Introduction
According to the Heart Disease and Stroke Statistics
2010 Update, coronary heart disease caused ~1 of every 6
mortalities in the United States in 2006 (1). Coronary heart disease mortality in
2006 was 425,425. In 2010, 785,000 Americans were estimated to have
a new coronary attack, and ~470,000 experience a recurrent attack
(1). In China, mortalities from
coronary heart disease were 57.1/100,000 among urban residents and
33.74/100,000 among rural residents. A total of 500,000 or more
individuals are affected by myocardial infarction (MI) each year
and the prevalence rate of MI patients is estimated to be
>2,000,000 in China (2).
Coronary heart disease has become a growing worldwide problem.
Acute coronary syndrome (ACS) is a cluster of coronary
atherosclerotic heart diseases, including unstable angina (UA),
non-ST-segment elevation MI (NSTEMI) and ST-segment elevation MI
(STEMI) (3). The most common
pathophysiological basis of ACS is disrupted atherosclerotic
plaques (4), caused by arterial
inflammation, endothelial injury, microembolization of platelet
aggregates and coronary spasm (5–7).
Ras homolog gene family, member A (RhoA) is one of
the best-known members of the Rho protein family that, in addition
to its effect on actin organization or through this effect,
regulates a wide range of fundamental cell functions, including
contraction, motility, proliferation and apoptosis (8). Stimulation of tyrosine kinase and G
protein-coupled receptors recruits and activates Rho guanine
nucleotide exchange factors (GEFs), leading to activation of RhoA,
the direct upstream activator of Rho-associated kinases (ROCKs)
(9).
Increasing evidence suggests that ROCK, a target of
small glutamyltranspeptidase (GTPase) Rho, mediates various
cellular physiological functions, including cell proliferation,
migration, adhesion, apoptosis and contraction (10–12).
Rho-kinase activity is increased in patients with atherosclerosis
(13), hypertension (14), diabetes (15), metabolic syndrome (MetS) (16), stroke (17) and hyperlipemia (18), and in cigarette smokers (19). Atherosclerosis is the underlying
disorder in the majority of patients with cardiovascular disease.
Atherosclerosis is a complex process involving inflammatory cells,
endothelial dysfunction, smooth muscle cell proliferation,
extracellular matrix alteration and thrombosis. ROCKs have been
shown to be upregulated in inflammatory arteriosclerotic lesions
and have the ability to cause coronary vasospastic responses
through the inhibition of myosin light-chain phosphatase (MLCP) in
a porcine model of coronary artery spasm (20) and arteriosclerotic human arteries
(21). Previously, the ROCK
pathway has been shown to be involved in atherosclerotic lesion
formation (22) and coronary
artery disease (23). Furthermore,
ROCK inhibitors, including fasudil and Y-27632, inhibited
atherosclerosis and attenuated vasospastic angina (22,24),
which suggests that ROCK may be important in the pathogenesis of
coronary artery disease.
Thus, we hypothesized that ROCK is increased in ACS.
In the present study, we measured peripheral leukocyte ROCK
activity in a Chinese population with ACS and determined whether
ROCK activity is an independent marker of ACS and whether it
correlates with other risk factors of ACS.
Materials and methods
Patients
Twenty-one patients with ACS (ACS group: 12 males, 9
females, mean age 58±8 years) and 20 age-matched control subjects
(control group: 10 males, 10 females, mean age 55±6 years) were
enrolled in the present study. All patients were from the
Department of Cardiology, Tongji Hospital, Tongji Medical College,
Huazhong University of Science and Technology (Wuhan, Hubei,
China). Patients with ACS were diagnosed under the American College
of Cardiology/American Heart Association (ACC/AHA) 2007 guidelines
(3). This included patients who
had typical acute chest pain syndrome ≥20 min and electrocardiogram
(ECG) showing persistent ST-segment depression ≥1 mm, or patients
who had typical acute chest pain syndrome ≥20 min and ECG showing
persistent ST-segment elevation ≥1 mm in 2 contiguous leads (≥2 mm
in V1–V3 leads), or patients with acute chest pain without
ST-segment elevation; however, with elevated troponin levels.
Patients with stable angina or history of prior MI
were excluded from the study. Other exclusion criteria included
patients who had severe heart failure or any significant
arrhythmias within 3 months of the study (25), patients with severe anemia or
dysfunction of the kidney, liver or brain, those with a history of
diabetes mellitus and patients taking statins prior to enrollment.
Control subjects were those without any risk factors for coronary
heart disease, symptoms and signs of heart disease or coronary
angiography suggestive of atherosclerosis. The study was approved
by the Human Research Committee at Tongji Hospital and written
informed consent was obtained from all subjects.
Analytical methods
Blood samples (20 ml) were collected from the
cubital vein of all subjects in sterile tubes containing
ethylenediaminetetraacetic acid (EDTA) and stored at 4°C for <1
h. Fasting serum lipids [total cholesterol (TC), low-density
lipoprotein (LDL-C), high-density lipoprotein (HDL) and
triglycerides] and glucose were measured in the clinical laboratory
of Tongji Hospital. Mean arterial pressure (MAP) was approximated
by dividing the pulse pressure by three and adding the value to the
diastolic pressure. Blood pressure measurements were made with the
patient sitting or recumbent, and were conducted using Korotkoff’s
method.
Leukocyte isolation
To isolate human leukocytes, whole blood samples
were centrifuged at 2190 × g for 10 min at room temperature, and
the supernatant was aspirated and discarded. The leukocyte pellet
and 5-fold volume of the red cell lysis buffer (Red Blood Cell
Lysing Buffer-R7757; Sigma, St. Louis, MO, USA) were added into a
15 ml centrifuge tube. The tube was then centrifuged at 716 × g for
10 min, and the supernatant was discarded. The leukocyte pellet was
resuspended in 10 ml Hanks’ balanced salt solution (HBSS) by
pipetting the solution up and down, and the suspension was
centrifuged at 716 × g for 10 min. The supernatant was discarded
and the pellet was resuspended in 4 ml of M199 (Sigma). The trypan
blue (Sigma) exclusion test was used to determine cell yield and
viability, and the suspension was diluted with HBSS to achieve a
concentration of 5×106 cells/ml. After mixing the
diluted cells with a transfer pipette to ensure a uniform
suspension, 400 μl leukocyte suspension was transferred to sterile
1.5 ml tubes along with 400 μl fixative solution [50%
trichloroacetic acid (Sigma), 50 mmol/l
dichlorodiphenyltrichloroethane (Sigma) and protease inhibitors].
The suspension was vortexed and then centrifuged at 4°C for 5 min
at 14,000 × g. The supernatant was removed carefully and completely
with a micropipette. The precipitate was stored at −80°C for
western blot analysis.
Measurement of ROCK activity by
immunoblotting
Leukocyte pellets were diluted with 10 μl of 1 mol/l
Tris base and 100 μl of extraction buffer (8 mol/l urea, 2% sodium
dodecyl sulfate, 5% sucrose and 5% 2-mercaptoethanol). Equal
amounts of protein extracts were used for separation by 8% SDS-PAGE
and transferred onto a PVDF membrane. NIH3T3 cell lysates were used
as a positive control. The experiments were repeated >3 times in
order to standardize the results of western blot analysis. The
proteins were detected with antibodies against MBS (Covance,
Emeryville, CA, USA) and phospho-Thr696-MBS polyclonal antibody
(Millipore, Temecula, CA, USA). Immunoblotting was performed
according to the procedure described previously (26). Rho kinase activity was presented as
the ratio between the phospho-myosin-binding subunit (P-MBS) and
MBS normalized to the control.
Statistical analysis
SPSS 13.0 software was used to perform statistical
analysis on the data. All quantitative data from the two groups are
expressed as the means ± standard deviation (SD). The frequencies
between ACS and controls were compared using Chi-square analysis.
Due to heterogeneity of variance, age, heart rate, fasting glucose
and triglycerides were analyzed using nonparametric methods. The
student’s unpaired t-test or Wilcoxon Rank Sum test were used to
determine the significant differences between the two groups.
Correlation of ROCK activity to the lipid levels and MAP was
assessed by analysis of Pearson’s correlation coefficient. All
reported P-values were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics
The subjects in the control and ACS groups were age
and gender matched. The risk factors for coronary artery disease
are shown for the control and ACS groups (Table I). The two groups were comparable
in heart rate, history of smoking, fasting glucose and triglyceride
levels (P>0.05). Compared with the control group, the average
body mass index, MAP, TC and LDL-C were significantly higher, and
the average HDL was significantly lower, in the ACS group
(P<0.05; Table I). These
results were consistent with a previous study (16).
 | Table IClinical characteristics of controls
and ACS patients. |
Table I
Clinical characteristics of controls
and ACS patients.
| Control (n=20) | ACS (n=21) | P-value |
|---|
| Age (years) | 55.0±6.0.. | 58.0±8.0.. | 0.146 |
| Male (no.) | 10 (50.0%). | 12 (57.1%).. | 0.647 |
| BMI
(kg/m2) | 23.0±2.9.. | 25.2±2.3.. | 0.012a |
| HR (bpm) | 78.0±6.0.. | 80.0±7.0.. | 0.089 |
| MAP (mmHg) | 91.6±9.0.. | 100.0±14.0.. | 0.028a |
| Smokers (no.) | 7 (35.0%) | 9 (42.9%) | 0.606 |
| Glucose (mmol/l) | 5.7±1.6 | 6.1±3.4 | 0.240 |
| Total cholesterol
(mmol/l) | 4.6±0.5 | 5.3±0.5 | 0.001a |
| LDL-C (mmol/l) | 2.6±0.5 | 3.2±0.5 | 0.002a |
| HDL (mmol/l) | 1.4±0.3 | 1.2±0.3 | 0.043a |
| Triglycerides
(mmol/l) | 1.6±0.4 | 2.0±0.8 | 0.130 |
ROCK activity
Compared with the control subjects, ROCK activity,
as measured by P-MBS/MBS, was significantly increased in ACS
patients. The mean leukocyte ROCK activity levels were 0.45±0.04 in
control subjects. In patients with ACS, the mean activity levels
were 0.69±0.07 (P<0.001, Fig.
1). However, the ROCK activity levels did not significantly
differ between the acute MI (AMI) patients and the UA patients
(P=0.2).
Correlation between ROCK activity and
parameters
To determine whether ROCK activity is a novel risk
marker of ACS and whether it correlates with other risk factors of
ACS, correlation analysis was performed. Consequently, there was no
correlation between the lipid levels (TC and LDL-C) and ROCK
activity (r=0.17, P>0.05; r=0.08, P>0.05; respectively).
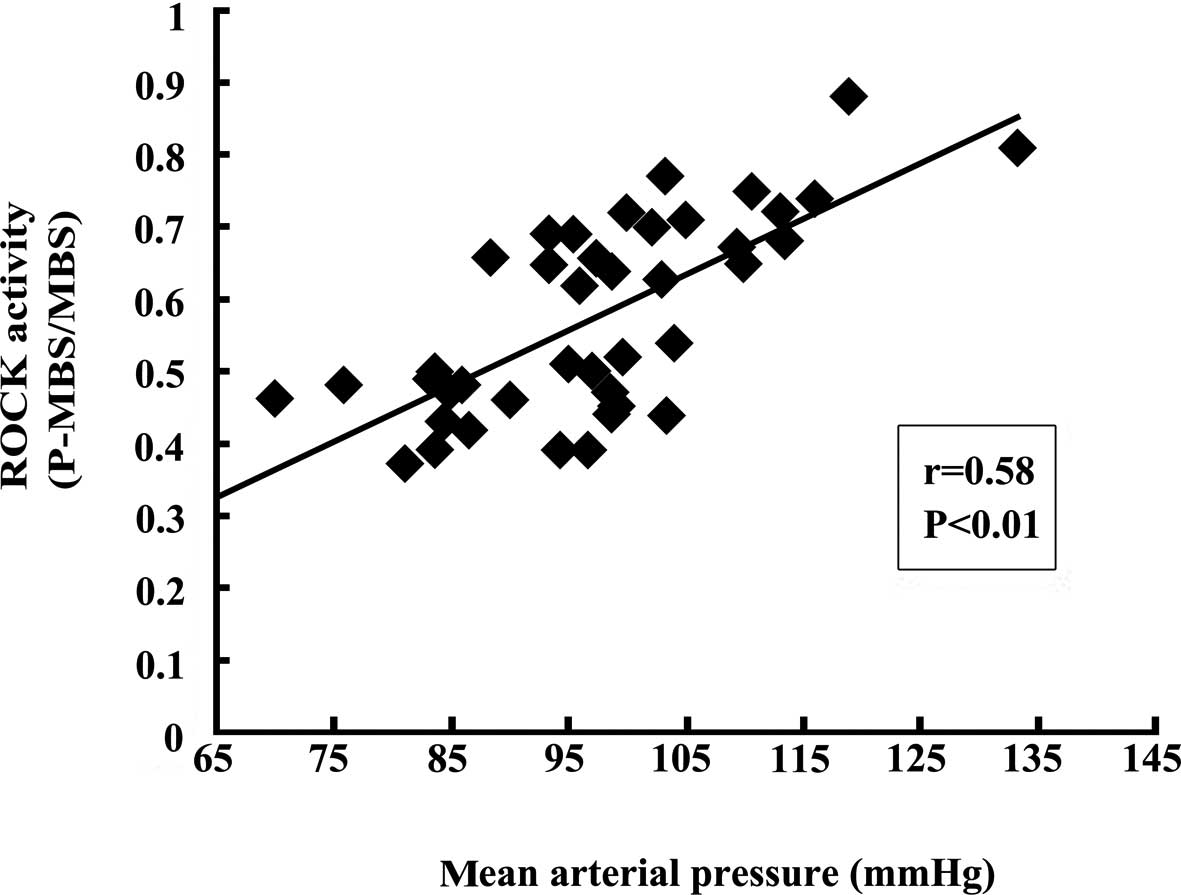
However, MAP was significantly correlated with ROCK activity
(r=0.58, P<0.01; Fig. 2).
Multivariate analysis was performed to explain variability (blood
pressure and smoking status being associated with ROCK activity).
Thus, ACS status was an independent predictor of ROCK activity.
Discussion
The results of the present study demonstrate that
peripheral leukocyte ROCK activity increased in patients with ACS.
However, there were no significant differences between UA and AMI
patients. In general, leukocyte ROCK levels represent the ROCK
activity from systemic circulation and the ROCK levels in the blood
vessels and myocardium represent tissue ROCK activity. The
relevance of each level is currently unclear (27,28).
Furthermore, the results revealed that higher ROCK activity was not
correlated with lipid levels, whereas it was significantly
positively correlated with MAP. These findings suggest that
increased ROCK activity may be a novel marker of ACS, the
activation of ROCK may contribute to the pathogenesis of ACS and
therapies that inhibit ROCK may be clinically useful in the
treatment of ACS.
It has been suggested that ROCK activity contributes
to the development of early atherosclerosis, possibly through its
modulation of NF-κB and activation of T lymphocyte proliferation
(22). Furthermore, accumulating
evidence indicates that coronary dysfunction and coronary
arteriosclerosis are attenuated by inhibition of ROCKs (23,24,29,30).
In the present study, ROCK activity was increased in subjects with
ACS and there were no significant differences between AMI and UA
patients, despite the possible higher ROCK activity in AMI
patients.
Although the precise mechanism of increased ROCK
activity in ACS patients is unclear, several possible mechanisms
may explain the observed findings. Firstly, inflammation has a
critical role in the occurrence and development of ACS. Recruitment
of mononuclear leukocytes to the intima is one of the earliest
events in the formation of an inflammatory infiltrate and the
active inflammation within plaques leads to plaque disruption
(4,31). ROCK-mediated leukocyte recruitment
in the vessel wall and enhanced inflammatory activity of the vessel
wall contribute to the development of ACS (32). Furthermore, endothelial injury and
dysfunction play a critical role in patients with atherosclerosis
and acute coronary events. Notably, coronary artery spasm is one of
the most significant features of ACS. Overactivity of ROCK in
humans with atherosclerosis leads to reduced nitric oxide (NO)
bioavailability and upregulated myosin light-chain (MLC)
phosphorylation, which in turn leads to cellular contraction by
inhibition of MLC phosphatase through phosphorylation of its
regulatory MBS (20,23). Hence, Rho-kinase may contribute to
the development of ACS. In addition, higher Rho-kinase activity in
AMI patients may be due to increased damage to myocardial
cells.
Blood pressure, fasting glucose, LDL-C, TG, BMI,
hs-CRP and waist circumference were greater among the MetS subjects
and lower HDL levels were observed in MetS patients. In the present
study fasting glucose, TG, BMI, hs-CRP and waist circumference were
positively associated with increased ROCK activity and HDL levels
were negatively associated with ROCK activity (16). However, LDL-C and TC were not
correlated with ROCK activity in ACS subjects. An earlier study
demonstrated that the Rho-kinase inhibitor fasudil increased
flow-mediated vasodilatation without altering lipid levels in
patients with elevated baseline TC and LDL-C (23). This suggests that increased ROCK
activity may be independent of lipid levels in ACS subjects.
Notably, in our study, ROCK activity significantly
correlated with MAP in ACS subjects. Our results are supported by
several studies demonstrating that ROCKs are involved in the
pathogenesis of increased peripheral vascular resistance in
hypertension (14,33). In cigarette smokers with normal
blood pressure, a significant correlation was noted between the
activity of ROCK and systolic blood pressure (19). Therefore, the current results
indicate that activation of ROCK leads to VSMC contraction and
contributes to coronary spasm in ACS patients.
Fasudil, a potent and selective inhibitor of
Rho-kinase, is clinically used for the treatment of cerebral
vasospasms following subarachnoid hemorrhage (34). It has been demonstrated that
hydroxyfasudil, a major active metabolite of fasudil following oral
administration, has a more selective inhibitory effect on ROCK
compared with its parent drug (35). Fasudil inhibits Rho-kinase by
competing with ATP for binding to the catalytic site of the kinase
and therefore is equipotent in terms of inhibiting ROCK1 and ROCK2.
Therefore, fasudil is currently being developed for the treatment
of acute stroke and cardiovascular disorders. Inhibition of ROCK in
atherosclerosis patients has been investigated in several previous
studies using fasudil. A multicenter study demonstrated that
fasudil significantly improved stable angina (36). In humans, leukocyte ROCK activity
was increased in patients with acute ischemic stroke and reached
maximal activity ~48 h after stroke onset. Fasudil additionally
offers a safe option for the treatment of cerebral infarction in
patients with acute thrombosis as well as cerebral vasospasm
(17,37). These studies indicate that
atherosclerosis and vascular injury may contribute to ROCK
activity. The present findings suggest that fasudil inhibition of
ROCK activity may have therapeutic benefits in patients with
ACS.
This study has several limitations. Firstly, we
measured the activity of ROCK by only measuring the ratio between
P-MBS and MBS. Thus, further studies are required with antibodies
from distal targets of ROCK in order to obtain precise results and
to develop methods to distinguish between ROCK1 and ROCK2
activities. Secondly, all subjects in this study were Chinese.
Therefore, the results may not be applicable to other ethnicities.
As we were unable to determine the time of occurrence of unstable
angina, we could not use the inhibitor of ROCK in these patients.
Accordingly, future investigations should aim to use fasudil in
patients with ACS and study the outcome.
In conclusion, we were able to demonstrate that
peripheral leukocyte ROCK activity was increased in patients with
ACS. This result suggests that inhibition of Rho-kinase may be
regarded as a novel therapeutic method for the treatment of acute
coronary events in humans. However, the precise molecular mechanism
of increased ROCK in coronary atherosclerosis and its effects on
subsequent acute coronary events remain to be elucidated.
Acknowledgements
The authors would like to thank Dr James K. Liao
(Vascular Medicine Research Unit, Brigham and Women’s Hospital and
Harvard Medical School, Boston, MA, USA) for his assistance with
the technology of measuring ROCK activity.
References
|
1
|
Lloyd-Jones D, Adams RJ, Brown TM, et al:
Heart disease and stroke statistics - 2010 update: a report from
the American Heart Association. Circulation. 121:e46–e215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Center for Cardiovascular
Diseases. Report on Cardiovascular Diseases in China 2007.
Encyclopedia of China Publishing House; Beijing: pp. 1002007
|
|
3
|
Anderson JL, Adams CD, Antman EM, et al:
ACC/AHA 2007 guidelines for the management of patients with
unstable angina/non-ST-Elevation myocardial infarction: a report of
the American College of Cardiology/American Heart Association Task
Force on Practice Guidelines (Writing Committee to Revise the 2002
Guidelines for the Management of Patients With Unstable
Angina/Non-ST-Elevation Myocardial Infarction) developed in
collaboration with the American College of Emergency Physicians,
the Society for Cardiovascular Angiography and Interventions, and
the Society of Thoracic Surgeons endorsed by the American
Association of Cardiovascular and Pulmonary Rehabilitation and the
Society for Academic Emergency Medicine. J Am Coll Cardiol.
50:e1–e157. 2007.
|
|
4
|
Davies MJ: The pathophysiology of acute
coronary syndromes. Heart. 83:361–366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fichtlscherer S, Breuer S and Zeiher AM:
Prognostic value of systemic endothelial dysfunction in patients
with acute coronary syndromes: further evidence for the existence
of the ‘vulnerable’ patient. Circulation. 110:1926–1932.
2004.PubMed/NCBI
|
|
6
|
Libby P: Inflammation in atherosclerosis.
Nature. 420:868–874. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mizuno K, Satomura K, Miyamoto A, Arakawa
K, Shibuya T, Arai T, Kurita A, Nakamura H and Ambrose JA:
Angioscopic evaluation of coronary-artery thrombi in acute coronary
syndromes. N Engl J Med. 326:287–291. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
9
|
Hart MJ, Jiang X, Kozasa T, Roscoe W,
Singer WD, Gilman AG, Sternweis PC and Bollag G: Direct stimulation
of the guanine nucleotide exchange activity of p115 RhoGEF by
Galpha13. Science. 280:2112–2114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amano M, Chihara K, Kimura K, Fukata Y,
Nakamura N, Matsuura Y and Kaibuchi K: Formation of actin stress
fibers and focal adhesions enhanced by Rho-kinase. Science.
275:1308–1311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar
|
|
12
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyata K, Shimokawa H, Kandabashi T, Higo
T, Morishige K, Eto Y, Egashira K, Kaibuchi K and Takeshita A:
Rho-kinase is involved in macrophage-mediated formation of coronary
vascular lesions in pigs in vivo. Arterioscler Thromb Vasc Biol.
20:2351–2358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masumoto A, Hirooka Y, Shimokawa H,
Hironaga K, Setoguchi S and Takeshita A: Possible involvement of
Rho-kinase in the pathogenesis of hypertension in humans.
Hypertension. 38:1307–1310. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sandu OA, Ragolia L and Begum N: Diabetes
in the Goto-Kakizaki rat is accompanied by impaired
insulin-mediated myosin-bound phosphatase activation and vascular
smooth muscle cell relaxation. Diabetes. 49:2178–2189. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu PY, Chen JH, Lin LJ and Liao JK:
Increased Rho kinase activity in a Taiwanese population with
metabolic syndrome. J Am Coll Cardiol. 49:1619–1624. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feske SK, Sorond FA, Henderson GV, Seto M,
Hitomi A, Kawasaki K, Sasaki Y, Asano T and Liao JK: Increased
leukocyte ROCK activity in patients after acute ischemic stroke.
Brain Res. 1257:89–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rikitake Y and Liao JK: Rho-kinase
mediates hyperglycemia-induced plasminogen activator inhibitor-1
expression in vascular endothelial cells. Circulation.
111:3261–3268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hidaka T, Hata T, Soga J, Fujii Y, Idei N,
Fujimura N, Kihara Y, Noma K, Liao JK and Higashi Y: Increased
leukocyte rho kinase (ROCK) activity and endothelial dysfunction in
cigarette smokers. Hypertens Res. 33:354–359. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kandabashi T, Shimokawa H, Miyata K,
Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S,
Kaibuchi K and Takeshita A: Inhibition of myosin phosphatase by
upregulated rho-kinase plays a key role for coronary artery spasm
in a porcine model with interleukin-1beta. Circulation.
101:1319–1323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kandabashi T, Shimokawa H, Mukai Y, Matoba
T, Kunihiro I, Morikawa K, Ito M, Takahashi S, Kaibuchi K and
Takeshita A: Involvement of rho-kinase in agonists-induced
contractions of arteriosclerotic human arteries. Arterioscler
Thromb Vasc Biol. 22:243–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mallat Z, Gojova A, Sauzeau V, Brun V,
Silvestre JS, Esposito B, Merval R, Groux H, Loirand G and Tedgui
A: Rho-associated protein kinase contributes to early
atherosclerotic lesion formation in mice. Circ Res. 93:884–888.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nohria A, Grunert ME, Rikitake Y, Noma K,
Prsic A, Ganz P, Liao JK and Creager MA: Rho kinase inhibition
improves endothelial function in human subjects with coronary
artery disease. Circ Res. 99:1426–1432. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masumoto A, Mohri M, Shimokawa H, Urakami
L, Usui M and Takeshita A: Suppression of coronary artery spasm by
the Rho-kinase inhibitor fasudil in patients with vasospastic
angina. Circulation. 105:1545–1547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kishi T, Hirooka Y, Masumoto A, Ito K,
Kimura Y, Inokuchi K, Tagawa T, Shimokawa H, Takeshita A and
Sunagawa K: Rho-kinase inhibitor improves increased vascular
resistance and impaired vasodilation of the forearm in patients
with heart failure. Circulation. 111:2741–2747. 2005. View Article : Google Scholar
|
|
26
|
Xiao B, Li X, Yan J, Yu X, Yang G, Xiao X,
Voltz JW, Zeldin DC and Wang DW: Overexpression of cytochrome P450
epoxygenases prevents development of hypertension in spontaneously
hypertensive rats by enhancing atrial natriuretic peptide. J
Pharmacol Exp Ther. 334:784–794. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Q, Gensch C and Liao JK:
Rho-associated coiled-coil-forming kinases (ROCKs): potential
targets for the treatment of atherosclerosis and vascular disease.
Trends Pharmacol Sci. 32:167–173. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dong M, Yan BP and Yu CM: Current status
of rho-associated kinases (ROCKs) in coronary atherosclerosis and
vasospasm. Cardiovasc Hematol Agents Med Chem. 7:322–330. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Morishige K, Shimokawa H, Eto Y,
Kandabashi T, Miyata K, Matsumoto Y, Hoshijima M, Kaibuchi K and
Takeshita A: Adenovirus-mediated transfer of dominant-negative
rho-kinase induces a regression of coronary arteriosclerosis in
pigs in vivo. Arterioscler Thromb Vasc Biol. 21:548–554. 2001.
View Article : Google Scholar
|
|
30
|
Wolfrum S, Dendorfer A, Rikitake Y,
Stalker TJ, Gong Y, Scalia R, Dominiak P and Liao JK: Inhibition of
Rho-kinase leads to rapid activation of phosphatidylinositol
3-kinase/protein kinase Akt and cardiovascular protection.
Arterioscler Thromb Vasc Biol. 24:1842–1847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee KW and Lip GY: Acute coronary
syndromes: Virchow’s triad revisited. Blood Coagul Fibrinolysis.
14:605–625. 2003.
|
|
32
|
Noma K, Rikitake Y, Oyama N, et al: ROCK1
mediates leukocyte recruitment and neointima formation following
vascular injury. J Clin Invest. 118:1632–1644. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loirand G and Pacaud P: The role of Rho
protein signaling in hypertension. Nat Rev Cardiol. 7:637–647.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Budzyn K, Marley PD and Sobey CG:
Targeting Rho and Rho-kinase in the treatment of cardiovascular
disease. Trends Pharmacol Sci. 27:97–104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimokawa H and Rashid M: Development of
Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol
Sci. 28:296–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimokawa H, Hiramori K, Iinuma H, Hosoda
S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T,
Nakashima M and Kato K: Anti-anginal effect of fasudil, a
Rho-kinase inhibitor, in patients with stable effort angina: a
multicenter study. J Cardiovasc Pharmacol. 40:751–761. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shibuya M, Hirai S, Seto M, Satoh S and
Ohtomo E; Fasudil Ischemic Stroke Study Group. Effects of fasudil
in acute ischemic stroke: results of a prospective
placebo-controlled double-blind trial. J Neurol Sci. 238:31–39.
2005. View Article : Google Scholar : PubMed/NCBI
|