Introduction
Radiation therapy is important for the treatment of
malignant tumors, with ~60% of cancer patients requiring
radiotherapy as a component of their cancer treatment (1). However, since it is genotoxic,
radiation also activates various radiation response genes.
Activation of these genes leads to the production of cytokines that
are able to alter the microenvironment surrounding tumor cells,
which in turn increases the invasion and metastasis of surviving
tumor cells (2,3). Previous studies have demonstrated
that radiation promotes the invasion or metastasis of various types
of cancer cells, including pancreatic, glioma, hepatocellular,
breast and melanoma (4–7). In addition, a clinical study revealed
that invasive recurrence increased following treatment of ductal
carcinoma in situ with radiation therapy (8).
Cancer cell invasion and metastasis are complicated
processes involving cell proliferation at the primary site,
adhesion to and invasion of basement membranes, migration through
the extracellular matrix (ECM), penetration into lymphatic or blood
vessels and angiogenesis at the metastatic site (9). During the invasion of basement
membranes by cancer cells, matrix metalloproteinases (MMPs),
members of the matrixin subfamily of the zinc metalloproteinases,
play key roles in degradation of the ECM (10), and elevated levels of MMPs have
been found in a number of tumor types (11). MMP-2 degrades denatured
interstitial collagens I and III, as well as native collagen IV,
which is an important component of the basement membrane. Activated
MMP-2 triggers tumor invasion and metastasis and increased
expression of MMP-2 is associated with poor prognosis in cancer
patients (12). Notably, the
expression and activity of MMP-2 is enhanced by radiation in a
number of cells, including astrocytes and mesangial and kidney
tubule epithelial cells in rats and bronchial epithelial cells in
humans (13–16). However, the biological function of
elevated MMP-2 expression induced by radiation remains unclear.
The transcription factor signal transducer and
activator of transcription 3 (STAT3), a member of the STAT family,
plays a critical role in mediating cell survival, proliferation,
invasion and angiogenesis (17).
STAT3 includes a conserved tyrosine residue at position 705
(Tyr705), which is phosphorylated by Janus-activated kinase 2
(18). STAT3 phosphorylation
promotes its dissociation from the receptor and its
homodimerization. The dimer then translocates to the nucleus, where
it regulates the transcription of target genes (19), including MMP-2 and MMP-9. Blocking
STAT3 signaling in highly metastatic mouse melanoma cells was
reported to significantly inhibit the invasion of tumor cells by
suppressing expression of the MMP-2 gene (20). Inhibition of STAT3 also prevents
the metastasis of breast cancer cells (21). Collectively, previous studies have
indicated that STAT3 affects tumor invasion, growth and
metastasis.
E-cadherin is important for cell-to-cell cohesion,
cell-to-cell recognition and epithelial polarity (22), and analysis of E-cadherin protein
expression levels is an important indicator of cell invasion
ability.
Despite the therapeutic role of radiation in lung
cancer, particularly in non-small cell lung cancer (NSCLC),
radiation also promotes the invasion of NSCLC (23). NSCLC invasion is associated with
poor prognosis since it increases the invasive growth and
metastasis of tumors; for example, in mouse Lewis lung cancer
(24).
In the current study, the hypothesis that radiation
promotes the invasion of lung adenocarcinoma A549 cells was
investigated. In addition, the effect of radiation on the secretion
of MMP-2 in A549 cells and the molecular mechanism by which A549
cell invasion is induced by radiation was determined. Radiation was
found to promote the invasion of A549 cells and the secretion of
MMP-2, which may represent the molecular mechanism of
radiation-induced A549 cell invasion. The previous observation that
STAT3 plays a key role in radiation-induced A549 cell invasion was
further confirmed in this study.
Materials and methods
Cell lines
The A549 cell line was obtained from the Cell
Culture Center of The Institute of Basic Medical Sciences (Chinese
Academy of Medical Sciences, Beijing, China). Cells were maintained
in Dulbecco’s Modified Eagle’s Medium (DMEM) plus 10% fetal bovine
serum at 37°C within humidified 5% CO2 air. The present
study was approved by the Ethics committee of The Beijing Institute
of Radiation Medicine, Beijing, China.
Radiation and chemical reagents
A549 cells were irradiated using a Cobalt-60 unit
(Beijing Institute of Radiation Medicine, Beijing, China) at a
source-skin distance of 4 m. The dose rate was 2.17 Gy/min. The
chemical inhibitor, AG490 (Sigma-Aldrich, St. Louis, MO, USA), was
used to suppress the radiation-induced invasion of A549 cells.
Invasion assay
The invasion of A549 cells was measured as the
number of cells invading through Matrigel-coated transwell inserts,
as described previously (25). In
brief, transwell inserts with 8-μm pores were coated with Matrigel
(20 μg/well; BD Biosciences, Franklin Lakes, NJ, USA). A total of
2×105 cells were suspended in 200 μl serum-free DMEM and
seeded into the upper chamber. DMEM supplemented with 10% fetal
bovine serum (FBS) was placed in the lower chamber as a
chemoattractant. Cells were allowed to invade for 20 h. Following
incubation, non-invaded cells on the upper side of the membrane
were removed using a cotton swab. The invasive cells on the lower
surface of the Matrigel-coated membrane were fixed with 100%
methanol and stained using Wright’s stain (Sigma-Aldrich). Invasion
was determined by counting cells in 5 microscopic fields/well and
the extent of invasion was expressed as an average number of
cells/microscopic field.
Cell adhesion assay
Firstly, 96-well plates were coated with 10 μg/ml
fibronectin in phosphate buffered saline (PBS; both Sigma-Aldrich)
overnight at 4°C. Wells were then rinsed and blocked with 1% bovine
serum albumin in PBS for 1 h. Cells were harvested during the
logarithmic phase of growth by trypsin and plated at
5×104 cells/well. Following 1 h incubation at 37°C,
wells were rinsed to remove non-adherent cells. Adhered cells were
treated with 100 μl 0.05%
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
(MTT) dissolved in PBS and incubated for 1 h. Following incubation,
the MTT solution was removed and formazan formed by the cells was
resuspended in 100% ethanol. Absorbance was measured at 590 nm
using a Versamax microplate reader (Amersham Pharmacia Biotech,
Amersham, UK).
Western blot analysis
Cells were harvested 12 or 24 h following
irradiation and were then directly lysed in a lysis buffer
containing a mammalian protease cocktail to obtain total protein
content. For nuclear extracts, cells were lysed in a NE-PER
extraction reagent (Pierce Biotechnology, Inc., Rockford, IL, USA),
according to the manufacturer’s instructions. Protein concentration
was measured using the Bradford method. Protein (50 μg) was
separated on a 12% sodium dodecyl sulfate-polyacrylamide gel and
transferred onto nitrocellulose membranes. Membranes were blocked
in TBST buffer (10 mM Tris-HCl, pH 8.0; 0.15 M NaCl; and 0.05%
Tween 20) containing 5% skimmed milk. Next, membranes were
incubated overnight with monoclonal antibodies against p-STAT3
(Y705; Cell Signaling Technology, Inc., Danvers, MA, USA), MMP-2
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), vascular
endothelial growth factor (VEGF; Sigma-Aldrich), β-actin (Santa
Cruz Biotechnology, Inc.) and E-cadherin (clone ECH-6; Cell Marque
Corp., Rocklin, CA, USA). Finally, horseradish peroxidase-linked
anti-mouse IgG (Zhongshan Goldenbridge Biotechnology Co., Beijing,
China) was added to allow enhanced chemiluminescence visualization
of the bands.
Gelatin zymography
Subconfluent A549 cells (70–80% confluency) were
washed and refreshed with serum-free DMEM, and then irradiated and
incubated for 24 h. The activity of electrophoretically separated
gelatinolytic enzymes in the conditioned medium of A549 cells was
determined by gelatin zymography, as described previously (24). Zones of gelatinolytic activity were
detected as clear bands against a blue background.
Reverse transcription-PCR (RT-PCR)
Total cellular RNA content was extracted with TRIzol
(Gibco-BRL, Carlsbad, CA, USA), according to the manufacturer’s
instructions. Complementary first-strand DNA was generated using an
ImProm-II reverse transcription system (Promega, Shanghai, China),
according to the manufacturer’s instructions. The PCR protocol
involved an initial denaturation step at 94°C for 5 min, followed
by 30 cycles at 94°C for 30 sec, 57°C for 30 sec and 72°C for 30
sec. Oligonucleotide primer sequences were as follows: MMP-2,
5′-GGCCAAGTGGTCCGTGTG-3′ (sense) and 5′-GAGGCCCCATAGAGCTCC-3′
(antisense); and β-actin, 5′-CATCTCTTGCTCGAAGTCCA-3′ (sense) and
5′-ATCATGTTTGAGACCTTCAACA-3′ (antisense). PCR was performed using a
PCR Mix kit [Tiangen Biotech (Beijing) Co., Ltd., Beijing, China],
according to the manufacturer’s instructions.
Statistical analysis
All experiments were performed in triplicate or
quadruplicate and 3 measurements/data point were obtained in each
experiment. A two-tailed Student’s t-test was used for statistical
analysis of comparative data using SPSS software (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
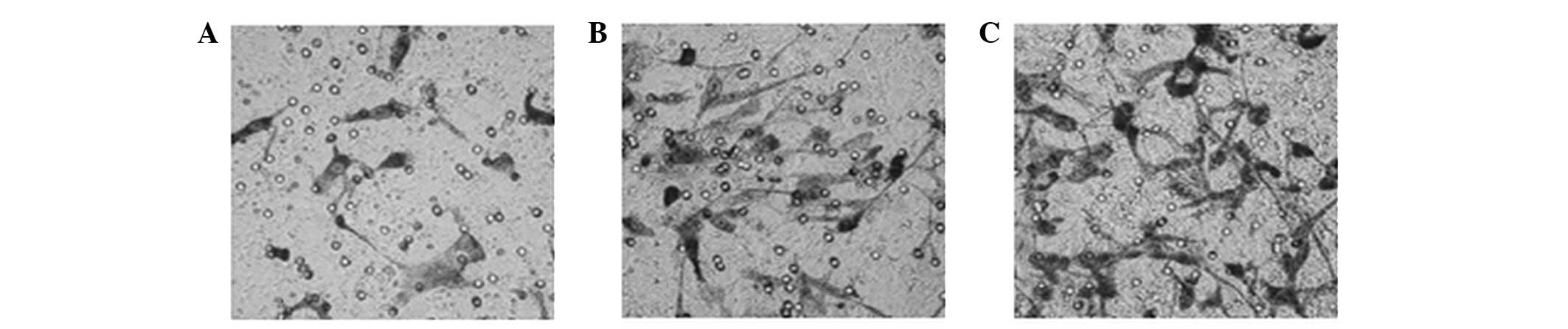
Radiation promotes the invasion of A549
cells but has no effect on cell adhesion
Radiation doses of 2 or 4 Gy were found to
significantly promote the invasion of A549 cells in a
dose-dependent manner compared with the untreated controls
(Fig. 1). Cell adhesion, another
important factor that affects tumor metastasis, was unchanged by
radiation.
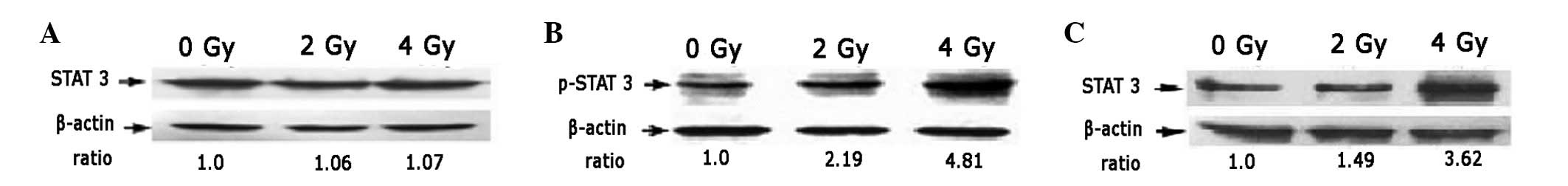
Radiation induces STAT3 activation and
promotes its nuclear localization
The expression of STAT3 in A549 cells was not
affected by 2 or 4 Gy radiation (Fig.
2A). However, levels of phosphorylated STAT3, the functional
form of STAT3, in A549 cells were observed to increase
significantly 12 h after 2 or 4 Gy radiation compared with the
untreated controls (Fig. 2B).
These observations indicate that 2 and 4 Gy radiation induces the
activation of STAT3. Total STAT3 in the nucleus of A549 cells
irradiated with 2 or 4 Gy radiation was identified to be
significantly higher than in untreated cells (Fig. 2C).
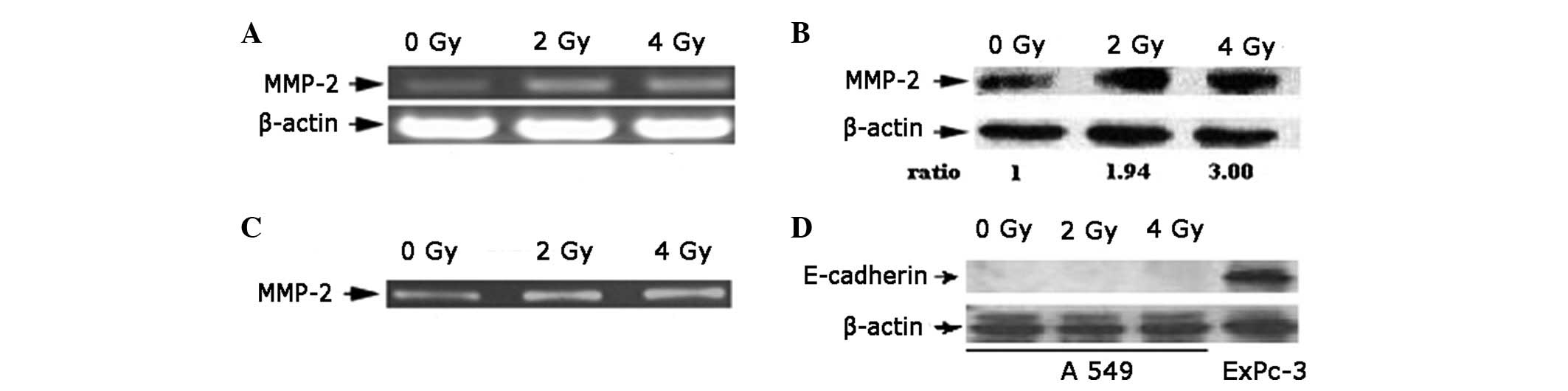
Radiation increases the expression and
activity of MMP-2
RT-PCR results indicate that the expression of MMP-2
mRNA in A549 cells increased significantly 24 h after 2 or 4 Gy
radiation compared with the untreated controls (Fig. 3A). An upregulated expression of
MMP-2 protein in A549 cells 24 h after radiation was also detected
(Fig. 3B), and the expression of
MMP-2 protein at 2 Gy was higher than levels at 4 Gy. The activity
of the MMP-2 enzyme in the conditioned medium increased following
the irradiation of A549 cells (Fig.
3C). The expression of E-cadherin was not affected by radiation
(Fig. 3D), consistent with results
of the cell adhesion assay. E-cadherin protein was not detected in
A549 cells prior to and following irradiation.
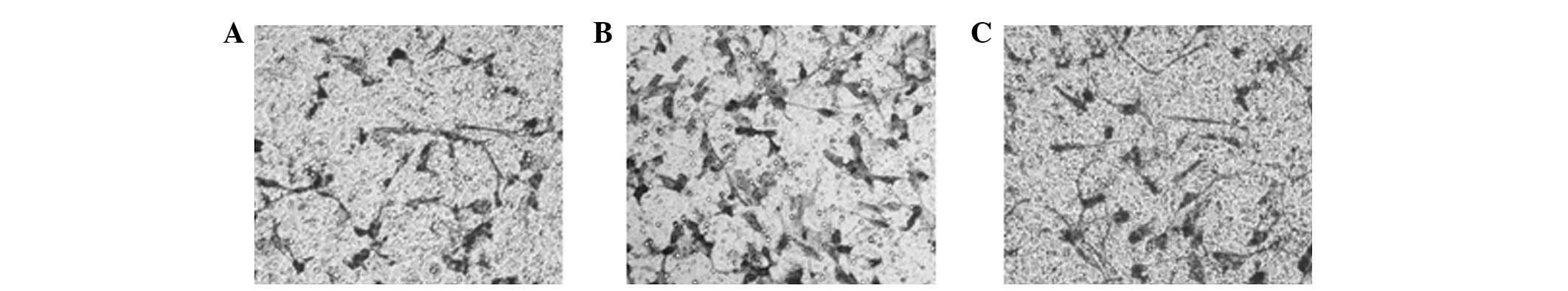
Inhibition of STAT3 phosphorylation
suppresses the radiation-induced expression of MMP-2 and invasion
of A549 cells
The increase in radiation-induced MMP-2 expression
triggered by 4 Gy radiation was suppressed when A549 cells were
treated with 20 or 40 μM AG490 (Fig.
4A). In addition, AG490 inhibited the enzymatic activity of
MMP-2 (Fig. 4B). Since
radiation-induced activation of STAT3 in A549 cells leads to the
elevated expression of MMP-2, we investigated whether inhibition of
STAT3 eliminates the increased invasion of A549 cells. Results
demonstrate that 20 μM AG490 suppressed the radiation-induced
invasion of A549 cells (Fig.
4C).
Radiation increases the expression of
VEGF via activation of STAT3
Doses of 2 or 4 Gy irradiation were found to
significantly increase the transcription of VEGF, indicating that
irradiation regulated VEGF expression at the transcriptional level
(Fig. 5A and B). Further
immunoblotting assays revealed that VEGF protein expression was
also significantly elevated by 2 or 4 Gy irradiation. The
radiation-induced elevation of VEGF (Fig. 5C) was significantly suppressed by
AG490, consistent with MMP-2 expression.
Discussion
Lung cancer is the leading cause of cancer mortality
worldwide and radiotherapy is the primary mode of treatment,
particularly in cases of non-resectable lung cancer. Although
radiotherapy generally increases survival in lung cancer patients,
the majority of patients are likely to suffer from metastases in
distant organs. In the current study, irradiation was found to
enhance A549 cell invasion, consistent with the observation that
radiotherapy of the primary tumor is associated with unpredictable
metastatic effects (24). In
addition, a previous in vivo study revealed that irradiation
promoted the metastatic growth of Lewis lung cancer, a type of
mouse pulmonary adenocarcinoma (26). In the present study, increased
irradiation-induced invasion of A549 cells was, at least in part,
mediated by increased MMP-2 expression. Additional analyses
demonstrated that the activation of STAT3 was involved in
irradiation-induced MMP-2 expression and activation, ultimately
promoting the invasion of A549 cells. Notably, the activation of
STAT3 by irradiation also increased the expression of VEGF in A549
cells.
The malignant characteristics of tumor cells include
rapid cell growth, migration, invasion and metastasis. Metastasis
itself is the most insidious and life-threatening property of
cancer cells, and is preceded by migration and invasion. In a
previous study, it was demonstrated that ionizing irradiation
increases A549 cell migration (27). Results of the present study were
consistent with these observations, whereby the invasive ability of
A549 cells was also enhanced following 2 and 4 Gy irradiation. In
addition, VEGF expression was found to be significantly increased
in irradiated A549 cells. Since specific radioresistant tumor cells
are able to survive radiation therapy, these results indicated that
irradiation therapy may increase tumor metastasis due to the
increased migration, invasion and pro-angiogenic ability of
surviving cells.
MMPs degrade and modify the ECM, facilitating cancer
cell invasion, and have been reported to be involved in mammalian
tumor progression and metastasis. Altered expression of MMPs is a
recognized hallmark of invasive tumor cells (28), and MMPs have been found to be
upregulated in cancer cells. Previous studies have revealed that
irradiation promotes MMP-9 expression in hepatocellular carcinoma
cells (29) and MMP-2 expression
in glioma cells (30), and
elevated expression levels of MMP-2 or MMP-9 have been revealed to
promote irradiation-induced cancer cell invasion. Similarly,
results of the present study demonstrated that MMP-2 is also
upregulated in pulmonary adenocarcinoma cells following
irradiation. Radisky and Radisky (31) previously demonstrated that MMP-2
promotes and mediates epithelial-mesenchymal transition (EMT), a
process implicated in tumor progression and promotion of the
migration and invasion of cancer cells. Notably,
irradiation-induced EMT-associated changes in A549 were observed in
a previous study (27). Combined
with observations of the present study, these results indicate that
irradiation-induced increases in MMP-2 regulated the invasion of
A549 cells by promoting EMT-associated changes, as well as by
having a direct pro-invasive activity.
STAT3, which has been found to be phosphorylated in
several clinical cancer cells, is activated by numerous cytokines,
growth factors and oncogenic proteins (32). In the current study, STAT3 was also
found to be activated by irradiation. Similarly, Singh-Gupta et
al(33) found that irradiation
promoted the phosphorylation of STAT3 in prostate cancer cells.
Abdulghani et al(34) reported that STAT3 is associated
with metastatic progression in various types of cancer, including
lung, skin, liver, ovarian, kidney, colon and prostate cancer.
Activation of STAT3 may activate the expression of genes associated
with the regulation of cell migration, invasion and angiogenesis at
the transcriptional level. The expression of these genes, including
MMPs and VEGF, promote tumor metastasis. In the current study,
irradiation-induced STAT3 activation was identified to be involved
in the upregulation of MMP-2, which facilitated the invasion of
A549 cells. In addition, the activation of STAT3 was involved in
irradiation-induced VEGF expression, indicating that STAT3
activation mediates tumor metastasis by several mechanisms.
In conclusion, in the present study, γ-ray radiation
was found to enhance A549 cell invasion, largely via the
upregulation of MMP-2 expression mediated by STAT3. The ability of
irradiation to kill tumor cells is definitive and has been
demonstrated in a number of in vitro and in vivo
studies (35–37). However, at present, only a limited
number of studies on the effect of irradiation on tumor cell
invasion have been performed. Based on the widespread application
of radiotherapy for lung cancer and the poor outcomes associated
with increased invasion, results of the present study indicate the
importance of suppression of the irradiation-induced invasion of
lung cancer cells. Identification of the key mediator involved in
this process is likely to lead to development of a specific
inhibitor to block metastatic signaling, while retaining the
therapeutic benefit of irradiation for lung cancer therapy.
Acknowledgements
The present study was supported by Science Funds for
Young Scholar of Chinese Center for Disease Control and Prevention
(nos. 2011A202), and grants from the National Natural Science
Foundation of China (nos. 81001216, 81172130 and 81202151).
References
|
1
|
Klepper L: An interactive method for
optimization of irradiation plans in radiation therapy of malignant
tumors. Med Tekh. 25–30. 2006.(In Russian).
|
|
2
|
Rastogi S, Boylan M, Wright EG and Coates
PJ: Interactions of apoptotic cells with macrophages in
radiation-induced bystander signaling. Radiat Res. 179:135–145.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Y and Nelson PS: Molecular pathways:
involving microenvironment damage responses in cancer therapy
resistance. Clin Cancer Res. 18:4019–4025. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Madani I, De Neve W and Mareel M: Does
ionizing radiation stimulate cancer invasion and metastasis? Bull
Cancer. 95:292–300. 2008.PubMed/NCBI
|
|
5
|
Kaur H, Buettner H, Salomao DR and Marks
RS: Transcleral orbital invasion by a radiation and
chemotherapy-resistant choroidal metastasis of a pulmonary
adenocarcinoma. Am J Ophthalmol. 143:369–370. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itoh YH, Nakatsugawa S, Oguri T and Miyata
N: Increase of cellular fibrinolysis in human lung cancer cell line
by radiation: relationship between urokinase-type plasminogen
activator (uPA) and metastasis and invasion. Nihon Igaku Hoshasen
Gakkai Zasshi. 56:747–749. 1996.(In Japanese).
|
|
7
|
Boutrus R, Abi-Raad R, Niemierko A, et al:
Does lymphovascular invasion predict regional nodal failure in
breast cancer patients with zero to three positive lymph nodes
treated with conserving surgery and radiotherapy? Implications for
regional radiation. Int J Radiat Oncol Biol Phys. 78:793–798. 2010.
View Article : Google Scholar
|
|
8
|
Guerra LE, Smith RM, Kaminski A, Lagios MD
and Silverstein MJ: Invasive local recurrence increased after
radiation therapy for ductal carcinoma in situ. Am J Surg.
196:552–555. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fidler IJ, Kim SJ and Langley RR: The role
of the organ microenvironment in the biology and therapy of cancer
metastasis. J Cell Biochem. 101:927–936. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kargozaran H, Yuan SY, Breslin JW, et al:
A role for endothelial-derived matrix metalloproteinase-2 in breast
cancer cell transmigration across the endothelial-basement membrane
barrier. Clin Exp Metastasis. 24:495–502. 2007. View Article : Google Scholar
|
|
11
|
Kerkelä E and Saarialho-Kere U: Matrix
metalloproteinases in tumor progression: focus on basal and
squamous cell skin cancer. Exp Dermatol. 12:109–125.
2003.PubMed/NCBI
|
|
12
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis, and angiogenesis.
Surg Oncol Clin N Am. 10:383–392. 2001.PubMed/NCBI
|
|
13
|
Araya J, Maruyama M, Sassa K, et al:
Ionizing radiation enhances matrix metalloproteinase-2 production
in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol.
280:L30–L38. 2001.PubMed/NCBI
|
|
14
|
Sawaya R, Tofilon PJ, Mohanam S, et al:
Induction of tissue-type plasminogen activator and 72-kDa type-IV
collagenase by ionizing radiation in rat astrocytes. Int J Cancer.
56:214–218. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, O’Malley Y and Robbins ME:
Irradiation of rat mesangial cells alters the expression of gene
products associated with the development of renal fibrosis. Radiat
Res. 152:160–169. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao W, O’Malley Y, Wei S and Robbins ME:
Irradiation of rat tubule epithelial cells alters the expression of
gene products associated with the synthesis and degradation of
extracellular matrix. Int J Radiat Biol. 76:391–402. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lui VW, Boehm AL, Koppikar P, et al:
Antiproliferative mechanisms of a transcription factor decoy
targeting signal transducer and activator of transcription (STAT)
3: the role of STAT1. Mol Pharmacol. 71:1435–1443. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ju H, Venema VJ, Liang H, Harris MB, Zou R
and Venema RC: Bradykinin activates the Janus-activated
kinase/signal transducers and activators of transcription
(JAK/STAT) pathway in vascular endothelial cells: localization of
JAK/STAT signalling proteins in plasmalemmal caveolae. Biochem J.
351:257–264. 2000. View Article : Google Scholar
|
|
19
|
Shen Y, Devgan G, Darnell JE Jr and
Bromberg JF: Constitutively activated Stat3 protects fibroblasts
from serum withdrawal and UV-induced apoptosis and antagonizes the
proapoptotic effects of activated Stat1. Proc Natl Acad Sci USA.
98:1543–1548. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Wang Y, Xie C, Qiu X and Wang E:
Expression of MMP-2, MMP-9, TIMP-1 and their relationship with
prognosis in NSCLC. Zhongguo Fei Ai Za Zhi. 7:497–500. 2004.(In
Chinese).
|
|
21
|
Gariboldi MB, Ravizza R, Molteni R, Osella
D, Gabano E and Monti E: Inhibition of Stat3 increases doxorubicin
sensitivity in a human metastatic breast cancer cell line. Cancer
Lett. 258:181–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeanes A, Gottardi CJ and Yap AS:
Cadherins and cancer: how does cadherin dysfunction promote tumor
progression? Oncogene. 27:6920–6929. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsutsumi K, Tsuda M, Yazawa N, et al:
Increased motility and invasiveness in tumor cells that survive 10
Gy irradiation. Cell Struct Funct. 34:89–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Camphausen K, Moses MA, Beecken WD, Khan
MK, Folkman J and O’Reilly MS: Radiation therapy to a primary tumor
accelerates metastatic growth in mice. Cancer Res. 61:2207–2211.
2001.PubMed/NCBI
|
|
25
|
Zhang W, Nwagwu C, Le DM, Yong VW, Song H
and Couldwell WT: Increased invasive capacity of
connexin43-overexpressing malignant glioma cells. J Neurosurg.
99:1039–1046. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayo JG: Biologic characterization of the
subcutaneously implanted Lewis lung tumor. Cancer Chemother Rep 2.
3:325–330. 1972.PubMed/NCBI
|
|
27
|
Jung JW, Hwang SY, Hwang JS, Oh ES, Park S
and Han IO: Ionising radiation induces changes associated with
epithelial-mesenchymal transdifferentiation and increased cell
motility of A549 lung epithelial cells. Eur J Cancer. 43:1214–1224.
2007. View Article : Google Scholar
|
|
28
|
Béliveau A, Bérubé M, Rousseau A,
Pelletier G and Guérin SL: Expression of integrin alpha5beta1 and
MMPs associated with epithelioid morphology and malignancy of uveal
melanoma. Invest Ophthalmol Vis Sci. 41:2363–2372. 2000.PubMed/NCBI
|
|
29
|
Cheng JC, Chou CH, Kuo ML and Hsieh CY:
Radiation-enhanced hepatocellular carcinoma cell invasion with
MMP-9 expression through PI3K/Akt/NF-kappaB signal transduction
pathway. Oncogene. 25:7009–7018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park CM, Park MJ, Kwak HJ, et al: Ionizing
radiation enhances matrix metalloproteinase-2 secretion and
invasion of glioma cells through Src/epidermal growth factor
receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt
signaling pathways. Cancer Res. 66:8511–8519. 2006. View Article : Google Scholar
|
|
31
|
Radisky ES and Radisky DC: Matrix
metalloproteinase-induced epithelial-mesenchymal transition in
breast cancer. J Mammary Gland Biol Neoplasia. 15:201–212. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh-Gupta V, Zhang H, Banerjee S, et al:
Radiation-induced HIF-1alpha cell survival pathway is inhibited by
soy isoflavones in prostate cancer cells. Int J Cancer.
124:1675–1684. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdulghani J, Gu L, Dagvadorj A, et al:
Stat3 promotes metastatic progression of prostate cancer. Am J
Pathol. 172:1717–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lynch DH, Gurish MF and Daynes RA: The
effects of ultraviolet irradiation on the generation of anti-tumor
cytotoxic effector cell responses in vitro. J Immunol.
127:1163–1168. 1981.PubMed/NCBI
|
|
36
|
Frey B, Rubner Y, Wunderlich R, et al:
Induction of abscopal anti-tumor immunity and immunogenic tumor
cell death by ionizing irradiation - implications for cancer
therapies. Curr Med Chem. 19:1751–1764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jürgenliemk-Schulz IM, Renes IB, Rutgers
DH, et al: Anti-tumor effects of local irradiation in combination
with peritumoral administration of low doses of recombinant
interleukin-2 (rIL-2). Radiat Oncol Investig. 5:54–61.
1997.PubMed/NCBI
|