. This suggests that SPION labeling
did not interfere with the proliferation of RCECs.
Expression patterns of marker proteins are
frequently utilized in CEC characterization. Intercellular tight
junction-associated proteins of CEC cells, such as ZO-1, cell
markers of proliferation proteins, such as Ki-67, and intermediate
filament proteins, such as nestin, are crucial in maintaining
corneal dehydration and transparency (19,21,24).
Furthermore, certain adhesion junction-associated proteins mediate
and strengthen close cell-cell and cell-matrix associations
(25). In this study, the results
of fluorescent immunocytochemistry revealed that RCEC (SPION)
maintained the stable expression of ZO-1, Ki-67 and nestin,
suggesting that SPIONs do not change these RCEC characteristics
following labeling at a concentration of 16 μg/ml for 36 h.
In conclusion, our study demonstrated that SPION
labeling of RCECs at a specific concentration and time does not
affect cell functions in any of the four assays that were used.
Acknowledgements
We would like to thank Dr Felix Bock and Dr Claus
Cursiefen (Department of Ophthalmology, University Hospital of
Cologne, Cologne, Germany) for technical advice and assistance with
ophthalmic imaging and Dr Siwei Liu for preparing the donor
tissues. Funding for this study came from the Natural Science
Foundation of China (NSFC: 30973247/C170601, to Yanlong Bi) and
Shanghai Excellent University Teacher Foundation (1500144019, to
Yanlong Bi). Mingfeng Wu and Fei Du were partially supported by a
stem cell traineeship from the Huadong Stem Cell Bank of China.
References
|
1
|
Okumura N, Ueno M, Koizumi N, et al:
Enhancement on primate corneal endothelial cell survival in vitro
by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 50:3680–3687. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joyce NC: Proliferative capacity of
corneal endothelial cells. Exp Eye Res. 95:16–23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamazoe K, Yamaguchi T, Hotta K, et al:
Outcomes of cataract surgery in eyes with a low corneal endothelial
cell density. J Cataract Refract Surg. 37:2130–2136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pescosolido N, Komaiha C, Dapoto L,
Lenarduzzi F and Nebbioso M: Corneal haze in course of Fuchs’
endothelial dystrophy. Clin Ter. 163:e169–e171. 2012.
|
|
5
|
Wang X, Wang W, Xu J and Wang Y: Analysis
of causes of bullous keratopathy in East China: a 10-year
retrospective study. Graefes Arch Clin Exp Ophthalmol. 250:307–308.
2012.PubMed/NCBI
|
|
6
|
Quilendrino R, Yeh RY, Dapena I, et al:
Large diameter Descemet membrane endothelial keratoplasty in
buphthalmic eyes. Cornea. Nov 26–2012.(Epub ahead of print).
|
|
7
|
Ang M, Mehta JS, Lim F, Bose S, Htoon HM
and Tan D: Endothelial cell loss and graft survival after
Descemet’s stripping automated endothelial keratoplasty and
penetrating keratoplasty. Ophthalmology. 119:2239–2244. 2012.
|
|
8
|
Jhanji V, Mehta JS, Sharma N, Sharma B and
Vajpayee RB: Targeted corneal transplantation. Curr Opin
Ophthalmol. 23:324–329. 2012. View Article : Google Scholar
|
|
9
|
Peh GS, Beuerman RW, Colman A, Tan DT,
Mehta JS, et al: Human corneal endothelial cell expansion for
corneal endothelium transplantation: an overview. Transplantation.
91:811–819. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel SV, Bachman LA, Hann CR, Bahler CK
and Fautsch MP: Human corneal endothelial cell transplantation in a
human ex vivo model. Invest Ophthalmol Vis Sci. 50:2123–2131. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mimura T, Yamagami S, Usui T, et al:
Long-term outcome of iron-endocytosing cultured corneal endothelial
cell transplantation with magnetic attraction. Exp Eye Res.
80:149–157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mimura T, Yamagami S, Yokoo S, et al:
Cultured human corneal endothelial cell transplantation with a
collagen sheet in a rabbit model. Invest Ophthalmol Vis Sci.
45:2992–2997. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue K, Guduru R, Hong J, Liang P, Nair M
and Khizroev S: Magneto-electric nano-particles for non-invasive
brain stimulation. PLoS One. 7:e440402012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang F, Jin C, Subedi S, et al: Emerging
inorganic nanomaterials for pancreatic cancer diagnosis and
treatment. Cancer Treat Rev. 38:566–579. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raju HB, Hu Y, Vedula A, Dubovy SR and
Goldberg JL: Evaluation of magnetic micro- and nanoparticle
toxicity to ocular tissues. PLoS One. 6:e174522011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun JH, Zhang YL, Qian SP, et al:
Assessment of biological characteristics of mesenchymal stem cells
labeled with superparamagnetic iron oxide particles in
vitro. Mol Med Rep. 5:317–320. 2012.PubMed/NCBI
|
|
17
|
Balakumaran A, Pawelczyk E, Ren J, et al:
Superparamagnetic iron oxide nanoparticles labeling of bone marrow
stromal (mesenchymal) cells does not affect their ‘stemness’. PLoS
One. 5:e114622010.
|
|
18
|
Busin M, Scorcia V, Patel AK, Salvalaio G
and Ponzin D: Donor tissue preparation for Descemet membrane
endothelial keratoplasty. Br J Ophthalmol. 95:1172–1173. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu YT, Hayashida Y, Kheirkhah A, He H,
Chen SY and Tseng SC: Characterization and comparison of
intercellular adherent junctions expressed by human corneal
endothelial cells in vivo and in vitro. Invest Ophthalmol Vis Sci.
49:3879–3886. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Zhou Q, Qu M, Yang L, Wang Y and
Shi W: In vitro culture of human fetal corneal endothelial cells.
Graefes Arch Clin Exp Ophthalmol. 249:663–669. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roh DS and Funderburgh JL: Rapid changes
in connexin-43 in response to genotoxic stress stabilize cell-cell
communication in corneal endothelium. Invest Ophthalmol Vis Sci.
52:5174–5182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatou S, Yamada M, Akune Y, et al: Role of
insulin in regulation of Na+-/K+-dependent ATPase activity and pump
function in corneal endothelial cells. Invest Ophthalmol Vis Sci.
51:3935–3942. 2010.
|
|
23
|
Hatou S, Yamada M, Mochizuki H, Shiraishi
A, Joko T and Nishida T: The effects of dexamethasone on the
Na,K-ATPase activity and pump function of corneal endothelial
cells. Curr Eye Res. 34:347–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chandrasekaran S, Geng Y, DeLouise LA and
King MR: Effect of homotypic and heterotypic interaction in 3D on
the E-selectin mediated adhesive properties of breast cancer cell
lines. Biomaterials. 33:9037–9048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugrue SP and Zieske JD: ZO1 in corneal
epithelium: association to the zonula occludens and adherens
junctions. Exp Eye Res. 64:11–20. 1997. View Article : Google Scholar : PubMed/NCBI
|