Introduction
Organic dust is the dried particles of plants,
animals, fungi or bacteria that are capable of being windborne due
to their fine structure. Organic dust is commonly found in a large
number of occupational and agricultural production environments
(1,2). Exposure to organic dust may cause
several types of inflammatory lung disease, such as asthma and
hypersensitivity pneumonitis (HP) (2,3). The
etiology of HP in organic dust processing environments includes
numerous types of extrinsic substances. Fungi are known to
constitute one of the main pathogenic causes of HP. 1,3-β-Glucan is
a biomarker of fungi exposure and a major cell wall component of
fungi (4–6). A strong association between the level
of 1,3-β-glucan and respiratory symptoms has been reported
previously (7).
Many studies have shown that 1,3-β-glucan affects
the Th1 immune response in different ways. Berner et
al(8) showed that the synergy
between interferon-γ (IFN-γ) and 1,3-β-glucan upregulates the mRNA
expression of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and
IL-1β, as well as the secretion of these pro-inflammatory cytokines
in mouse peritoneal macrophages in vitro. Furthermore, a
number of studies have shown that dietary 1,3-β-glucan increases
the production of IL-12 and IFN-γ from splenocytes, as well as the
number of IFN-γ-producing cells (9,10).
However, Wu et al(11)
demonstrated that dietary supplementation with 1,3-β-glucan
significantly inhibited the delayed-type Th1 immune reaction
(11). According to an additional
study (12), 1,3-β-glucan was
suggested to have a beneficial action on restoring Th2 function.
Th1 and Th2 cytokines were found to counteract each other. It was
reported that some 1,3-β-glucan inhibited the Th1 immune response
(13). 1,3-β-Glucan could increase
the levels of IL-10 and transforming growth factor-β (TGF-β) to
suppress the Th1 immune response (12,14).
The aim of the present study was to investigate
whether 1,3-β-glucan affected the pattern of Th1 and Th2 cytokine
secretion and regulated the Th1/Th2 balance by secreting
anti-inflammatory cytokines. Therefore, macrophages and lymphocytes
were extracted from mice and co-cultured in vitro.
Enzyme-linked immunosorbent assay (ELISA) was used to detect the
levels of cytokines in co-culture media and real-time reverse
transcription (RT)-polymerase chain reaction (PCR) was used to
determine the mRNA expression of forkhead box p3 (Foxp3) in
lymphocytes.
Materials and methods
Animals
Healthy, female C57BL/6 mice (6–8 weeks old) were
purchased from the SLAC Laboratory Animal Co., Ltd. (Shanghai,
China). All of the animals were housed in a specific pathogen-free
environment and maintained on standard mouse chow with free access
to food and water. The study was approved by the Animal Care and
Use Committee of the China Medical University (Shenyang, Liaoning,
China; permit no. CMU62043010), and complied with the National
Institute of Health Guide for the Care and Use of Laboratory
Animals.
1,3-β-Glucan exposure
Zymosan A from Saccharomyces cerevisiae
(Z4250), purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA),
was dissolved in phosphate-buffered saline (PBS) to a final
concentration of 3 mg/ml. Twenty mice were randomly allocated into
two groups: the PBS and the 1,3-β-glucan group. All of the animals
were anesthetized by an intraperitoneal injection of 2%
pentobarbital sodium (45 mg/kg body weight). The trachea was
exposed by opening the neck skin and blunt dissection. A 7-gauge
needle was inserted transorally into the trachea. The mice in the
1,3-β-glucan or the PBS group were administered 0.1 ml zymosan
solution (3 mg/ml) or 0.1 ml sterile PBS, respectively. The site of
surgery was sutured and cleaned with penicillin. The mice were
allowed to recover until they were sacrificed.
Macrophage isolation
All of the mice were sacrificed 7 days following
exposure to 1,3-β-glucan (Zymosan A) or PBS. The lungs were removed
and washed twice in cold PBS. Bronchoalveolar lavage fluid (BALF)
was obtained by cannulating the trachea, injecting and retrieving
1-ml aliquots of sterile physiological saline several times to
obtain 6 ml liquid. The BALF was centrifuged at 200 × g for 8 min
at 4°C; the pellet was washed and re-suspended with 0.5 ml
RPMI-1640. Cell suspension (10 μl) was used to count the cells
under a microscope. RPMI-1640 supplemented with 10%
heat-inactivated fetal bovine serum (FBS) was then added to a final
concentration of 1×105 cells/ml. BALF cells from mice in
the PBS group were placed in 24-well tissue culture plates (Costar)
in four wells. The plates were then placed in an incubator
supplemented with 5% CO2 at 37°C. Macrophages were
allowed to adhere for 2 h, and the wells were then washed with
RPMI-1640 thrice to rinse away most non-adherent cells.
Lymphocyte isolation
The spleens were removed from the mice and placed on
35-mm plates with 4–5 ml mouse lymphocyte separation medium
(EZ-Sep™ Mouse 1X). After being grinded and mechanically disrupted,
the splenocytes were isolated and carefully transferred to a 15-ml
centrifuge tube and covered with 200–500 μl RPMI-1640. Following
centrifugation for 30 min at 360 × g, lymphocytes were obtained and
washed once with RPMI-1640 supplemented with 10% FBS by
centrifugation, followed by re-suspension in RPMI-1640. The cells
were counted using a hemocytometer. Subsequently, cell
concentration was adjusted to 5×106. Lymphocytes (1 ml)
from mice treated with PBS were transferred to the four wells
containing macrophages.
Co-culture in vitro
Lymphocytes were cultured in 24-well flat-bottom
plates pre-coated with macrophages. Macrophages from mice treated
with PBS were the same in the four experimental groups. The four
experimental groups were the following: the
LG−G−, LG−G+,
LG+G− and LG+G+ group.
LG−, lymphocytes isolated from PBS-treated mice;
LG+, lymphocytes isolated from 1,3-β-glucan-treated
mice; G−, lymphocytes co-cultured in vitro and
treated with PBS; and G+, lymphocytes co-cultured in
vitro treated with 1,3-β-glucan (100 μg/ml). Each group
contained ConA to stimulate survival. The cells were cultured for
24 or 48 h at 37°C under a 5% CO2 atmosphere.
Separating and conserving
Following 24 or 48 h of culture, mixtures of cells
and culture media were transferred to 1.5-ml Eppendorf tubes,
followed by centrifugation for 8 min at 4°C. Subsequently, culture
media were stored for the investigation of cytokine protein levels.
TRIzol reagent was added to the lymphocytes to avoid RNA
degradation. The cells and culture media were maintained at
−70°C.
Enzyme-linked immunosorbent assay
(ELISA)
The ELISA plate was coated with 100 μl capture
antibody in coating buffer/well of the ELISA kit (eBioscience, San
Diego, CA, USA) and incubated at 4°C overnight. The plate was
washed with 250 μl washing buffer. The cells in each well were then
blocked with 200 μl assay diluent and incubated for 1 h at room
temperature. A volume of 100 μl culture supernatants or the
different dilutions of standard (for standard curve) were added to
each well and incubated for 2 h at room temperature. The cells in
each well were incubated with 100 μl detection antibody for 1 h at
room temperature, followed by incubation with 100 μl avidin-HRP for
30 min at room temperature. Substrate solution (100 μl)was added to
each well for a 15-min incubation at room temperature. Stop
solution (50 μl) was added to stop the reaction. The absorbance was
read at 450 nm. ELISA was performed in triplicate.
RNA extraction and real-time RT-PCR
Total RNA was extracted from lymphocytes using the
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
according to the manufacturer’s protocol. The RNA concentration and
the ratio of A260/280 were determined using an ultraviolet (UV)
spectrophotometer. The primers were designed using Primer3
(http://frodo.wi.mit.edu/primer3) and the
sequences were blasted (http://blast.ncbi.nlm.nih.gov/Blast.cgi). PrimeScript
RT reagent kit (DRR037A; Takara, Shiga, Japan) and SYBR Premix Ex
Taq II (DRR081A; Takara) were used for real-time RT-PCR. The primer
sequences used were the following: Foxp3, sense
5′-CAGCTCTGCTGGCGAAAGTG-3′ and antisense,
5′-TCGTCTGAAGGCAGAGTCAGGA-3′; GAPDH, sense,
5′-CAATGTGTCCGTCGTGGATCT-3′ and antisense,
5′-GTCCTCAGTGTAGCCCAAGATG-3′. Total RNA (0.4 μg) of each group at
48 h was reverse transcribed in a volume of 20 μl and the following
PCR conditions were used: 37°C for 15 min and 85°C for 5 sec. cDNA
(2 μl) was used in a 25-μl PCR reaction volume. Each sample was
assayed in triplicate. The difference of the amplification
efficiency between the target gene and the housekeeping gene were
identified by comparing the slopes of the standard curves. The PCR
reactions were run on ABI 7500 (Applied Biosystems) using the
following conditions: 95°C for 30 sec, and 40 cycles of 95°C for 5
sec and 60°C for 34 sec. Analysis was performed using the ABI 7500
system software.
Statistical analysis
The SPSS 16.0 software was used to perform
statistical analyses. The differences between values were evaluated
using one-way analysis of variance (ANOVA) followed by pair-wise
comparison with the LSD and Student-Newman-Keuls test. P<0.05
was considered to indicate a statistically significant difference.
All of the experiments were repeated five times. The results were
expressed as the means ± SEM.
Results
1,3-β-Glucan exposure decreases the
secretion of Th1 cytokines in vitro
To investigate the role of 1,3-β-glucan in the
secretion of Th1 cytokines, macrophages isolated from mice treated
with PBS were allocated into 4 groups. The levels of Th1 cytokines
(IL-2 and IFN-γ) in the culture media of cells treated with
1,3-β-glucan in vitro (LG−G+ group)
significantly decreased compared with the
LG−G− group 24 and 48 h following in
vitro treatment (Figs. 1 and
2). The levels of IL-2 in the
culture media of cells in the LG−G+ and
LG+G+ groups significantly decreased compared
with the LG−G− and
LG+G− groups 24 and 48 h following treatment
with 1,3-β-glucan or PBS in vitro (Fig. 1). The levels of IFN-γ in the
LG+G+ group significantly decreased compared
with the LG+G− group at both time points
(Fig. 2).
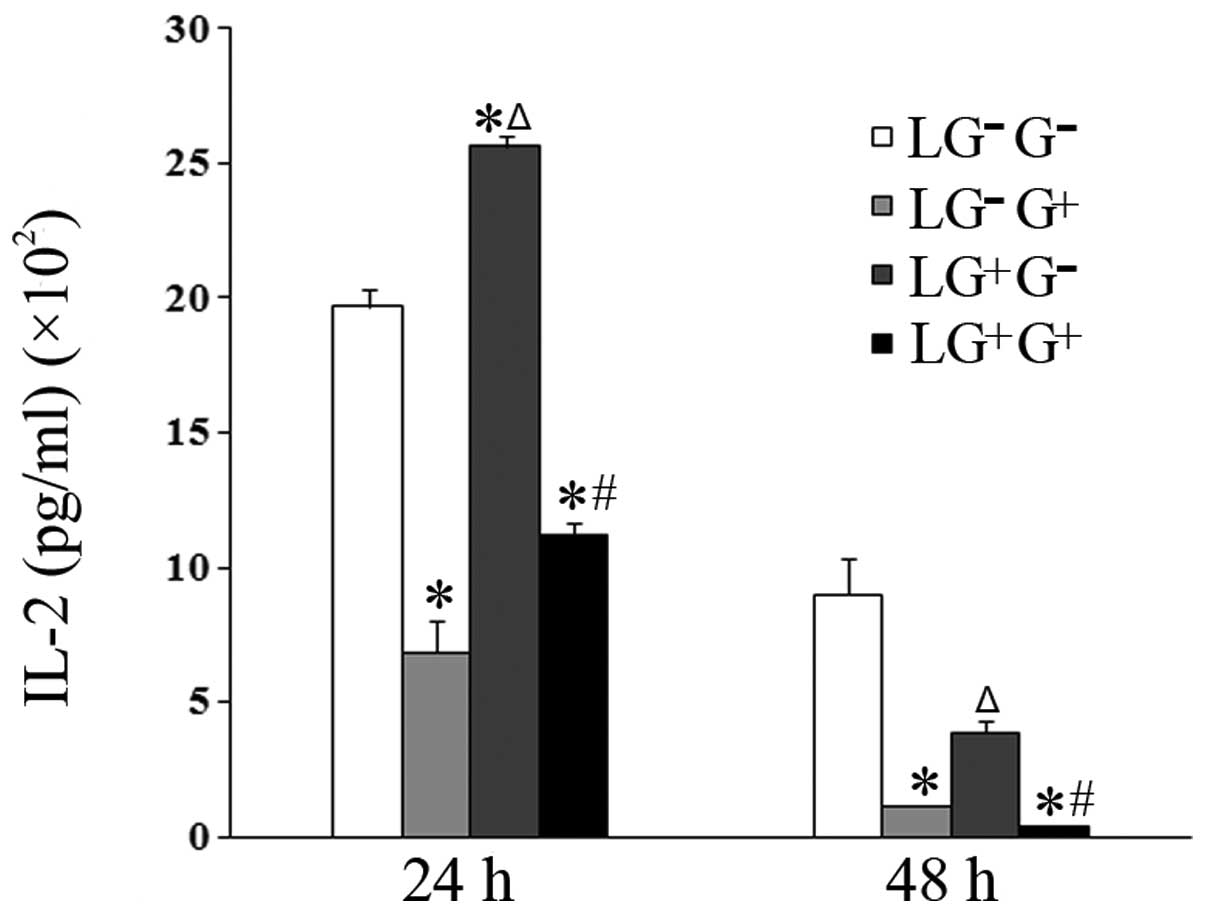 | Figure 1Levels of IL-2 in the culture media.
The expression levels of IL-2 in the culture media were determined
using ELISA (n=5). The levels of IL-2 in all the cell groups for
the various treatment durations are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group; #P<0.05 compared
with the LG+G− group. IL, interleukin; ELISA,
enzyme-linked immunosorbent assay; PBS, phosphate-buffered
saline. |
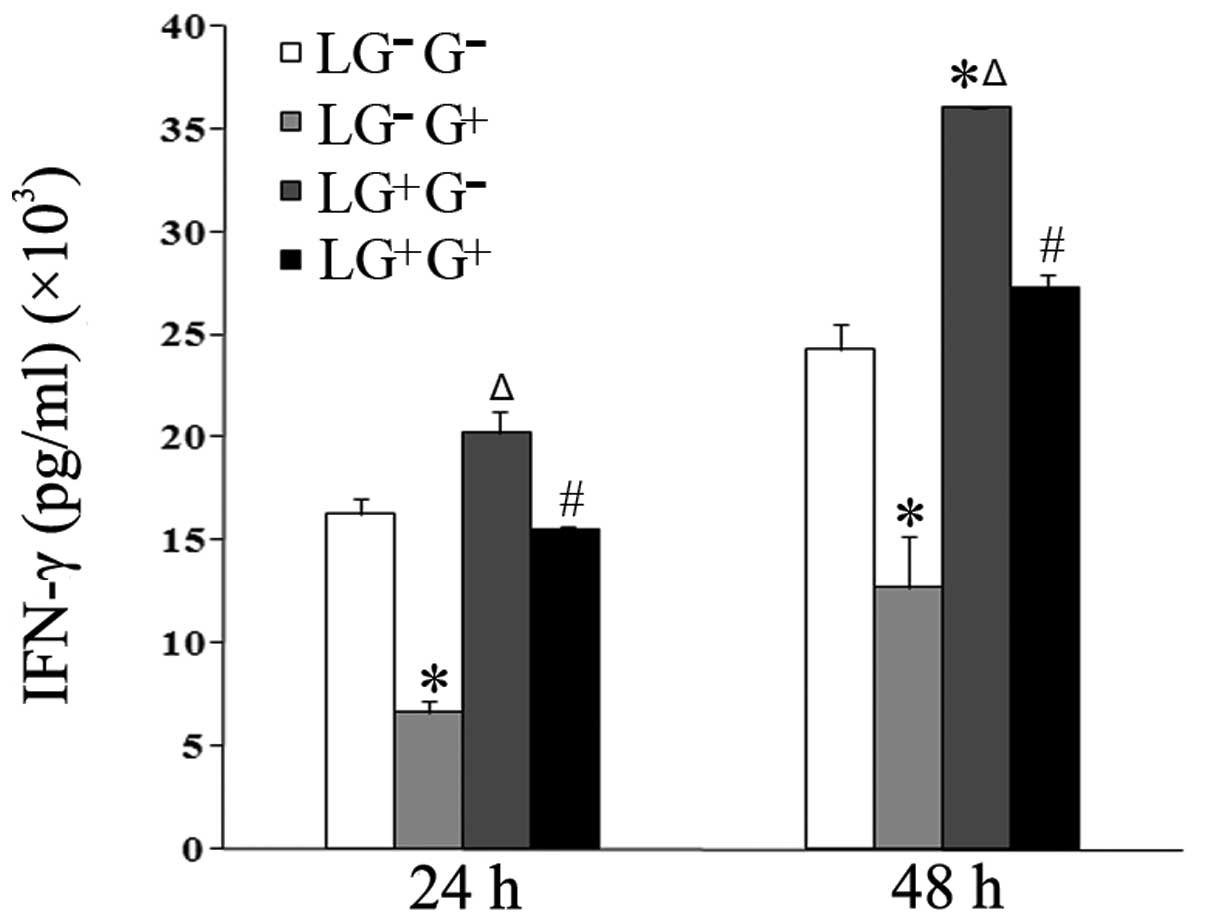 | Figure 2Levels of IFN-γ in the culture media.
The expression levels of IFN-γ in the culture media were determined
using ELISA (n=5). The levels of IFN-γ in all the cell groups for
the various treatment durations are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group; #P<0.05 compared
with the LG+G− group. IL, interleukin; ELISA,
enzyme-linked immunosorbent assay; PBS, phosphate-buffered
saline. |
The levels of IFN-γ in the culture media of the
cells treated with 1, 3-β-glucan in vivo
(LG+G− group) significantly increased
compared with LG−G− and
LG−G+ groups 48 h following in vitro
treatment (Fig. 2). The levels of
IL-2 in the culture media of the cells treated with 1,3-β-glucan
in vivo (LG+G− group) significantly
increased compared with LG−G− and
LG−G+ group 24 h following in vitro
treatment (Fig. 1). This suggests
that 1,3-β-glucan inhibits the secretion of Th1 cytokines in
vitro.
1,3-β-Glucan exposure increases the
secretion of the Th2 cytokine IL-4 in vitro
The secretion of the Th2 cytokine IL-4 in response
to 1,3-β-glucan treatment in vitro was examined. The levels
of IL-4 increased in the culture media of the cells in the
LG+G+ group compared with the
LG+G− group 24 h following in vitro
treatment (Fig. 3). The levels of
IL-4 in the culture media of the LG−G+ and
LG+G+ groups increased compared with the
LG−G− and LG+G− groups
48 h after treatment with 1,3-β-glucan or PBS in vitro
(Fig. 3). This result suggests
that 1,3-β-glucan increases the expression levels of IL-4 in
vitro.
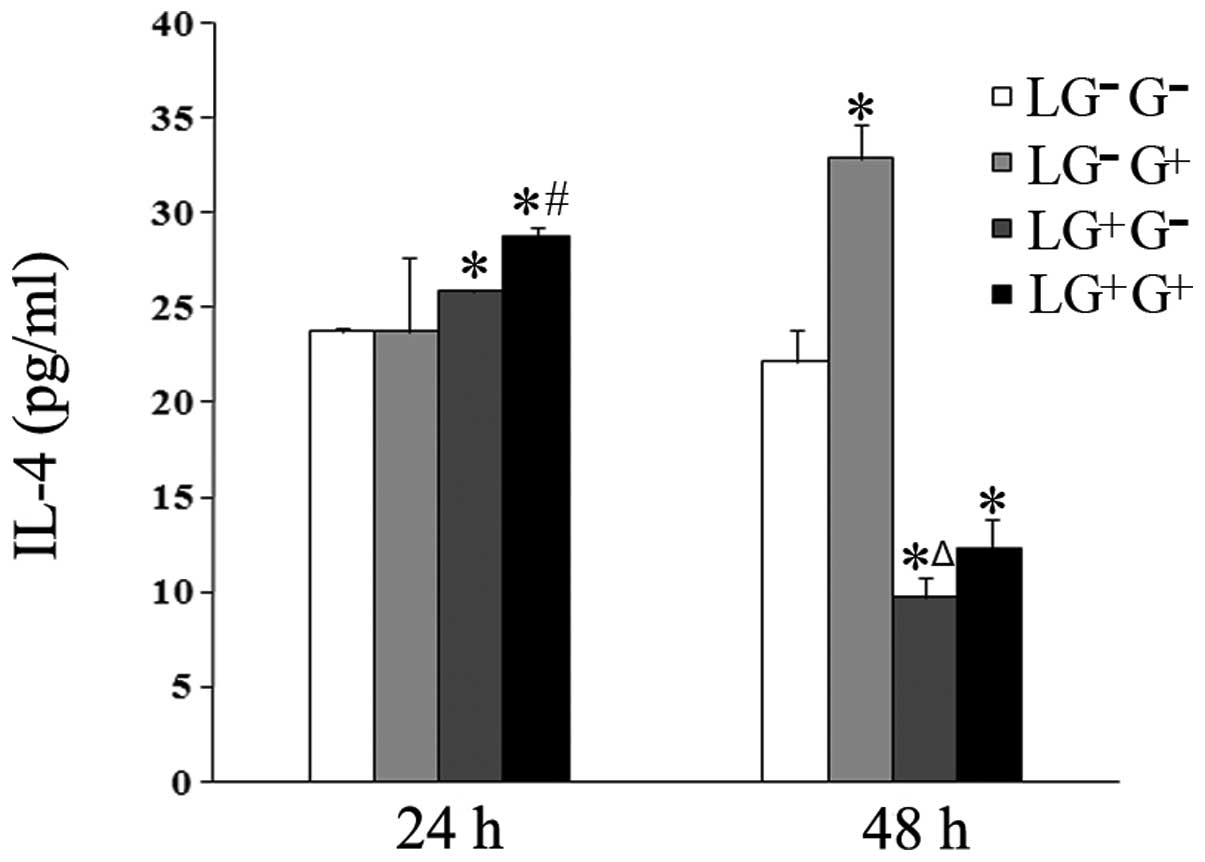 | Figure 3Levels of IL-4 in the culture media.
The expression levels of IL-4 in the culture media were determined
using ELISA (n=5). The levels of IL-4 in all the cell groups for
the various treatment durations are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group; #P<0.05 compared
with the LG+G− group. IL, interleukin; ELISA,
enzyme-linked immunosorbent assay; PBS, phosphate-buffered
saline. |
1,3-β-Glucan exposure promotes the
secretion of anti-inflammatory cytokines in vitro
The expression levels of anti-inflammatory cytokines
were then assessed. The expression levels of IL-10 in the culture
media of the LG−G+ and
LG+G+ groups significantly increased compared
with the LG−G− and
LG+G− groups 24 and 48 h following in
vitro treatment (Fig. 4).
Thus, 1,3-β-glucan increases the secretion of IL-10 in
vitro.
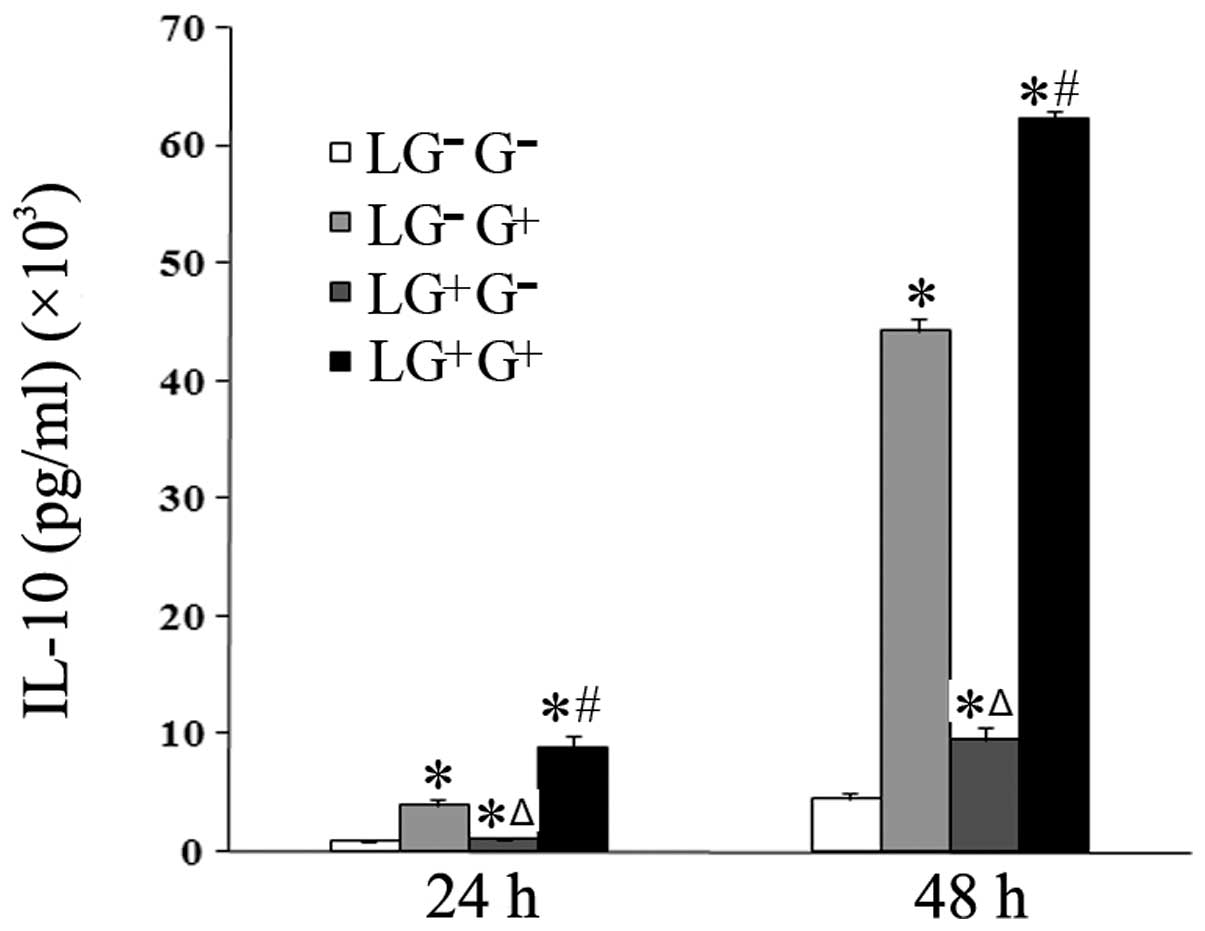 | Figure 4Levels of IL-10 in the culture media.
The expression levels of IL-10 in the culture media were determined
using ELISA (n=5). The levels of IL-10 in all the cell groups for
the various treatment durations are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group; #P<0.05 compared
with the LG+G− group. IL, interleukin; ELISA,
enzyme-linked immunosorbent assay; PBS, phosphate-buffered
saline. |
The expression levels of TGF-β in the culture media
of the LG+G+ group increased compared with
the LG+G− group at 24 h. At 48 h, the levels
of TGF-β in the LG−G+ and
LG+G+ groups increased compared with the
LG−G− and LG+G− groups
(Fig. 5). These results suggest
that 1,3-β-glucan alters the Th1/Th2 balance through an IL-10
dependent rather than an TGF-β-dependent pathway.
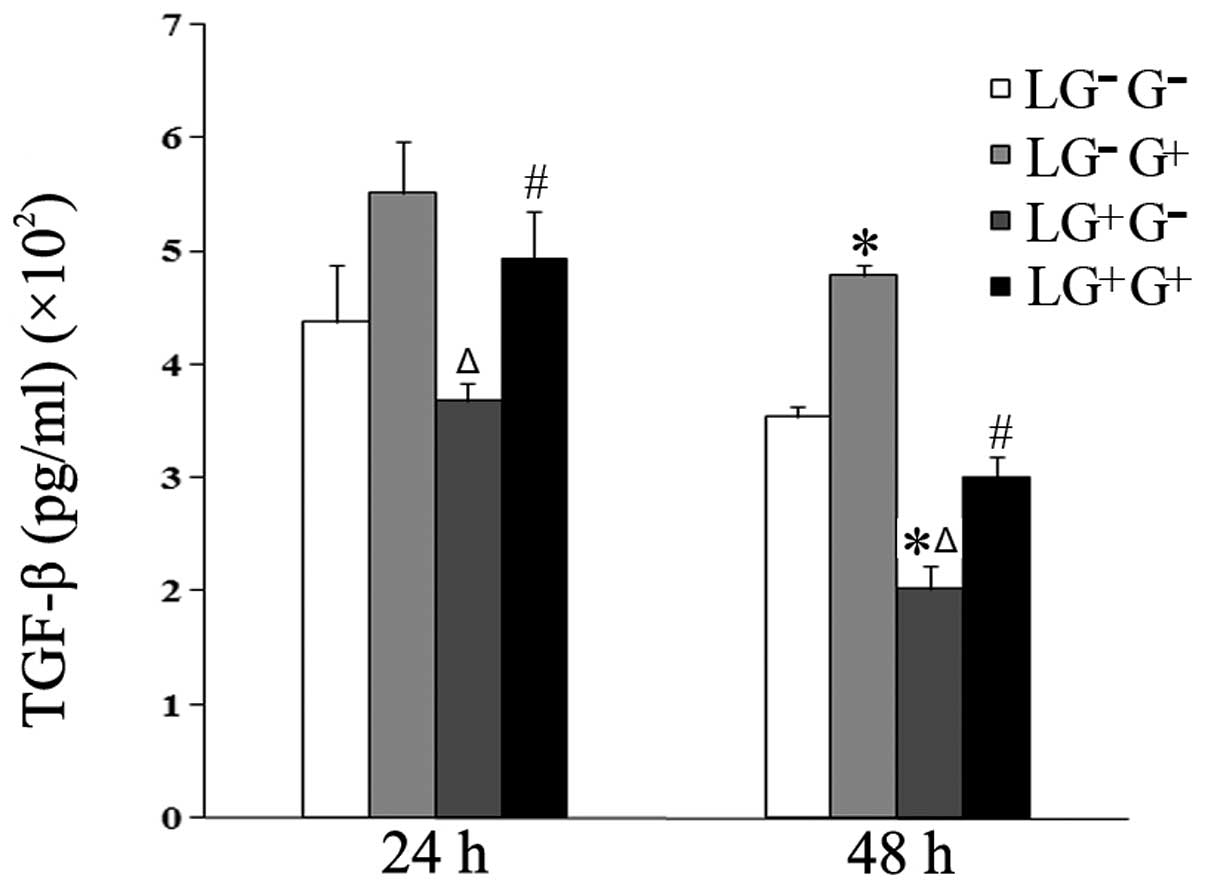 | Figure 5Levels of TGF-β in the culture media.
The expression levels of TGF-β in the culture media were determined
using ELISA (n=5). The levels of TGF-β in all the cell groups for
the various treatment durations are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group; #P<0.05 compared
with the LG+G− group. TGF-β, transforming
growth factor-β; ELISA, enzyme-linked immunosorbent assay; PBS,
phosphate-buffered saline. |
1,3-β-Glucan exposure may increase the
expression of Foxp3 mRNA in vitro
As shown in Fig. 6,
although the mRNA expression of Foxp3 increased in response to
1,3-β-glucan treatment in vitro, no statistically
significant difference among the 4 cell groups was observed. This
result suggests that 1,3-β-glucan exposure may increase the
expression of Foxp3 in lymphocytes.
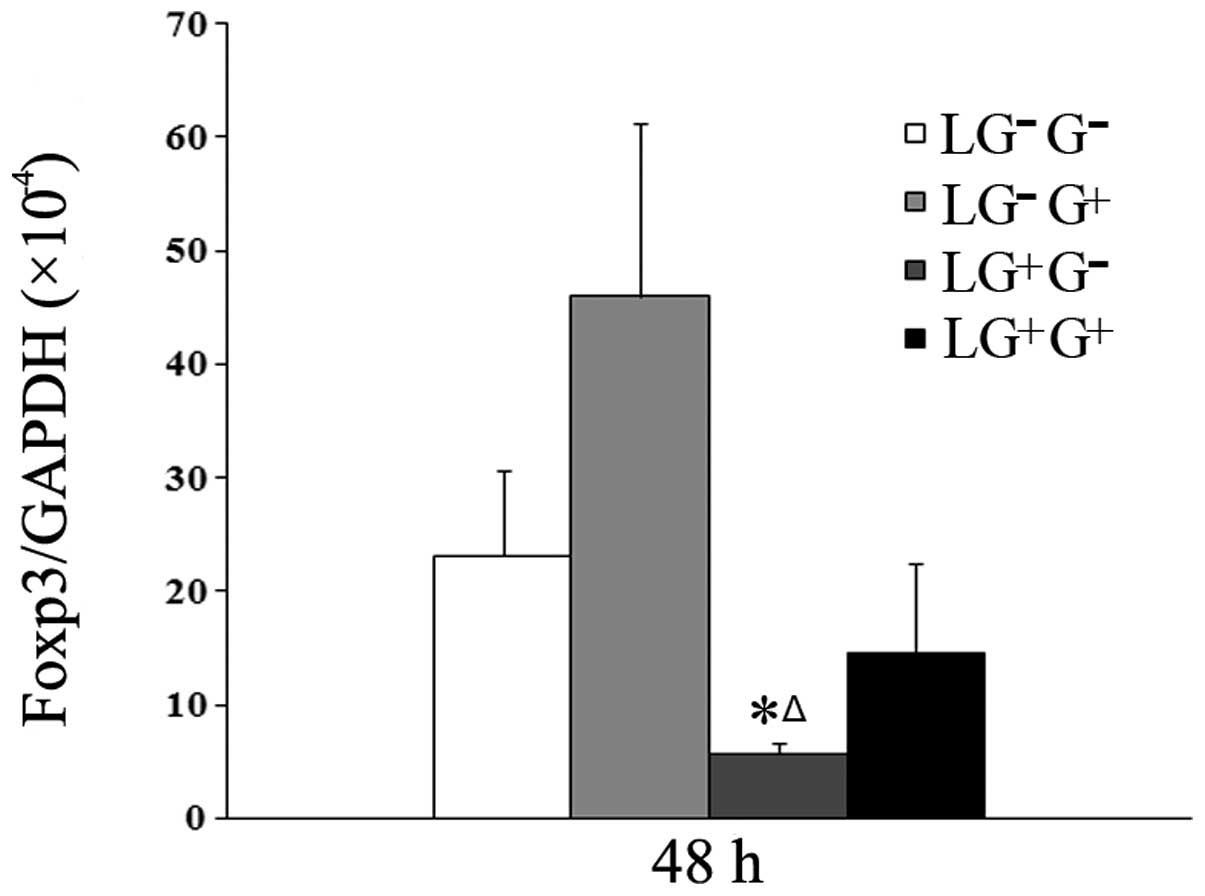 | Figure 6Levels of Foxp3 mRNA expression in
lymphocytes. The expression of Foxp3 mRNA at 48 h was assessed by
real-time RT-PCR using the −ΔΔCt method (n=5). The levels of Foxp3
mRNA expression in all the cell groups 48 h following in
vitro treatment are shown in the graph.
LG−G− group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with PBS;
LG−G+ group, lymphocytes isolated from
PBS-treated mice, co-cultured in vitro and treated with
1,3-β-glucan; LG+G− group, lymphocytes
isolated from 1,3-β-glucan-treated mice, co-cultured in
vitro and treated with PBS; LG+G+ group,
lymphocytes isolated from 1,3-β-glucan-treated mice, co-cultured
in vitro and treated with 1,3-β-glucan.
*P<0.05 compared with the LG−G−
group; ΔP<0.05 compared with the
LG−G+ group. Foxp3, forkhead box p3; PBS,
phosphate-buffered saline. |
Discussion
Th1/Th2 balance is the major framework used to
address adaptive immunity (15),
and Th1 and Th2 cells are characterized by specific cytokine
signatures (16). IL-2 and IFN-γ
are considered to be hallmark Th1 cytokines (15,17,18),
while IL-4 is the hallmark Th2 cytokine. Many studies have
demonstrated that the secretion of pro-inflammatory cytokines is
upregulated in macrophages exposed to soluble or particulate
β-glucans (8,19–22).
In the present study, macrophages and lymphocytes were extracted
from mice and co-cultured in vitro in order to investigate
the role of 1,3-β-glucan in Th1/Th2 balance. According to our
results, the expression of Th1 cytokines, such as IFN-γ and IL-2,
was inhibited, while the expression of the Th2 cytokine IL-4 was
increased following 1,3-β-glucan stimulation. Moreover,
1,3-β-glucan exposure in vitro increased the secretion of
IL-10 and TGF-β in the culture media of cells. These results
suggest that 1,3-β-glucan may alter the Th1/Th2 balance towards a
Th2-dependent response by promoting the secretion of
anti-inflammatory cytokines.
IL-10 and TGF-β are considered as anti-inflammatory
cytokines (23,24). In the present study, the expression
of these two anti-inflammatory cytokines increased when the cells
were treated with 1,3-β-glucan in vitro, which was
consistent with the results of previous studies (12,24,25).
In response to antigen stimulation, naive lymphocytes may
differentiate into regulatory lineages which could regulate the
Th1/Th2 balance via IL-10 and/or TGF-β (26–28).
Foxp3 plays an important role in the development and function of
regulatory lymphocytes. In the present study, the increased
tendency of Foxp3 upon 1,3-β-glucan stimulation suggested that
regulatory T or B cells may regulate the Th1/Th2 balance via IL-10
and/or TGF-β (22). The underlying
mechanism of action has yet to be fully elucidated.
Antigen presentation cells (APC) which are able to
initiate innate immunity, such as macrophages, express specific
surface receptors, such as Dectin-1, to recognize 1,3-β-glucan
(29,30). These processed then initiated the
adaptive immune responses, accompanied by the secretion of
pro-inflammatory cytokines (6,30,31).
In the present study, macrophages were isolated from mice treated
with 1,3-β-glucan, and co-cultured with lymphocytes in
vitro. The results obtained were highly identical (data not
shown). These data suggest that macrophages, which possess
1,3-β-glucan-binding sites on their cell surface, direct the
differentiation of lymphocytes (19,20).
The results of the present study showed that in
vivo exposure to 1,3-β-glucan upregulated the secretion of the
Th1 cytokines, IFN-γ and IL-2. These cytokines corresponded to the
early stage of inflammation in mice, which was Th1 dominant
(22). According to our previous
study, TGF-β was reduced in the early stage of 1,3-β-glucan-induced
inflammation (22). Exposure to
1,3-β-glucan in vitro alone increased the secretion of
TGF-β, while in vivo exposure to 1,3-β-glucan led to a
decrease in TGF-β expression. TGF-β may be secreted in the earliest
phase and rapidly inhibited by increasing Th1 production in
vivo.
In conclusion, the present study demonstrates that
1,3-β-glucan exposure in vitro promotes the secretion of
anti-inflammatory cytokines, leading to a decrease in Th1 and
increase in Th2 cytokine expression. 1,3-β-Glucan is capable of
inducing regulatory lymphocytes, which could partly contribute to
an increased secretion of anti-inflammatory cytokines in
co-cultured mouse macrophages and lymphocytes in vitro.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (No. 30771791) and a
Program for Liaoning Excellent Talents in University (No.
LR201039).
References
|
1
|
Rylander R: Airborne (1–3)-β-D-glucan and
airway disease in a day-care center before and after renovation.
Arch Environ Health. 52:281–285. 1997.
|
|
2
|
Rylander R, Thorn J and Attefors R:
Airways inflammation among workers in a paper industry. Eur Respir
J. 13:1151–1157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ronald LA, Davies HW, Bartlett KH, et al:
Beta(1–3)-glucan exposure levels among workers in four British
Columbia sawmills. Ann Agric Environ Med. 10:21–29. 2003.
|
|
4
|
Rylander R: Investigations of the
relationship between disease and airborne (1–3)-beta-D-glucan in
buildings. Mediators Inflamm. 6:275–277. 1997.
|
|
5
|
Rylander R: Indoor air-related effects and
airborne (1–3)-beta-D-glucan. Environ Health Perspect. 107(Suppl
3): 501–503. 1999.
|
|
6
|
Brown GD and Gordon S: Fungal beta-glucans
and mammalian immunity. Immunity. 19:311–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rylander R and Lin RH:
(1–3)-beta-D-glucan-relationship to indoor air-related symptoms,
allergy and asthma. Toxicology. 152:47–52. 2000.
|
|
8
|
Berner MD, Sura ME, Alves BN and Hunter KW
Jr: IFN-gamma primes macrophages for enhanced TNF-alpha expression
in response to stimulatory and non-stimulatory amounts of
microparticulate beta-glucan. Immunol Lett. 98:115–122. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kimura Y, Sumiyoshi M, Suzuki T, Suzuki T
and Sakanaka M: Inhibitory effects of water-soluble
low-molecular-weight β-(1,3–1,6) d-glucan purified from
Aureobasidium pullulans GM-NH-1A1 strain on food allergic
reactions in mice. Int Immunopharmacol. 7:963–972. 2007.
|
|
10
|
Xiao ZG, Trincado CA and Murtaugh MP:
Beta-glucan enhancement of T cell IFNgamma response in swine. Vet
Immunol Immunopathol. 102:315–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu HH, Weng BBC, Chen KL, Chiou PWS and Yu
B: Effect of dietary supplementation of β-1,3–1,6-glucan on
reproductive performance and immunity of New Zealand White does and
their pups. Livest Sci. 135:70–75. 2011.
|
|
12
|
Sarinho E, Medeiros D, Schor D, et al:
Production of interleukin-10 in asthmatic children after
Beta-1–3-glucan. Allergol Immunopathol (Madr). 37:188–192.
2009.
|
|
13
|
Zhou LD, Zhang QH, Zhang Y, Liu J and Cao
YM: The shiitake mushroom-derived immuno-stimulant lentinan
protects against murine malaria blood-stage infection by evoking
adaptive immune-responses. Int Immunopharmacol. 9:455–462. 2009.
View Article : Google Scholar
|
|
14
|
Olman MA and Matthay MA: Transforming
growth factor-β induces fibrosis in immune cell-depleted lungs. Am
J Physiol Lung Cell Mol Physiol. 285:L522–L526. 2003.
|
|
15
|
Mosmann TR and Coffman RL: TH1 and TH2
cells: different patterns of lymphokine secretion lead to different
functional properties. Annu Rev Immunol. 7:145–173. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei WC, Su YH, Chen SS, Sheu JH and Yang
NS: GM-CSF plays a key role in zymosan-stimulated human dendritic
cells for activation of Th1 and Th17 cells. Cytokine. 55:79–89.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coffman RL, Mocci S and O’Garra A: The
stability and reversibility of Th1 and Th2 populations. Curr Top
Microbiol Immunol. 238:1–12. 1999.PubMed/NCBI
|
|
18
|
Mosmann TR, Cherwinski H, Bond MW, Giedlin
MA and Coffman RL: Two types of murine helper T cell clone. I
Definition according to profiles of lymphokine activities and
secreted proteins. J Immunol. 136:2348–2357. 1986.
|
|
19
|
Hoffman OA, Olson EJ and Limper AH: Fungal
beta-glucans modulate macrophage release of tumor necrosis
factor-alpha in response to bacterial lipopolysaccharide. Immunol
Lett. 37:19–25. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakurai T, Ohno N and Yadomae T: Effects
of fungal beta-glucan and interferon-gamma on the secretory
functions of murine alveolar macrophages. J Leukoc Biol.
60:118–124. 1996.PubMed/NCBI
|
|
21
|
Abel G and Czop JK: Stimulation of human
monocyte beta-glucan receptors by glucan particles induces
production of TNF-alpha and IL-1 beta. Int J Immunopharmacol.
14:1363–1373. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu FW, Weng D, Chen Y, et al: Depletion
of CD4+CD25+Foxp3+ regulatory T
cells with anti-CD25 antibody may exacerbate the
1,3-β-glucan-induced lung inflammatory response in mice. Arch
Toxicol. 85:1383–1394. 2011.
|
|
23
|
Ding L and Shevach EM: IL-10 inhibits
mitogen-induced T cell proliferation by selectively inhibiting
macrophage costimulatory function. J Immunol. 148:3133–3139.
1992.PubMed/NCBI
|
|
24
|
Young SH, Ye J, Frazer DG, Shi X and
Castranova V: Molecular mechanism of tumor necrosis factor-alpha
production in 1,3-beta-glucan (zymosan)-activated macrophages. J
Biol Chem. 276:20781–20787. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gantner BN, Simmons RM, Canavera SJ, Akira
S and Underhill DM: Collaborative induction of inflammatory
responses by dectin-1 and Toll-like receptor 2. J Exp Med.
197:1107–1117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O’Garra A, Barrat FJ, Castro AG, Vicari A
and Hawrylowicz C: Strategies for use of IL-10 or its antagonists
in human disease. Immunol Rev. 223:114–131. 2008.
|
|
28
|
Moore KW, O’Garra A, de Waal Malefyt R,
Vieira P and Mosmann TR: Interleukin-10. Annu Rev Immunol.
11:165–190. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kankkunen P, Teiril L, Rintahaka J,
Alenius H, Wolff H and Matikainen S: (1,3)-beta-glucans activate
both dectin-1 and NLRP3 inflammasome in human macrophages. J
Immunol. 184:6335–6342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li B, Cramer D, Wagner S, et al: Yeast
glucan particles activate murine resident macrophages to secrete
proinflammatory cytokines via MyD88- and Syk kinase-dependent
pathways. Clin Immunol. 124:170–181. 2007. View Article : Google Scholar
|
|
31
|
Ganner A, Nitsch S, Erlacher K, Klimitsch
A and Schatzmayr G: Ex vivo effect of yeast beta-glucan on
lymphocyte viability and plasma IL-18 in weaning piglets. Livest
Sci. 133:246–248. 2010. View Article : Google Scholar
|




















