Introduction
Attention-deficit hyperactivity disorder (ADHD) is a
common neurobehavioral disorder that typically arises during
childhood. Globally, 5–10% of school-aged children have ADHD and
its prevalence is 3 times higher in males than females (1). ADHD is characterized by 3 major
behavioral symptoms, inattention, hyperactivity and
impulsivity. The exact causes of ADHD are unknown, although
genetic, social, physical and environmental factors have been
suggested to contribute to the etiology of the disease. ADHD is
highly heritable and ~75% of cases are genetically associated
(2). Hyperactivity is hypothesized
to be caused by a genetic disorder, and the genetic alteration of
dopamine transporters has since been suggested as a major cause of
ADHD (3).
Dopamine is a neurotransmitter that regulates motor
activity, attention and reward-seeking behaviors (4). Clinical and experimental studies have
demonstrated a correlation between the transmission of dopamine
with inattentive, hyperactive, aggressive and impulsive behaviors
(5,6). The axons of dopamine neurons located
in the substantia nigra are mainly situated in the dorsolateral
striatum, forming the nigrostriatal pathway (7). Dopaminergic activity in the
nigrostriatal pathway is affected by social interactions and social
status (8). Disrupted dopamine
signaling is implicated in the pathogenesis of numerous
neurological disorders, including ADHD and Parkinson’s disease
(9,10). Tyrosine hydroxylase (TH) catalyzes
the rate-limiting step in the synthesis of catecholamine
neurotransmitters, including dopamine, epinephrine and
norepinephrine. TH converts L-tyrosine to
L-3,4-dihydroxyphenylalanine (L-DOPA). TH activity is progressively
decreased in correlation with the loss of dopamine neurons in the
substantia nigra and striatum (10,11).
TH immunohistochemistry is widely used to detect the injury or
death of dopaminergic fibers and cell bodies (12,13).
Dopaminergic signaling also affects physical activity (14). Hyperactivity is an important
symptom of ADHD and anorexia nervosa, both of which are associated
with altered dopaminergic signaling (2,15,16).
Dopamine receptors form the main determinants of the
dopamine pathway. Dopamine receptors are divided into 2 classes,
D1-like (D1 and D5) and
D2-like (D2, D3 and D4)
dopamine receptors. In particular, the dopamine D2
receptor is closely associated with neuropsychiatric disorders,
including ADHD (3), schizophrenia
(17) and depression (18). Dopamine D2 receptors
belong to a family of G protein-coupled receptors and its
activation inhibits synaptogenesis (19). Several studies have examined the
positive effects of dopamine D2 receptor activation on
physical activity (20–22).
First-line treatment for ADHD consists of the
α2-adrenoreceptor agonists, clonidine and guanfacin
(23). Methylphenidate, the
historical gold standard treatment for ADHD, inhibits dopamine and
norepinephrine transporters. By contrast, atomoxetine is highly
specific for norepinephrine reuptake inhibitors, with a low
affinity for other monoamine transporters or neurotransmitter
receptors (24). Atomoxetine is a
well-tolerated non-stimulant medication that functions differently
from other medications approved for the treatment of ADHD (24). However, these medications induce
side-effects, including loss of appetite, sleep problems and mood
disturbances (25). Thus, there is
a need to develop alternative therapeutic drugs that possess fewer
side-effects.
Physical exercise improves neurological impairments
by increasing neurogenesis and the release of neurotransmitters,
facilitating neuronal maturation and enhancing learning ability and
memory capability in neuropsychiatric disorders (26–29).
In pediatric ADHD patients, physical movement improved working
speed and social behavioral problems, and reduced hyperactivity
(30). The effect of treadmill
exercise in ameliorating the symptoms of ADHD in spontaneous
hypertensive rats (SHRs) has been reported (31). Swimming exercise has been
demonstrated to be beneficial for the treatment of ADHD, for
example, in the case of the Olympic athlete Michael Phelps
(32). The possibility that
swimming exercise is effective for relieving the symptoms of ADHD
has been examined; however, the effects of swimming exercise on
ADHD in correlation with the levels of dopamine and dopamine
receptor expression have yet to be assessed.
In the present study, we investigated the effects of
swimming exercise on several behavioral characteristics, including
activity, impulsivity, non-aggressive and aggressive behaviors and
short-term memory in ADHD rats. The open-field test, social
interaction test, elevated plus maze test and step-through
avoidance test were conducted. The effects of swimming exercise on
the expression levels of TH and the dopamine D2 receptor
in the prefrontal cortex, substantia nigra and striatum were
evaluated. The expression of TH and the dopamine D2
receptor were detected by immunohistochemistry and western
blotting, respectively. The effectiveness of swimming exercise was
compared with atomoxetine treatment.
Materials and methods
Experimental animals and treatment
Adult male SHRs and Wister-Kyoto rats, weighing
180±5 g (6 weeks old), were obtained from a commercial breeder
(Orient Bio Co., Seoul, Korea). SHRs were used as animal models of
ADHD, since they exhibit the major symptoms of ADHD, including
inattention, hyperactivity and impulsiveness (33). In the present study, SHRs that
demonstrated hyperactivity in the open-field test were used as the
ADHD rats, while Wistar-Kyoto rats, which did not demonstrate
hyperactivity, were used as the control rats, according to
previously described methods (31,34).
The experimental procedures were performed in
accordance with the animal care guidelines of the National
Institutes of Health (NIH) and the Korean Academy of Medical
Sciences. The animals were housed under controlled temperature
(23±2°C) and lighting (08:00–20:00 h) conditions, with food and
water available ad libitum. The animals were randomly
divided into 4 groups (n=10 in each group): The control group
(Wistar-Kyoto rats), the ADHD group (SHRs), the ADHD and swimming
exercise group and the ADHD and atomoxetine treatment group. Rats
in the atomoxetine treatment group received 1 mg/kg atomoxetine
(Strattera®, Eli Lilly Co., Indianapolis, IN, USA)
orally once a day for 28 consecutive days.
Open-field test
To confirm hyperactivity in the ADHD rats, the
open-field test was conducted 1 day prior to the initiation of the
experiment using a previously described method (31). The animals were randomly assigned
to an order of testing and placed in a white square open-field
arena (100×100 cm) made of wood, enclosed by 40-cm-high walls and
exposed to strong illumination (200 lux). The arena was divided
into 25 squares (20×20 cm), consisting of 9 central and 16
peripheral squares. The animals were placed in the center of the
arena and were allowed to explore the environment for 1 min.
Following this time, the number of squares crossed was recorded for
5 min. The activity of each rat was repeatedly assessed using the
open-field test at 9, 18 and 26 days following the initiation of
the experiment.
Swimming exercise protocol
Rats in the swimming group were forced to swim for
30 min once a day, for a total of 28 days, according to a
previously described method (35).
The rats were placed individually in cylindrical tanks with a 30-cm
diameter and a height of 85 cm. The cylindrical tank was filled
with water at a temperature of 33±1°C and to a depth of 75 cm.
Social interaction test
The social interaction test was performed 27 days
following the initiation of the experiment, according to a
previously described method (36).
Two weight-matched rats selected from different cages were
examined. While the normal rat was used as the partner rat, the
test rat was marked with an oil pen and both rats were placed into
the center of a test box (60×60 cm open field with sixteen 15×15 cm
squares marked on the floor). The animals were closely observed and
the interactions exhibited by the index rat were recorded over a
period of 10 min. The social behaviors exhibited by the index rat
were measured visually. The number of non-aggressive behaviors
(genital investigation, sniffing, following and grooming) and
aggressive behaviors (kicking, boxing, biting, crawling under or
over the partner and touching the partner’s face) were recorded
during the test period.
Elevated plus maze test
The elevated plus maze test is a well-established
test used to determine the levels of impulsivity in rats. The
elevated plus maze test was performed 28 days following the
initiation of the experiment, according to a previously described
method (37). The plus maze
consisted of black plexiglas with two open arms (50×10×36 cm) and
two closed arms (50×10×36 cm), arranged so that the two arms were
opposite and connected by a central platform (10×10 cm). The whole
plus maze was elevated 60 cm above the floor and illuminated by a
100-watt light bulb fixed 2 m above the maze floor. During the
test, each rat was placed on the central platform of the maze with
their head facing an open arm. The total time spent in the open
arms was collected over a period of 7 min.
Step-through avoidance test
Short-term memory was evaluated using step-through
avoidance apparatus 29 days following the initiation of the
experiment (24 h following the last swimming exercise session),
according to a previously described method (26). The apparatus consisted of two
compartments, a dark compartment and a light compartment. A
guillotine door separated the two compartments. The dark
compartment had a stainless steel shock grid floor. During the
acquisition trial, each rat was placed in the light compartment.
Following a habituation period of 60 sec, the guillotine door was
opened. Immediately after the rat had entered the dark compartment,
the guillotine door was closed and an electric foot shock (75 V,
0.2 mA, 50 Hz) was delivered to the floor grids for 3 sec. The rat
was then removed from the dark compartment 5 sec later and returned
to the home cage. The latency of staying in the light compartment
was measured 2 h following the acquisition trial; however, a foot
shock was not delivered. The latency was recorded to a maximum of
300 sec.
Tissue preparation
The rats were sacrificed immediately following the
completion of the step-through avoidance test. The animals were
anesthetized using Zoletil 50® (10 mg/kg, i.p.; Virbac
Laboratories, Carros, France), transcardially perfused with 50 mM
phosphate-buffered saline (PBS) and fixed with a freshly prepared
solution consisting of 4% paraformaldehyde in 100 mM phosphate
buffer (PB; pH 7.4). The brains were dissected and post-fixed in
the same fixative overnight and transferred into a 30% sucrose
solution for cryoprotection. Coronal sections of 40-μm thickness
were cut using a freezing microtome (Leica, Nussloch, Germany). On
average, 10 slice sections in the prefrontal cortex, substantia
nigra and striatum were collected from each rat.
TH immunohistochemistry
For the immunolabeling of TH in the prefrontal
cortex, substantia nigra and striatum of each brain, TH
immunohistochemistry was performed as previously described
(13). Free-floating tissue
sections were incubated overnight with mouse anti-TH antibody
(1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and
the sections were then incubated for 1 h with biotinylated
anti-mouse secondary antibody (1:200; Vector Laboratories,
Burlingame, CA, USA). The sections were subsequently incubated with
avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at
room temperature. Immunoreactivity was visualized by incubating the
sections in a solution consisting of 0.05% 3,3′-diaminobenzidine
(DAB) and 0.01% H2O2 in 50 mM Tris-buffer (pH
7.6) for ~3 min. The sections were then washed 3 times with PBS and
mounted onto gelatin-coated slides. The slides were air-dried
overnight at room temperature and coverslips were mounted using
Permount® (Fisher Scientific, New Jersey, NJ, USA).
Western blotting for the detection of
dopamine D2 receptor expression
Western blotting was performed as previously
described (19,27). The prefrontal cortex, substantia
nigra and striatum tissues were collected and then immediately
frozen at −80°C. The tissues of each brain area were homogenized on
ice and lysed in a lysis buffer containing 50 mM Tris-HCl (pH 7.5),
150 mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% SDS, 1 mM
PMSF and 100 mg/ml leupeptin. The protein content was measured
using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules,
CA, USA). A total of 30 μg protein was separated on a
SDS-polyacrylamide gel and transferred onto a nitrocellulose
membrane. Mouse anti-actin antibody (1:500; Santa Cruz
Biotechnology, Inc.) and mouse anti-dopamine D2 receptor
antibody (1:1,000; Santa Cruz Biotechnology, Inc.) were used as the
primary antibodies. Horseradish peroxidase-conjugated anti-mouse
antibodies for actin and dopamine D2 receptor (1:2,000;
Vector Laboratories) were used as the secondary antibodies.
Experiments were performed in normal lab conditions and at room
temperature, with the exception of the membrane transfer. The
membrane transfer was performed at 4°C using a cold pack and
prechilled buffer. Band detection was performed using the enhanced
chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology,
Inc.).
Statistical analysis
The number of TH-positive cells in the substantia
nigra was counted using a light microscope (Olympus, Tokyo, Japan).
The optical densities of the TH-immunoreactive fibers in the
prefrontal cortex and striatum were measured in 100×100 μm square
images using an image analyzer (Multiscan, Fullerton, CA, USA). To
estimate the TH staining densities, the optical densities were
corrected for the non-specific background density, which was
measured in completely denervated parts of the prefrontal cortex
and striatum. The optical densities of the TH-positive fibers in
the prefrontal cortex and striatum were calculated as follows: The
optical density in the lesion side/the optical density in the
intact side. In order to assess the expression levels of the
dopamine D2 receptor in the prefrontal cortex,
substantia nigra and striatum, the detected bands were calculated
densitometrically using Image-Pro® Plus software (Media
Cybernetics Inc., Silver Spring, MD, USA). Statistical analysis was
performed using one-way ANOVA followed by the Duncan’s post-hoc
test. The results are expressed as the mean ± SEM. P<0.05 was
considered to indicate a statistically significant result.
Results
Effects of swimming exercise on activity,
impulsivity, short-term memory and non-aggressive and aggressive
behaviors
To determine the levels of activity, the open-field
test was performed 1 day prior to the initiation of the experiment
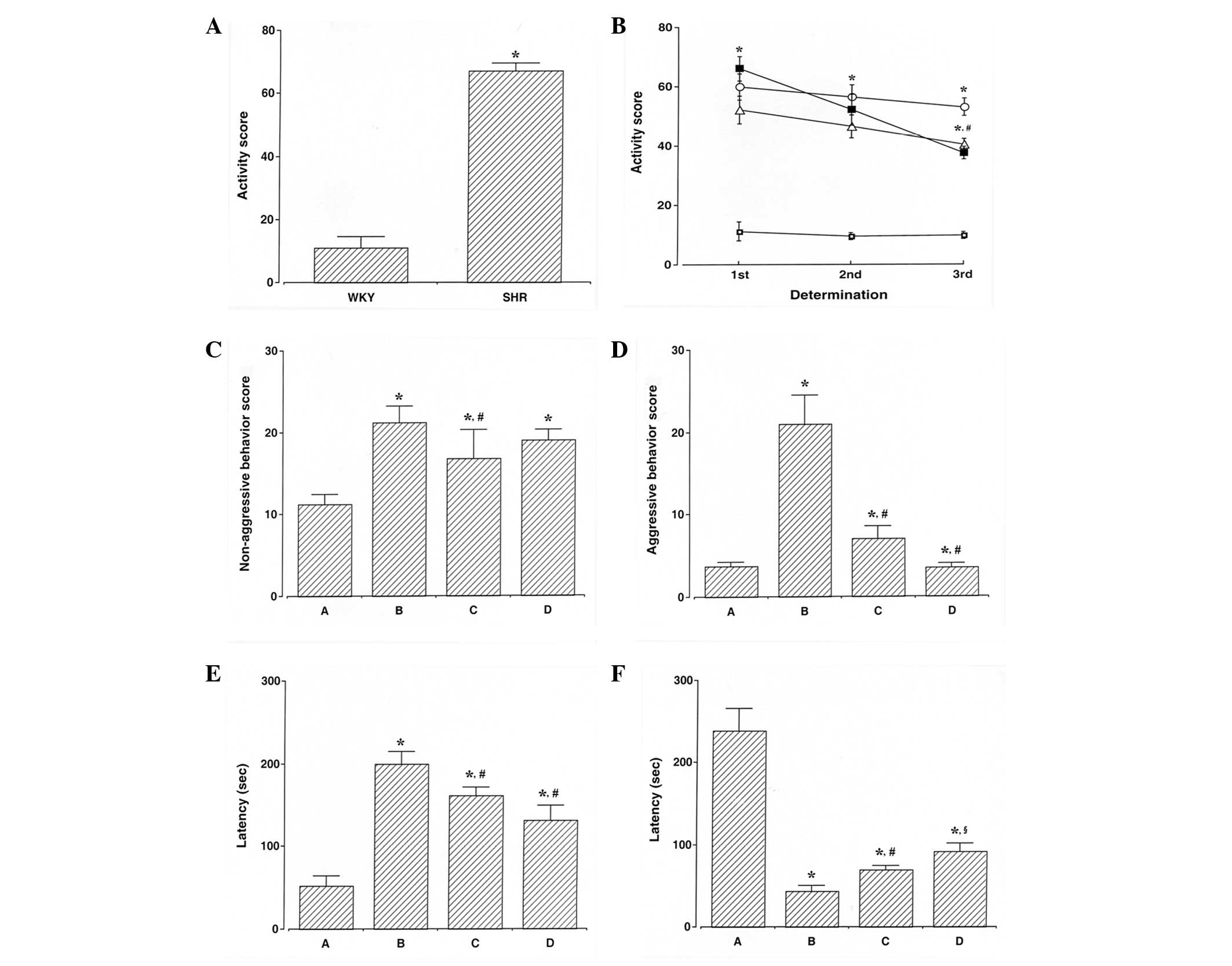
(Fig. 1A). ADHD rats were
hyperactive, demonstrating symptoms of ADHD. The activity score in
the open-field test was determined during the experimental period.
The activity score in the ADHD rats was higher compared with that
of the control rats (P<0.05), whereas swimming exercise and
atomoxetine treatment decreased the activity score observed in the
ADHD rats (P<0.05; Fig.
1B).
Non-aggressive and aggressive behaviors were
assessed using the social interaction test. The scores for
non-aggressive behaviors in the social interaction test are
presented in Fig. 1C. The score
for non-aggressive behaviors in the ADHD rats was higher compared
with that of the control rats (P<0.05), whereas swimming
exercise decreased the score for non-aggressive behaviors in the
ADHD rats (P<0.05). However, atomoxtine treatment exerted no
significant effect on the score for non-aggressive behaviors in the
ADHD rats. The scores for aggressive behaviors in the social
interaction test are presented in Fig.
1D. The score for aggressive behaviors in the ADHD rats was
higher compared with the control rats (P<0.05), whereas swimming
exercise and atomoxetine treatment decreased the score for
aggressive behaviors in the ADHD rats (P<0.05).
Impulsivity was assessed using the elevated plus
maze test. The latency of staying in the open arm in the elevated
plus maze test is presented in Fig.
1E. The ADHD rats demonstrated an enhanced impulsive activity
compared with the control rats (P<0.05), whereas swimming
exercise and atomoxetine treatment inhibited impulsivity in ADHD
rats (P<0.05).
Short-term memory was assessed using the
step-through avoidance test. The latency of staying in the light
compartment in the step-through avoidance test is presented in
Fig. 1F. ADHD rats demonstrated
short-term memory disturbance (P<0.05), whereas swimming
exercise and atomoxetine treatment alleviated short-term memory
disturbances in the ADHD rats (P<0.05).
Effects of swimming exercise on TH
expression levels in the prefrontal cortex, substantia nigra and
striatum
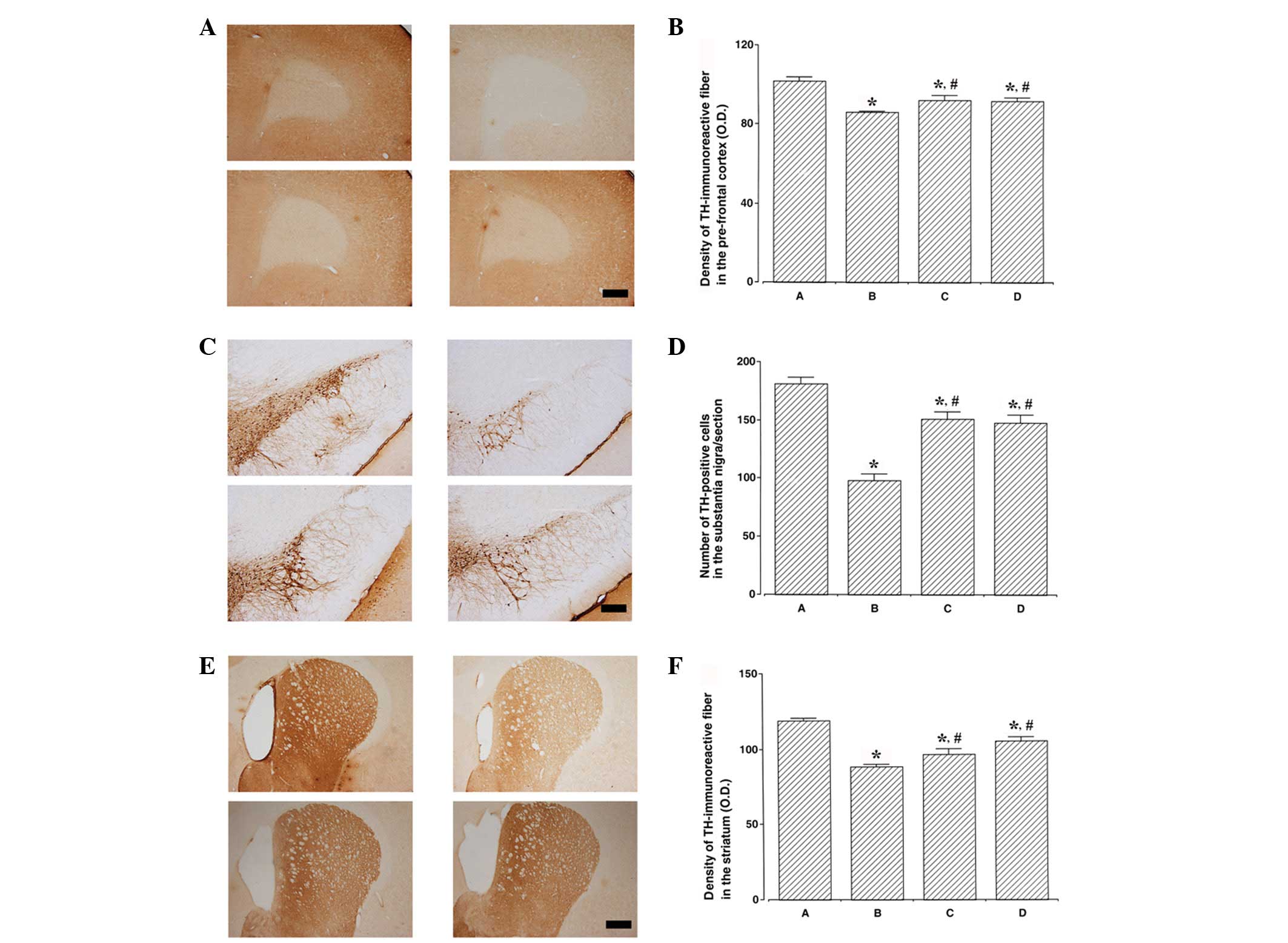
Photomicrographs of TH-immunoreactive fibers in the
prefrontal cortex, TH-positive cells in the substantia nigra and
TH-immunoreactive fibers in the striatum are presented in Fig. 2. TH expression levels were lower in
the ADHD rats compared with the control rats (P<0.05). By
contrast, swimming exercise and atomoxetine treatment increased TH
expression levels in the ADHD rats (P<0.05).
Effects of swimming exercise on dopamine
D2 receptor expression levels in the prefrontal cortex,
substantia nigra and striatum
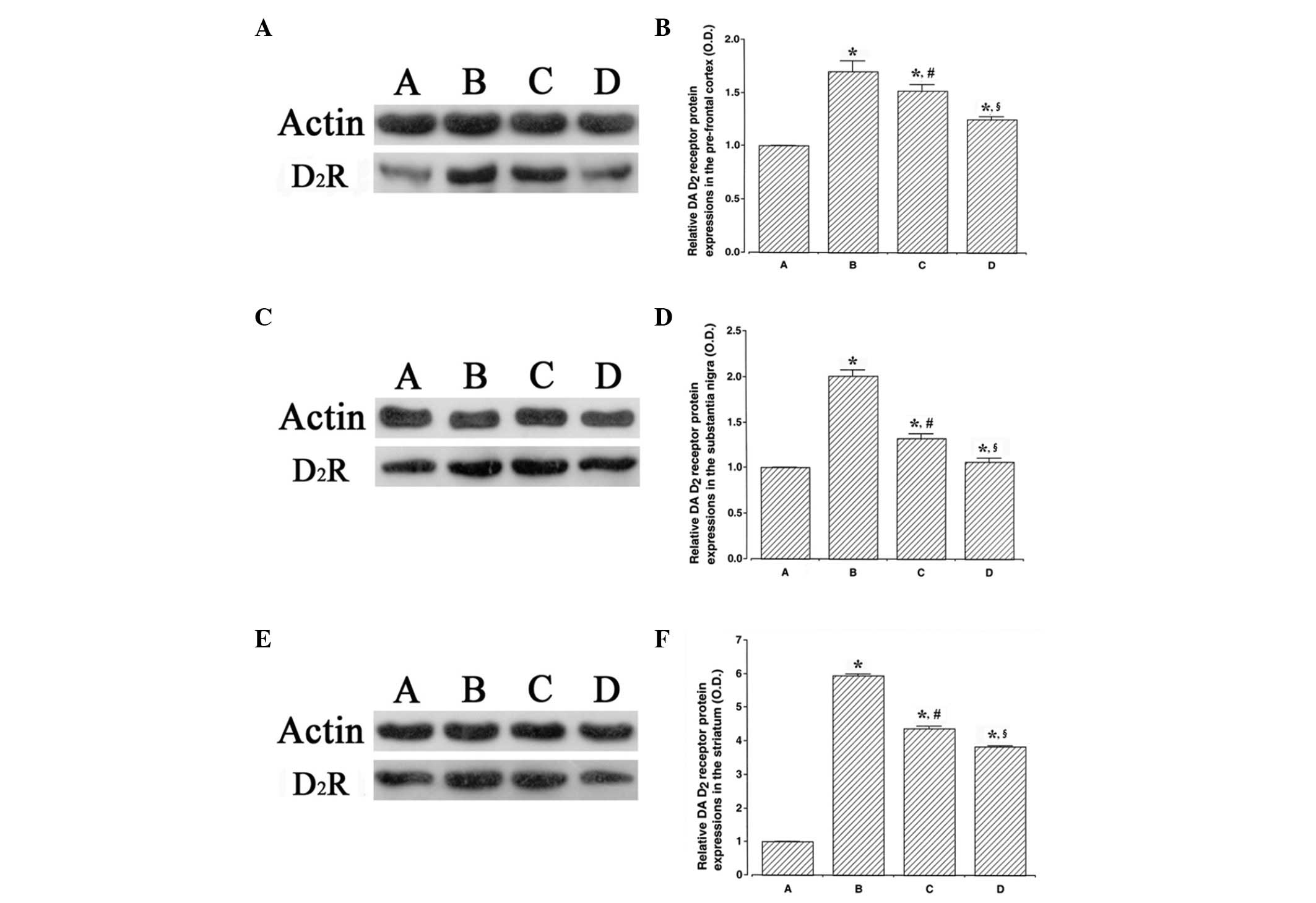
The results of the western blot analysis detecting
the expression of the dopamine D2 receptor in the
prefrontal cortex, substantia nigra and striatum are presented in
Fig. 3. The dopamine D2
receptor expression levels were higher in the ADHD rats compared
with the control rats (P<0.05). By contrast, swimming exercise
and atomoxetine treatment suppressed dopamine D2
receptor expression levels in the ADHD rats (P<0.05).
Discussion
SHRs were used as animal models of ADHD since they
exhibit hyperactivity, impulsivity, impaired ability to withhold
responses and poorly sustained attention compared with normotensive
Wistar-Kyoto control rats (31,34).
Physical exercise improved working speed and social behavioral
disorders, and also diminished hyperactivity in children with ADHD
(30). Kim et al(31) demonstrated that treadmill exercise
ameliorated hyperactivity and enhanced spatial learning memory in
ADHD rats. In particular, swimming has been recommended for
children suffering from ADHD-induced learning disabilities
(32).
In the present study, hyperactivity in ADHD rats was
suppressed by swimming exercise. In ADHD rats, non-aggressive and
aggressive behaviors, and impulsivity were inhibited by swimming
exercise. Swimming exercise also ameliorated ADHD-induced
short-term memory impairment. The results of the present study
demonstrated that swimming exercise improved the symptoms of ADHD
in SHRs (Fig. 1).
Dopamine is an important neurotransmitter in the
regulation of attention and cognitive function (16,38).
Hypofunction of the dopaminergic system decreased reinforcement of
previous behaviors. These conditions led to delayed aversion, the
development of hyperactivity in novel conditions, impulsiveness,
deficient sustained attention and increased behavioral variability
(38). The dysfunction of dopamine
signaling in the midbrain is one of the main mechanisms responsible
for causing hyperactivity and memory deficits (3,16).
Neuronal hypoactivity in the prefrontal cortex induced behavioral
alterations similar to those observed in ADHD, including attention
deficit and hyperactivity (2).
Microdialysis studies demonstrated that norepinephrine and dopamine
efflux was preferentially decreased within the prefrontal cortex of
SHRs (39,40). Exercise is known to protect
dopamine cell loss and increase dopamine levels, resulting in an
improvement of symptoms caused by Parkinson’s disease and stressful
conditions (10,41,42).
Exercise also enhanced the recovery from nigrostriatal dopamine
injury and altered dopaminergic neurotransmission in the
nigrostriatal system (29).
Treadmill exercise enhanced TH expression levels in the striatum
and substantia nigra of ADHD rats (31).
In the present study, the expression levels of TH,
which catalyzes the rate-limiting step of dopamine synthesis, in
the prefrontal cortex, substantia nigra and striatum were decreased
in ADHD rats. Swimming exercise increased TH expression levels in
ADHD rats (Fig. 2).
Swimming-induced increments in dopamine expression levels may
contribute to the improvement of symptoms observed in ADHD
rats.
Swimming exercise induced adaptive changes in the
functional responsiveness of dopamine receptors in the
nigrostriatal and mesolimbic systems, which were evident from the
modification of behavioral responses (43). Aggressive behaviors are closely
correlated with expression of dopamine D2 receptors in
animals and humans, suggesting that mesocorticolimbic dopamine may
be closely associated with permission of behaviors (44). Altered dopamine signaling may have
a causal association with motor hyperactivity and may be considered
as a potential endophenotype of ADHD (45). Chronic activation of dopamine
D2 receptors decreased dopamine release and inhibited
synapse formation (19). Dopamine
D1 and D2 receptor function within the
striatum may act to balance behavioral inhibition (46). Alterations in the activity of the
dopamine D2 receptor are implicated in several
neurological and psychiatric disorders, including schizophrenia,
Parkinson’s disease, Huntington’s disease, Tourette syndrome, ADHD
and drug addiction (47). Dopamine
D2 receptor activation enhanced physical activity
(20–22); however, impaired dopamine
D2 receptor function is associated with decreased
physical activity (48).
In the present study, the expression levels of the
dopamine D2 receptor in the prefrontal cortex,
substantia nigra and striatum were increased in ADHD rats compared
with control rats. Swimming exercise decreased the dopamine
D2 receptor expression levels in ADHD rats (Fig. 3). The suppressive effect of
swimming exercise on dopamine D2 receptor expression
levels may be associated with the relief of symptoms in ADHD
rats.
In this study, we demonstrated that swimming
exercise alleviated the symptoms of ADHD in SHRs by upregulating
the expression of dopamine and downregulating the expression of the
dopamine D2 receptor. The effectiveness of swimming
exercise appeared comparable to treatment with atomoxetine. Based
on the present results, swimming exercise may be a potential useful
therapeutic strategy for the treatment of ADHD.
Acknowledgements
This study was supported by the Research Fund from
Kyung Hee University granted in 2010 (KHU 20100648).
References
|
1
|
Scahill L and Schwab-Stone M: Epidemiology
of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am.
9:541–555. 2000.PubMed/NCBI
|
|
2
|
Arnsten AF: Fundamentals of
attention-deficit/hyperactivity disorder: circuits and pathways. J
Clin Psychiatry. 67(Suppl 8): 7–12. 2006.PubMed/NCBI
|
|
3
|
Bowton E, Saunders C, Erreger K, Sakrikar
D, Matthies HJ, Sen N, Jessen T, Colbran RJ, Caron MG, Javitch JA,
Blakely RD and Galli A: Dysregulation of dopamine transporters via
dopamine D2 autoreceptors triggers anomalous dopamine efflux
associated with attention-deficit hyperactivity disorder. J
Neurosci. 30:6048–6057. 2010. View Article : Google Scholar
|
|
4
|
Palmiter RD: Dopamine signaling in the
dorsal striatum is essential for motivated behaviors: lessons from
dopamine-deficient mice. Ann NY Acad Sci. 1129:35–46. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Castellanos FX and Tannock R: Neuroscience
of attention-deficit/hyperactivity disorder: the search for
endophenotypes. Nat Rev Neurosci. 3:617–628. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Winstanley CA, Eagle DM and Robbins TW:
Behavioral models of impulsivity in relation to ADHD: translation
between clinical and preclinical studies. Clin Psychol Rev.
26:379–395. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu Z, Cooper M, Crockett DP and Zhou R:
Differentiation of the midbrain dopaminergic pathways during mouse
development. J Comp Neurol. 476:301–311. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaplan RF and Stevens M: A review of adult
ADHD: a neuropsychological and neuroimaging perspective. CNS
Spectr. 7:355–362. 2002.PubMed/NCBI
|
|
9
|
Volkow ND, Wang GJ, Fowler JS and Ding YS:
Imaging the effects of methylphenidate on brain dopamine: new model
on its therapeutic actions for attention-deficit/hyperactivity
disorder. Biol Psychiatry. 57:1410–1415. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG,
Sung YH, Kim SE, Lee HH, Kim YP and Kim CJ: Treadmill exercise
suppresses nigrostriatal dopaminergic neuronal loss in
6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett.
423:12–17. 2007.PubMed/NCBI
|
|
11
|
Haavik J and Toska K: Tyrosine hydroxylase
and Parkinson’s disease. Mol Neurobiol. 16:285–309. 1998.
|
|
12
|
Hurley FM, Costello DJ and Sullivan AM:
Neuroprotective effects of delayed administration of
growth/differentiation factor-5 in the partial lesion model of
Parkinson’s disease. Exp Neurol. 185:281–289. 2004.PubMed/NCBI
|
|
13
|
Ko IG, Cho H, Kim SE, Kim JE, Sung YH, Kim
BK, Shin MS, Cho S, Pak YK and Kim CJ: Hypothermia alleviates
hypoxic ischemia-induced dopamine dysfunction and memory impairment
in rats. Anim Cells Syst. 15:279–286. 2011. View Article : Google Scholar
|
|
14
|
Millan MJ, Seguin L, Gobert A, Cussac D
and Brocco M: The role of dopamine D3 compared with D2 receptors in
the control of locomotor activity: a combined behavioural and
neurochemical analysis with novel, selective antagonists in rats.
Psychopharmacology (Berl). 174:341–357. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pirke KM, Broocks A, Wilckens T, Marquard
R and Schweiger U: Starvation-induced hyperactivity in the rat: the
role of endocrine and neurotransmitter changes. Neurosci Biobehav
Rev. 17:287–294. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Volkow ND, Wang GJ, Newcorn J, Telang F,
Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C
and Swanson JM: Depressed dopamine activity in caudate and
preliminary evidence of limbic involvement in adults with
attention-deficit/hyperactivity disorder. Arch Gen Psychiatry.
64:932–940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang M, Pei L, Fletcher PJ, Kapur S,
Seeman P and Liu F: Schizophrenia, amphetamine-induced sensitized
state and acute amphetamine exposure all show a common alteration:
increased dopamine D2 receptor dimerization. Mol Brain. 3:252010.
View Article : Google Scholar
|
|
18
|
Gründer G: Cariprazine, an orally active
D2/D3 receptor antagonist, for the potential treatment of
schizophrenia, bipolar mania and depression. Curr Opin Investig
Drugs. 11:823–832. 2010.PubMed/NCBI
|
|
19
|
Fasano C, Poirier A, DesGroseillers L and
Trudeau LE: Chronic activation of the D2 dopamine autoreceptor
inhibits synaptogenesis in mesencephalic dopaminergic neurons in
vitro. Eur J Neurosci. 28:1480–1490. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chausmer AL, Elmer GI, Rubinstein M, Low
MJ, Grandy DK and Katz JL: Cocaine-induced locomotor activity and
cocaine discrimination in dopamine D2 receptor mutant mice.
Psychopharmacology (Berl). 163:54–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sevak RJ, Owens WA, Koek W, Galli A, Daws
LC and France CP: Evidence for D2 receptor mediation of
amphetamine-induced normalization of locomotion and dopamine
transporter function in hypoinsulinemic rats. J Neurochem.
101:151–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanabe LM, Suto N, Creekmore E,
Steinmiller CL and Vezina P: Blockade of D2 dopamine receptors in
the VTA induces a long-lasting enhancement of the locomotor
activating effects of amphetamine. Behav Pharmacol. 15:387–395.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banaschewski T, Roessner V, Dittmann RW,
Santosh PJ and Rothenberger A: Non-stimulant medications in the
treatment of ADHD. Eur Child Adolesc Psychiatry. 13(Suppl 1):
i102–i116. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bymaster FP, Katner JS, Nelson DL,
Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM,
Gehlert DR and Perry KW: Atomoxetine increases extracellular levels
of norepinephrine and dopamine in prefrontal cortex of rat: a
potential mechanism for efficacy in attention deficit/hyperactivity
disorder. Neuropsychopharmacology. 27:699–711. 2002. View Article : Google Scholar
|
|
25
|
Cascade E, Kalali AH and Wigal SB:
Real-world data on: attention deficit hyperactivity disorder
medication side effects. Psychiatry (Edgmont). 7:13–15.
2010.PubMed/NCBI
|
|
26
|
Jee YS, Ko IG, Sung YH, Lee JW, Kim YS,
Kim SE, Kim BK, Seo JH, Shin MS, Lee HH, Cho HJ and Kim CJ: Effects
of treadmill exercise on memory and c-Fos expression in the
hippocampus of the rats with intracerebroventricular injection of
streptozotocin. Neurosci Lett. 443:188–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim
CJ, Kim SH, Baek SS, Lee EK and Jee YS: Treadmill exercise prevents
aging-induced failure of memory through an increase in neurogenesis
and suppression of apoptosis in rat hippocampus. Exp Gerontol.
45:357–365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim SE, Ko IG, Park CY, Shin MS, Kim CJ
and Jee YS: Treadmill and wheel exercise alleviate
lipopolysaccharide-induced short-term memory impairment by
enhancing neuronal maturation in rats. Mol Med Rep. 7:37–42.
2013.PubMed/NCBI
|
|
29
|
O’Dell SJ, Gross NB, Fricks AN, Casiano
BD, Nguyen TB and Marshall JF: Running wheel exercise enhances
recovery from nigrostriatal dopamine injury without inducing
neuroprotection. Neuroscience. 144:1141–1151. 2007.PubMed/NCBI
|
|
30
|
Majorek M, Tüchelmann T and Heusser P:
Therapeutic Eurythmy-movement therapy for children with attention
deficit hyperactivity disorder (ADHD): a pilot study. Complement
Ther Nurs Midwifery. 10:46–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim
SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW and Kim CJ:
Treadmill exercise and methylphenidate ameliorate symptoms of
attention deficit/hyperactivity disorder through enhancing dopamine
synthesis and brain-derived neurotrophic factor expression in
spontaneous hypertensive rats. Neurosci Lett. 504:35–39. 2011.
View Article : Google Scholar
|
|
32
|
Baron DA: The gold medal face of ADHD. J
Atten Disord. 13:323–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sagvolden T: Behavioral validation of the
spontaneously hypertensive rat (SHR) as an animal model of
attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav
Rev. 24:31–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prediger RD, Pamplona FA, Fernandes D and
Takahashi RN: Caffeine improves spatial learning deficits in an
animal model of attention deficit hyperactivity disorder (ADHD) -
the spontaneously hypertensive rat (SHR). Int J
Neuropsychopharmacol. 8:583–594. 2005. View Article : Google Scholar
|
|
35
|
Kim K, Chung E, Kim CJ and Lee S: Swimming
exercise during pregnancy alleviates pregnancy-associated long-term
memory impairment. Physiol Behav. 107:82–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dandekar MP, Singru PS, Kokare DM, Lechan
RM, Thim L, Clausen JT and Subhedar NK: Importance of cocaine- and
amphetamine-regulated transcript peptide in the central nucleus of
amygdala in anxiogenic responses induced by ethanol withdrawal.
Neuropsychopharmacology. 33:1127–1136. 2008. View Article : Google Scholar
|
|
37
|
Seo JH, Kim TW, Kim CJ, Sung YH and Lee
SJ: Treadmill exercise during pregnancy ameliorates post-traumatic
stress disorder-induced anxiety-like responses in maternal rats.
Mol Med Rep. 7:389–395. 2013.PubMed/NCBI
|
|
38
|
Sagvolden T, Russell VA, Aase H, Johansen
EB and Farshbaf M: Rodent models of attention-deficit/hyperactivity
disorder. Biol Psychiatry. 57:1239–1247. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Devilbiss DM and Berridge CW:
Cognition-enhancing doses of methylphenidate preferentially
increase prefrontal cortex neuronal responsiveness. Biol
Psychiatry. 64:626–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heal DJ, Smith SL, Kulkarni RS and Rowley
HL: New perspectives from microdialysis studies in freely-moving,
spontaneously hypertensive rats on the pharmacology of drugs for
the treatment of ADHD. Pharmacol Biochem Behav. 90:184–197. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mabandla MV, Kellaway LA, Daniels WM and
Russell VA: Effect of exercise on dopamine neuron survival in
prenatally stressed rats. Metab Brain Dis. 24:525–539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sung YH, Kim SC, Hong HP, Park CY, Shin
MS, Kim CJ, Seo JH, Kim DY, Kim DJ and Cho HJ: Treadmill exercise
ameliorates dopaminergic neuronal loss through suppressing
microglial activation in Parkinson’s disease mice. Life Sci.
91:1309–1316. 2012.PubMed/NCBI
|
|
43
|
Dey S and Singh RH: Modification of
apomorphine-induced behaviour following chronic swim exercise in
rats. Neuroreport. 3:497–500. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miczek KA, Fish EW, De Bold JF and De
Almeida RM: Social and neural determinants of aggressive behavior:
pharmacotherapeutic targets at serotonin, dopamine and
gamma-aminobutyric acid systems. Psychopharmacology (Berl).
163:434–458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jucaite A, Fernell E, Halldin C, Forssberg
H and Farde L: Reduced midbrain dopamine transporter binding in
male adolescents with attention-deficit/hyperactivity disorder:
association between striatal dopamine markers and motor
hyperactivity. Biol Psychiatry. 57:229–238. 2005. View Article : Google Scholar
|
|
46
|
Eagle DM, Wong JC, Allan ME, Mar AC,
Theobald DE and Robbins TW: Contrasting roles for dopamine D1 and
D2 receptor subtypes in the dorsomedial striatum but not the
nucleus accumbens core during behavioral inhibition in the
stop-signal task in rats. J Neurosci. 31:7349–7356. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Sasaoka T and Dang MT: A molecular
genetic approach to uncovering the differential functions of
dopamine D2 receptor isoforms. Methods Mol Biol. 964:181–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klinker F, Hasan K, Paulus W, Nitsche MA
and Liebetanz D: Pharmacological blockade and genetic absence of
the dopamine D2 receptor specifically modulate voluntary locomotor
activity in mice. Behav Brain Res. 242:117–124. 2013. View Article : Google Scholar : PubMed/NCBI
|