Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies and the third most common cause of
cancer-related mortality worldwide (1). The treatment of HCC is currently
challenging and may remain difficult in the future (2). Although a few novel methods have been
applied (3), pharmacotherapy
remains the routine treatment for HCC.
Venom from natural toxins has been considered for
development into anticancer drugs (4) and the value of their potential
biomedical application is promising. Animal toxins have also been
identified to have antitumor activity, including bee (5), snake (6) and scorpion venom (7). Recently, scorpion venom was shown to
induce cell apoptosis (8) and
snake toxin was demonstrated to inhibit the proliferation of PA-1
and SK-OV3 cells (human ovarian cancer cells) (9).
Spider venom isolated from Lycosa singorensis
has exhibited an ability to inhibit cancer cell growth in
vitro(10). Spider venom is a
liquid with offensive, defensive and digestive functions.
Furthermore, different species of spiders vary in toxicity;
homology is limited even in primary structures. There are a large
number of spider species in China; Chilobrachy jingzhao is
one of these and is mainly located in the Guangxi Province of
China, with the following classification: Phylum, Arthropoda;
class, Arachnids; order, Araneae; family, Theraphosidae.
Chilobrachy jingzhaotoxin (JZTX)-III is a common biological
resource that may play a role in directly killing tumor cells.
Therefore, JZTX-III has the potential to be used in the development
of anticancer drugs and has received increasing attention from
pharmaceutical scientists and molecular biologists.
The present study aimed to investigate the potential
use of recombinant Escherichia coli (E. coli)
Trx-JZTX-III in the treatment of HCC by examining the proliferation
of Hepa1-6 cells, a HCC cell line from mice, following treatment
with or without recombinant E. coli Trx-JZTX-III.
Materials and methods
Preparation of recombinant E. coli
Trx-JZTX-III
In this study, genetic engineering was utilized to
inhibit the tetrodotoxin-resistant sodium channel. JZTX-III was
inserted in the prokaryotic expression vector pET-32a(+) to create
a recombination plasmid which expressed the fusion protein
Trx-JZTX-III in E. coli, using Trx as a protein tag. The
recombinant E. coli Trx-JZTX-III was freeze-dried and stored
at −80°C until use. The study was approved by the ethics committee
of Harbin Medical University, Harbin, China.
Cell culture
The HCC cell line Hepa1-6 was obtained from the
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai Branch Cell Bank, Shanghai, China). The cells
were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Carlsbad, CA, USA) containing 10% (v/v) fetal bovine serum (FBS;
Gibco), 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in
a humidified atmosphere containing 5% CO2.
MTT assay
The growth and viability of Hepa1-6 cells were
evaluated using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT;
Sigma, St. Louis, MO, USA) assay. The cells were plated into
96-well microtiter plates at a density of 5×103
cells/well. Following incubation for 24 h, recombinant E.
coli Trx-JZTX-III was serially diluted to various
concentrations (1,000, 900, 800, 700, 600, 500, 400, 300, 200 and
100 μg/ml) and added to each well. A control group was treated with
phosphate-buffered saline (PBS; Gibco) alone. After incubation for
24 and 48 h, 200 μl MTT (5 mg/ml) solution was added to each well
and cultured for 4 h at 37°C. Formazan crystals were solubilized
using 150 μl DMSO (Sigma) and plates were agitated for 10 min. The
absorbance was measured at 490 nm and a colorimetric MTT assay was
performed to investigate cell growth. All the experiments were
repeated three times.
Western blot analysis
Hepa1-6 cells (5×105) were seeded in
6-well plates and treated with 0, 600, 800 and 1,000 μg/ml
recombinant E. coli Trx-JZTX-III for 48 h, and proteins were
then extracted from each group of cells. The proteins were
separated by 10% SDS-PAGE and subsequently transferred to PVDF
membranes. Nonfat milk (5%) in TBST was used to block the membranes
at room temperature for 2 h. The membranes were then incubated with
rabbit anti-proliferating cell nuclear antigen (PCNA; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) or mouse anti-β-actin
antibodies (Santa Cruz Biotechnology, Inc.) at 4°C overnight. Next,
the membranes were incubated with anti-rabbit or anti-mouse
secondary antibodies for 2 h. Finally, the membranes were washed
three times with TBST and exposed to X-ray film.
Colony formation assay
A colony formation assay was conducted by plating
Hepa1-6 cells into 6-well plates (200 cells/plate). Following a
24-h incubation, recombinant E. coli Trx-JZTX-III at various
concentrations (600, 800 and 1,000 μg/ml) was added; the control
group received no recombinant E. coli Trx-JZTX-III. After 2
weeks, the cells were stained with Giemsa. Colonies were counted
only when a single clone contained >50 cells.
Cell migration assay
Cell migration was determined using a wound-healing
assay. Hepa1-6 cells were seeded at a density of 5×105
cells/6-well plate in DMEM containing 10% FBS. A scratch wound was
created on the confluent cell monolayer using a 200-μl pipette tip,
and then the cells were treated with various concentrations of
recombinant E. coli Trx-JZTX-III (0, 600, 800 and 1,000
μg/ml). The wounded areas were observed and images were captured
using a microscope at 0 and 48 h after scraping.
Flow cytometry
Hepa1-6 cells were seeded into 6-well plates in DMEM
containing 10% FBS and treated with various concentrations of
recombinant E. coli Trx-JZTX-III (0, 600, 800 and 1,000
μg/m) for 48 h. The cells were harvested, washed in cold PBS and
then fixed in 75% alcohol at 4°C for ≥12 h. The fixed cells were
resuspended in PBS containing 50 g/l RNase A and 50 mg/l propidium
iodide (PI) for 30 min, and analyzed using flow cytometry (Becton
Dickinson, USA).
Statistical analysis
The data are presented as the mean ± SEM. P<0.05
was considered to indicate a statistically significant difference.
The results were analyzed by Student’s t-test using SPSS version
10.0 (SPSS, Inc., Chicago, IL, USA).
Results
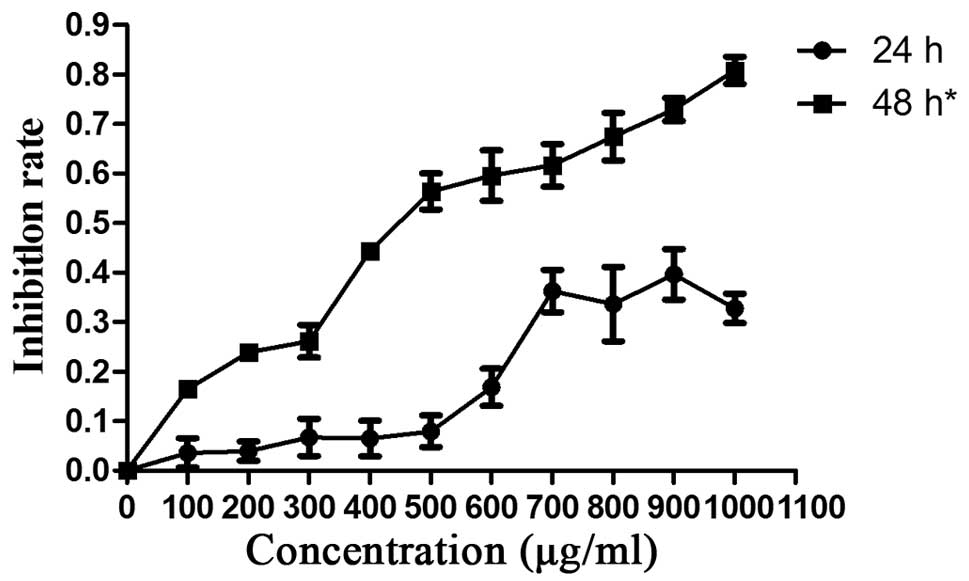
Recombinant E. coli Trx-JZTX-III inhibits
Hepa1-6 cell proliferation
Hepa1-6 cells were exposed to various concentrations
of E. coli Trx-JZTX-III (1,000, 900, 800, 700, 600, 500,
400, 300, 200 and 100 μg/ml). Growth inhibition was most
significant at a treatment time of >48 h (Fig. 1). Therefore, we further
investigated the in vitro inhibitory effects of recombinant
E. coli Trx-JZTX-III using a treatment time of 48 h
(P<0.05).
In order to obtain additional evidence, western blot
analysis was performed; PCNA was used as a reporter for
proliferation. Hepa1-6 cells were treated with recombinant E.
coli Trx-JZTX-III for 48 h and the expression of PCNA protein
was determined using western blot analysis. The expression of PCNA
was significantly lower with 600, 800 and 1,000 μg/ml E.
coli Trx-JZTX-III treatment compared with cells in the control
group (Fig. 2; P<0.05).
Recombinant E. coli Trx-JZTX-III
represses colony formation and cell migration
A colony formation assay was used to assess the
colony-forming ability of Hepa1-6 cells following treatment with or
without recombinant E. coli Trx-JZTX-III. Compared with
cells in the control group (Fig.
3D), cells treated with 600 (Fig.
3C), 800 (Fig. 3B) and 1,000
μg/ml (Fig. 3A) recombinant E.
coli Trx-JZTX-III formed fewer and smaller colonies.
Compared with the control group (Fig. 4A), a significant decrease in
Hepa1-6 cell migration was treated with recombinant E.coli
Trx-JZTX-III (Fig. 4B–D).
Recombinant E. coli Trx-JZTX-III induces
Hepa1-6 G0/G1 cell cycle arrest
As shown in Fig. 5,
cells treated with recombinant E. coli Trx-JZTX-III at
concentrations of 600 (Fig. 5C),
800 (Fig. 5B) or 1,000 μg/ml
(Fig. 5A) exhibited a
significantly increased cell population in the
G0/G1 phase when compared with the control
group (Fig. 5D). However, cells
treated with recombinant E. coli Trx-JZTX-III demonstrated a
significantly decreased cell population in the G2/M or S
phases (P<0.01; Table I).
 | Table ICell cycle distribution of Hepa1-6
cells (mean ± SEM). |
Table I
Cell cycle distribution of Hepa1-6
cells (mean ± SEM).
| Cell cycle
distribution (%) |
|---|
|
|
|---|
| Group |
G0/G1a | Sa |
G2/Ma |
|---|
| Trx-JZTX-III
(μg/ml) |
| 1000 | 72.2±3.5 | 13.96±1.9 | 28.4±1.3 |
| 800 | 45.7±2.0 | 29.45±3.7 | 54.3±2.4 |
| 600 | 42.1±2.9 | 31.18±4.2 | 57.9±2.0 |
| Control | 40.8±1.9 | 35.10±1.4 | 59.24±1.2 |
Discussion
Currently, several types of JZTXs are derived from
the spider Chilobrachys jingzhao, including JZTX-II
(11), -IX (12), -V (13), -XI (14), -IV (15) and -XIII (16). JZTX-III (molecular weight, 3919.3
Da) is a peptide toxin, containing 36 residues and three pairs of
intracellular disulfide bridges (I–IV, II–V, and III–VI) (17).
The venom from this spider has been shown to induce
apoptosis in the myelogenous leukemia K562 cell line (18) and in MCF-7 cells (19). Spider venom has been demonstrated
to be a novel tumor suppressor drug in HeLa cells (20) and was also shown to inhibit the
proliferation of HepG2 cells (21)
and induce BEL-7402 cell apoptosis (22) in HCC.
Spider venom is usually obtained by direct
extraction and artificial chemical synthesis. In this study, our
aim was to obtain a significant amount of recombinant fusion
protein of Trx-JZTX-III. Compared with natural JXTX-III,
recombinant E. coli Trx-JZTX-III has advantages with regard
to mass production and cost efficiency.
The MTT assay and quantification of PCNA expression
indicated that recombinant E. coli Trx-JZTX-III
significantly repressed the proliferation of Hepa1-6 cells.
Furthermore, the colony formation ability and migration of the
malignant cells were inhibited following treatment with recombinant
E. coli Trx-JZTX-III. Thus, recombinant E. coli
Trx-JZTX-III functioned as a tumor suppressor drug.
To explain the underlying mechanism of recombinant
E. coli Trx-JZTX-III in suppressing Hepa1-6 cell
proliferation, we investigated the cell cycle of Hepa1-6 cells
following treatment with or without recombinant E. coli
Trx-JZTX-III. Recombinant E. coli Trx-JZTX-III was shown to
induce G0/G1 cell cycle arrest; this may be
one of the mechanisms that mediate the antitumor function of
recombinant E. coli Trx-JZTX-III.
The present study demonstrated that recombinant
E. coli Trx-JZTX-III functioned as a tumor suppressor drug
in mouse HCC cell proliferation, potentially via the induction of
G0/G1 cell cycle arrest. These results
suggest that the antitumor activity of recombinant E. coli
Trx-JZTX-III may be associated with the proliferation of the mouse
cell line Hepa1-6. However, the effect of recombinant E.
coli Trx-JZTX-III on tumors in vivo remains unknown.
Future investigation with regard to the association between
recombinant E. coli Trx-JZTX-III and human hepatoma cells is
required to identify the specific molecules which are involved in
the antitumor activity and synthesis of recombinant E. coli
Trx-JZTX-III. Results of the present study suggest that recombinant
E. coli Trx-JZTX-III may be a promising candidate for
further development as a therapeutic agent due to its anticancer
efficacy. In conclusion, recombinant E. coli Trx-JZTX-III
represents a potentially important tool for the development of
novel drugs and antitumor agents in the treatment of HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81172616, 30771863 and
30910007).
References
|
1
|
Schütte K, Bornschein J and Malfertheiner
P: Hepatocellular carcinoma - epidemiological trends and risk
factors. Dig Dis. 27:80–92. 2009.PubMed/NCBI
|
|
2
|
Taieb J, Barbare JC, Boussaha T, et al:
Management of hepatocellular carcinoma. Where are we now? What’s
next? Bull Cancer. 96:19–34. 2009.(In French).
|
|
3
|
May BJ, Murthy R and Madoff DC: What’s new
in transarterial therapies for hepatocellular carcinoma?
Gastrointest Cancer Res. 5(Suppl 1): S14–S19. 2012.
|
|
4
|
Harvey A: Strategies for discovering drugs
from previously unexplored natural products. Drug Discov Today.
5:294–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Orsolić N, Sver L, Verstovsek S, Terzić S
and Basić I: Inhibition of mammary carcinoma cell proliferation in
vitro and tumor growth in vivo by bee venom. Toxicon. 41:861–870.
2003.PubMed/NCBI
|
|
6
|
de Carvalho DD, Schmitmeier S, Novello JC
and Markland FS: Effect of BJcuL (a lectin from the venom of the
snake Bothrops jararacussu) on adhesion and growth of tumor
and endothelial cells. Toxicon. 39:1471–1476. 2001.PubMed/NCBI
|
|
7
|
Liu YF, Ma RL, Wang SL, et al: Expression
of an antitumor-analgesic peptide from the venom of Chinese
scorpion Buthus martensii Karsch in Escherichia coli.
Protein Expr Purif. 27:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ning YN, Zhang WD and Wu LC: Study on the
mechanism of polypeptide extract from scorpion venom to promote the
restraint of cyclophosphamide on Lewis lung cancer. Zhongguo Zhong
Xi Yi Jie He Za Zhi. 32:537–542. 2012.(In Chinese).
|
|
9
|
Song JK, Jo MR, Park MH, et al: Cell
growth inhibition and induction of apoptosis by snake venom toxin
in ovarian cancer cell via inactivation of nuclear factor κB and
signal transducer and activator of transcription 3. Arch Pharm Res.
35:867–876. 2012.PubMed/NCBI
|
|
10
|
Liu Z, Deng M, Xiang J, et al: A novel
spider peptide toxin suppresses tumor growth through dual signaling
pathways. Curr Mol Med. 12:1350–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang M, Liu Q, Luo H, et al:
Jingzhaotoxin-II, a novel tarantula toxin preferentially targets
rat cardiac sodium channel. Biochem Pharmacol. 76:1716–1727. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng M, Kuang F, Sun Z, et al:
Jingzhaotoxin-IX, a novel gating modifier of both sodium and
potassium channels from Chinese tarantula Chilobrachys
jingzhao. Neuropharmacology. 57:77–87. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeng X, Deng M, Lin Y, Yuan C, Pi J and
Liang S: Isolation and characterization of Jingzhaotoxin-V, a novel
neurotoxin from the venom of the spider Chilobrachys
jingzhao. Toxicon. 49:388–399. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao Z, Yuan C, Deng M, et al: Solution
structure and functional characterization of jingzhaotoxin-XI: a
novel gating modifier of both potassium and sodium channels.
Biochemistry. 45:15591–15600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Diao J, Li J, et al: JZTX-IV, a
unique acidic sodium channel toxin isolated from the spider
Chilobrachys jingzhao. Toxicon. 52:871–880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan C, Liu Z, Hu W, Gao T and Liang S:
JZTX-XIII, a Kv channel gating modifier toxin from Chinese
tarantula Chilobrachys jingzhao. Toxicon. 59:265–271. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao Y, Tang J, Yang Y, et al:
Jingzhaotoxin-III, a novel spider toxin inhibiting activation of
voltage-gated sodium channel in rat cardiac myocytes. J Biol Chem.
279:26220–26226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Zhao Y, Li J, et al: The venom of
the spider Macrothele raveni induces apoptosis in the
myelogenous leukemia K562 cell line. Leuk Res. 36:1063–1066.
2012.PubMed/NCBI
|
|
19
|
Gao L, Yu S, Wu Y and Shan B: Effect of
spider venom on cell apoptosis and necrosis rates in MCF-7 cells.
DNA Cell Biol. 26:485–489. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao L, Shan BE, Chen J, Liu JH, Song DX
and Zhu BC: Effects of spider Macrothele raven venom on cell
proliferation and cytotoxicity in HeLa cells. Acta Pharmacol Sin.
26:369–376. 2005.
|
|
21
|
Gao L, Shen JB, Sun J and Shan BE: Effect
of the venom of the spider Macrothele raveni on the
expression of p21 gene in HepG2 cells. Sheng Li Xue Bao. 59:58–62.
2007.PubMed/NCBI
|
|
22
|
Gao L, Feng W, Shan BE and Zhu BC:
Inhibitory effect of the venom of spider Macrothele raveni
on proliferation of human hepatocellular carcinoma cell line
BEL-7402 and its mechanism. Ai Zheng. 24:812–816. 2005.(In
Chinese).
|