Introduction
Glucosamine, a naturally occurring monosaccharide,
acts as a preferred substrate for the biosynthesis of
glycosaminoglycan and has been used for the treatment of
osteoarthritis for >2 decades (1). Furthermore, glucosamine has been
shown to inhibit neutrophil functions, including superoxide
generation, phagocytosis, granule enzyme release, chemotaxis and
CD11b expression, thereby possibly exhibiting an anti-inflammatory
action (2,3). Glucosamine was recently reported to
inhibit intercellular adhesion molecule-1 (ICAM-1) and monocyte
chemoattractant protein-1 (MCP-1) expression in human umbilical
vein endothelial cells (HUVECs), thus showing anti-atherosclerotic
activities (4,5). A number of studies have reported that
glucosamine has an additional biological function, whereby it
inhibits tumor growth in vivo and in vitro(6,7).
Wang et al(8) reported that
the glucosamine sulfate-induced apoptosis of leukemia K562 cells is
associated with the translocation of cathepsin D and downregulation
of Bcl-xL. Another study has shown that D-glucosamine inhibits the
proliferation of human cancer cells through inhibition of p70S6K
(9). Although the study attempted
to investigate the mechanisms underlying this antitumor effect, the
exact mechanism remains to be fully elucidated. The regulation of
cell cycle checkpoints is important for the proper transition from
one phase of the cell cycle to the next. In particular, the
regulation of the G1 switching mechanisms between quiescence and
proliferation is defective in cancer cells, allowing them to
continuously grow (10,11). Cyclin E levels reach a peak in the
G1 phase, while cyclin E is rapidly degraded during the early S
phase. Cyclin E expression and, consequently, cyclin E/CDK2
activity is closely associated with the G1/S checkpoint (12). Various studies have indicated that
cyclin E is overexpressed in several tumor cells, such as breast
cancer cells, and it has been reported to be a prognostic biomarker
(13,14). p27Kip1 is one of the
most important cell cycle regulatory proteins. The Skp2-induced
ubiquitylation of p27Kip1 results in the recruitment of
p27Kip1 to the Skp1p-cullin-F-box protein (SCF) core
complex, but only after p27Kip1 is phosphorylated on
Thr187, a step that is required for Skp2 to recognize
p27Kip1. The important role of Skp2 in promoting entry
into the S phase, mainly by promoting p27Kip1
destruction, has been demonstrated in both cell-free systems and
cell cultures (15). Low levels of
p27Kip1 have been found to markedly impact tumor
progression and accurately predict poor prognosis in a large
variety of cancers, including breast, colorectal and prostate
carcinomas, as well as other epithelial carcinomas, sarcomas and
hematological malignancies (15,16).
These studies have suggested that loss of p27Kip1
contributes to uncontrolled tumor proliferation.
In the present study, the effects of glucosamine on
lung cancer cell proliferation, as well as on cyclin E and Skp2
expression levels were investigated. Cyclin E and Skp2 were found
to be overexpressed in the lung cancer cell lines A549 and H446,
indicating an association with lung cancer. Furthermore,
glucosamine was shown to inhibit cyclin E and Skp2 expression in
these cell lines. Therefore, this study suggests that glucosamine
may inhibit lung cancer cell proliferation by blocking G1/S
transition through the inhibition of cyclin E and Skp2 protein
expression.
Materials and methods
Reagents
D-glucosamine hydrochloride was purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Analysis of cyclin E and Skp2 protein
expression and their phosphorylation levels by western
blotting
Cells at 80% confluency were incubated with 2 mM
thymidine for 16 h. The medium was then changed to normal medium
and the cells were cultured for 9 h, followed by an additional
culture with 2 mM thymidine for 16 h. Various concentrations of
glucosamine (0.1–5 mM) were used in this study. To detect the
phosphorylation levels of cyclin E, thymidine-synchronized cells
were released into normal medium with or without 1 mM glucosamine
and cultured for 1.5 h. The cells were recovered in 250 μl of lysis
buffer (10 mM Tris-HCl, pH 7.4, 1% Triton X-100, 100 mM NaCl, 0.5%
sodium deoxycholate, 1 mM EDTA, 1 mM EGTA and 1 mM di-isopropyl
fluorophosphate) containing 1:25 v/v Complete™ (Roche Diagnostics,
Mannheim, Germany). The cells were not synchronized for the
detection of Skp2, since Skp2 is expressed throughout the cell
cycle. Cell lysates were placed on ice for 30 min and centrifuged
at 14,000 × g for 10 min. The supernatants were recovered, and
samples (10 μg protein/lane) were subjected to 10% sodium dodecyl
sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Separated
proteins were transferred to polyvinylidene difluoride (PVDF)
membranes (Millipore Corporation, Bedford, MA, USA). The membranes
were blocked with 5% skimmed milk and probed with rabbit anti-human
cyclin E, p27Kip1 polyclonal antibody or rabbit
anti-human phospho-cyclin E (Thr62), phospho-p27Kip1
(Thr 187) polyclonal antibodies, respectively (Cell Signaling
Technology, Beverly, MA, USA). After washing with
phosphate-buffered saline (PBS) with 0.05% Tween 20, the membranes
were further probed with horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG (Chemicon International, Temecula, CA, USA),
and the target bands were finally detected with a the
chemiluminescent substrate luminol (Pierce, Rockford, IL, USA). The
detected bands were quantified using a GIS-1000 image analyzer
(Fujitsu, Tokyo, Japan). The antibodies were then stripped from the
membranes using Restore Western Blot stripping buffer (Pierce).
GAPDH in each sample was detected using mouse anti-GAPDH monoclonal
antibody (MAB374; Chemicon International) and HRP-conjugated goat
anti-mouse IgG/IgM (Chemicon International). The protein content
was determined with a BCA protein assay kit (Pierce).
MTT assay
Cell viability was assayed using the CellTiter
96® aqueous one solution cell proliferation assay
(Promega, Medison, WI, USA) according to the manufacturer’s
instructions. Briefly, the cells were detached with trypsin and
collected in 10 ml of complete medium. Cells (2×103) in
a 90-μl volume were aliquoted in triplicate into each well of a
96-well plate. After 18 h, 10 μl of medium containing 0.1–5 mM
concentrations of glucosamine was added to each well and incubated
for 24 h. Then, 20 μl of a mixture containing phenazine
methosulfate in
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(-4-sulfophenyl)-2H-tetrazolium,
inner salt (MTS) and phenazine ethosulfate was added to each well.
The plates were incubated at 37ºC in 5% CO2 for 80 min.
Formazan product was detected by measuring the absorbance at 490
nm. Percent growth inhibition (% GI) was calculated according to
the following formula: 100 × (T-T0)/(C-T0), where T is the
absorbance of the test well after 72 h of exposure to test
chemicals, T0 is the absorbance at time zero and C is the control
absorbance.
Flow cytometry
For flow cytometric analysis of the DNA content,
~106 cells were fixed in 80% ethanol at −20ºC for 24 h.
Ethanol-fixed cells were stained with propidium iodide (PI)
staining solution (20 μg/ml PI, 0.1 mg/ml RNase A, 0.1% NP-40 and
0.1% trisodium citrate) for 30 min, and then analyzed using a FACS
analyzer (BD Labware, Franklin Lakes, NJ, USA).
Results
Glucosamine inhibits lung cancer cell
proliferation
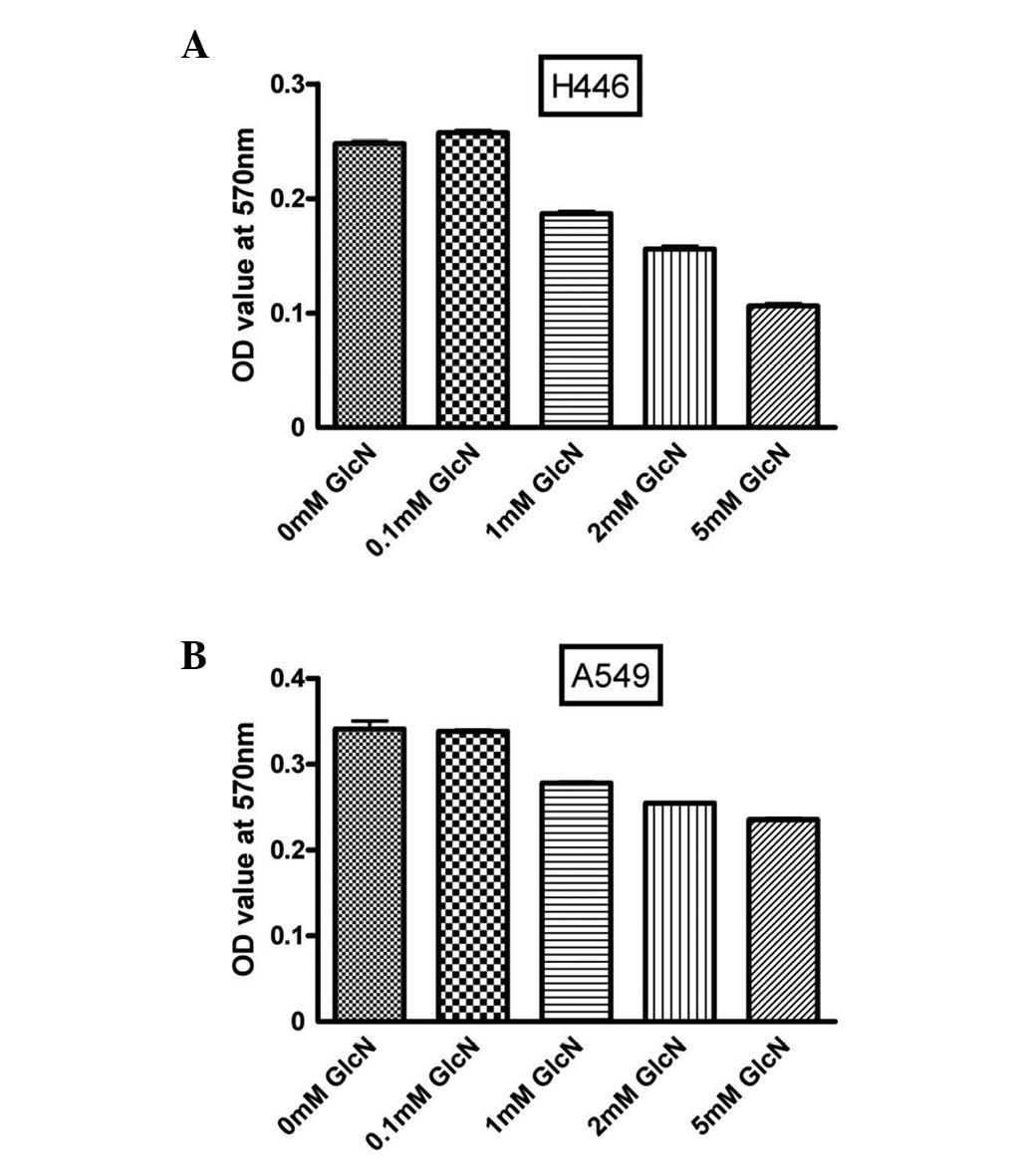
To evaluate the effect of glucosamine on lung cancer
cell proliferation, A549 and H446 cells were incubated with various
concentrations of glucosamine for 36 h. As shown in Fig. 1, glucosamine inhibited cell
proliferation in a dose-dependent manner.
Glucosamine treatment arrests cell cycle
in the G1 phase
Cell cycle progression is disordered in cancer
cells, and the inhibition of cell proliferation is important in
cancer therapy (16). Therefore,
we detected the effect of glucosamine on cell cycle progression.
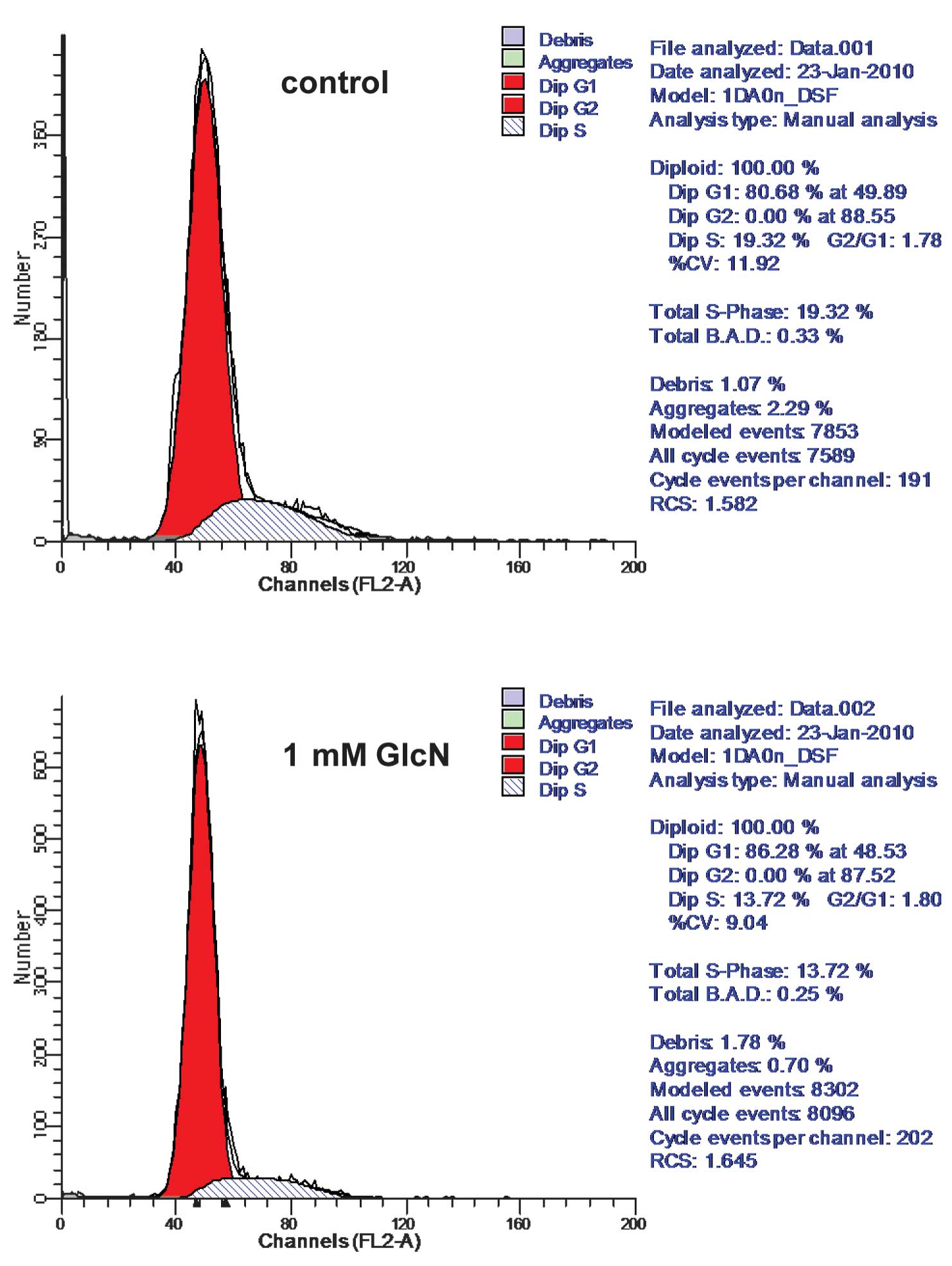
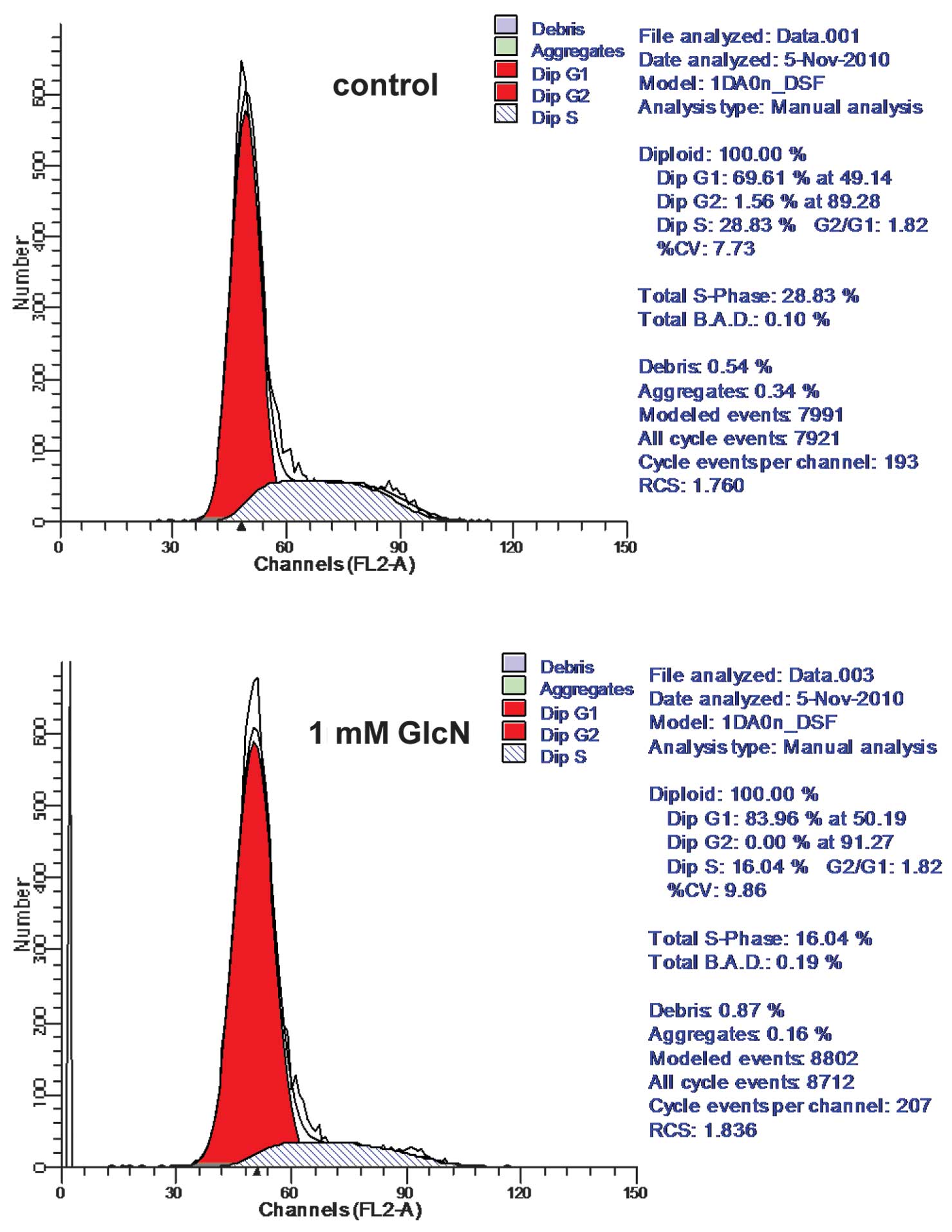
Glucosamine treatment altered lung cancer cell cycle progression
(Figs. 2 and 3). More specifically, the number of
thymidine-synchronized H446 cells arresting in the G1 phase was
increased by ~6%, and the number of normally cultured H446 cells in
the G1 phase was increased by ~14% (Fig. 2). Similar results were found in
A549 cells (Fig. 3).
Cyclin E and Skp2 are overexpressed in
lung cancer cells
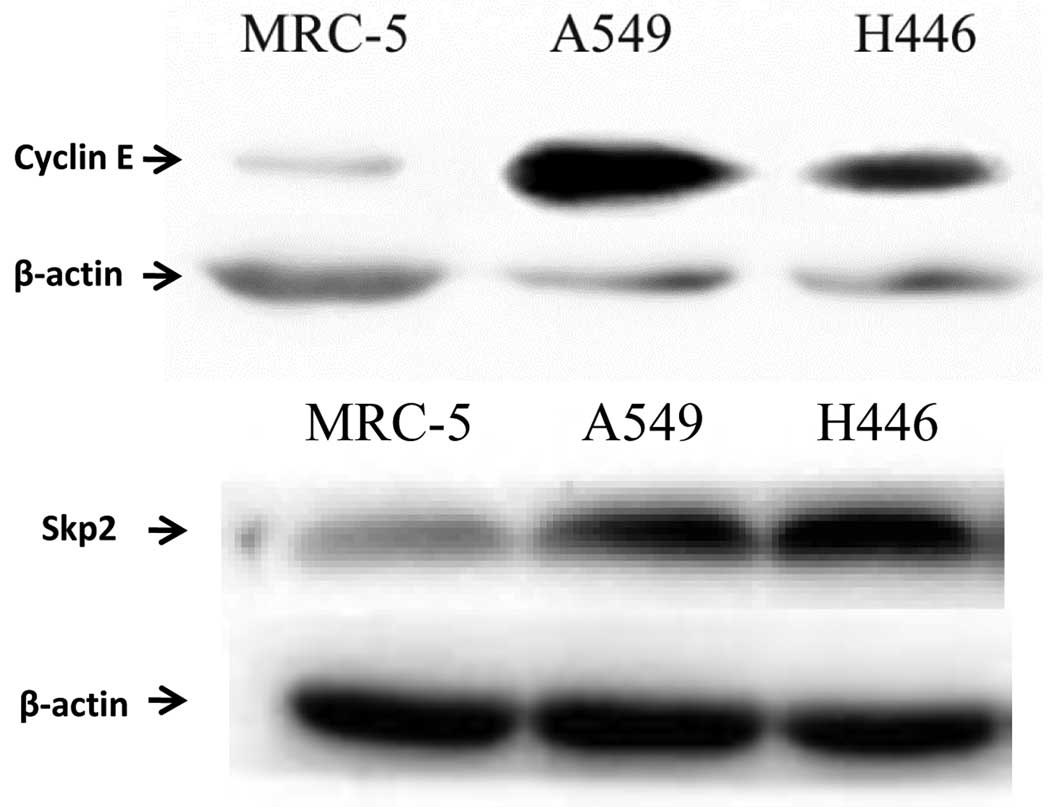
The accumulation and degradation of cyclin E and
Skp2 are important for G1/S transition. Previous research has shown
that cyclin E and Skp2 are overexpressed in various cancer cells
and tissues (12,16). Therefore, cyclin E and Skp2 protein
levels in the lung cancer cell lines A549 and H446 were
investigated. As expected, cyclin E and Skp2 were overexpressed in
these lung cancer cell lines compared with MRC-5 cells (human
embryonic lung cells), indicating an association between cyclin
E/Skp2 levels and lung cancer (Fig.
4).
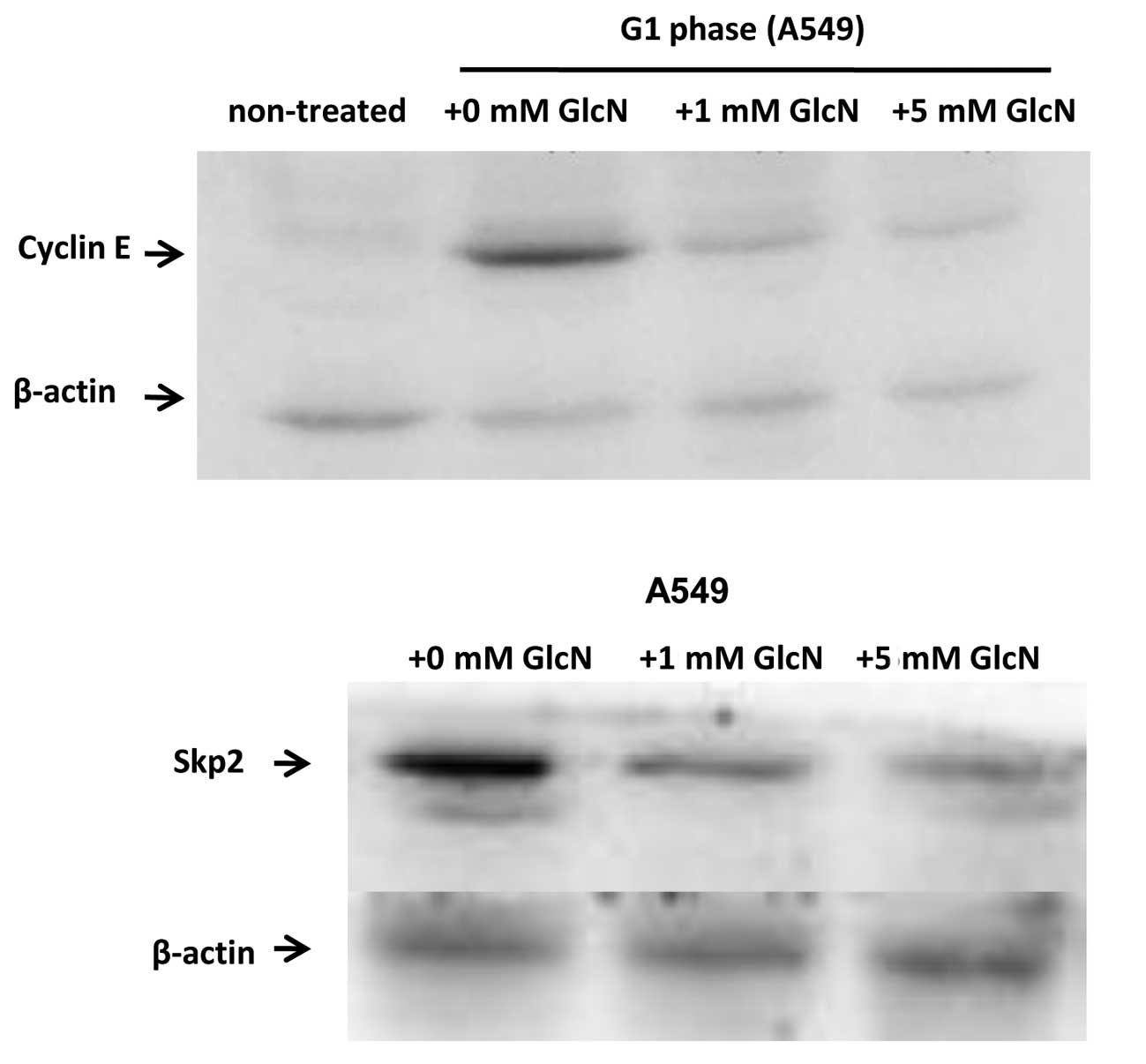
Glucosamine inhibits Skp2 and cyclin E
expression
The effect of glucosamine on cyclin E and Skp2
expression in the lung cancer cell lines A549 and H446 was
evaluated. As shown in Fig. 5,
glucosamine inhibited cyclin E and Skp2 expression in a
dose-dependent manner, indicating a potential inhibitory effect of
glucosamine on lung cancer cell proliferation. The same inhibitory
effect was observed in H446 cells (data not shown).
Glucosamine inhibits cyclin E degradation
via cyclin E phosphorylation
Cyclin E is important in G1/S transition.
Glucosamine was found to inhibit cyclin E Thr62 phosphorylation,
and to simultaneously reduce cyclin E degradation. These results
indicate that glucosamine inhibits G1/S transition through
affecting cyclin E degradation (Fig.
6).
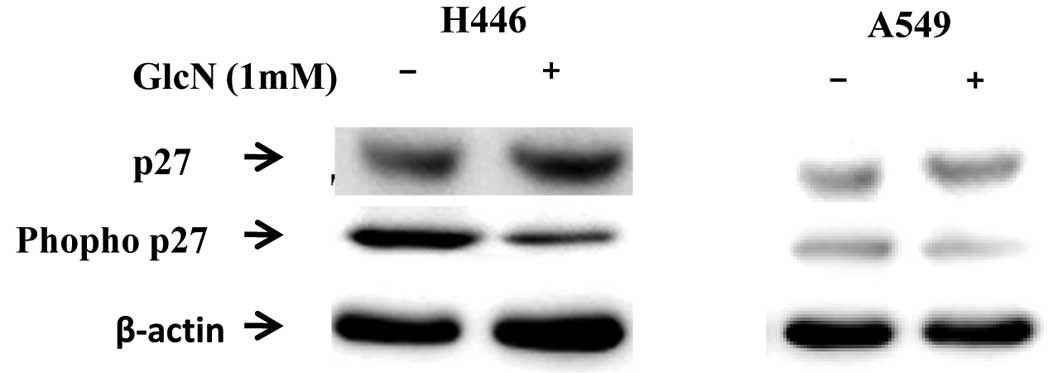
Glucosamine inhibits p27Kip1
phosphorylation at Thr182
p27Kip1 is one of the target proteins of
Skp2 ubiquitylation, and p27Kip1 phosphorylation at
Thr182 is essential for the recognition of p27Kip1.
p27Kip1 degradation promotes cell cycle progression into
the S phase (16). Therefore, we
detected the effect of glucosamine on p27Kip1 expression
and specific site phosphorylation. Glucosamine was found to inhibit
p27Kip1 phosphorylation and the p27Kip1
protein level was found to be upregulated in A549 and H446 cells
(Fig. 7).
Discussion
Lung cancer is the leading cause of cancer-related
mortality worldwide. Despite the fact that early-stage disease may
be cured with surgery, recurrence rates remain high. Cell cycle
abnormalities are important in tumorigenesis. In particular,
shortening of the G1 phase promotes S phase entry in the cell cycle
(12). Cyclin E and Skp2 are key
factors controlling G1/S phase transition, and the overexpression
of cyclin E and Skp2 induces cell proliferation (13). In the present study, we initially
investigated the association of cyclin E and Skp2 levels with cell
proliferation in the A549 and H446 lung cancer cell lines. Our
results showed that cyclin E and Skp2 are overexpressed in these
cell lines, indicating that cyclin E and Skp2 overexpression is
associated with cell cycle abnormalities.
Glucosamine is a naturally occurring monosaccharide
with anti-inflammatory and antitumor effects (2–5). Our
results indicated that glucosamine inhibited lung cancer cell
proliferation in a dose-dependent manner. In the present study, we
investigated the antitumor effect of glucosamine and the underlying
mechanism that regulates the G1/S checkpoint of the cell cycle.
More specifically, cyclin E and Skp2 expression at the mRNA and
protein levels with or without glucosamine treatment were examined.
Furthermore, the suppressive effect of glucosamine on cyclin E and
Skp2 expression was investigated, and our data indicated an
inhibitory action of glucosamine against lung cancer cell
proliferation in vitro. Cyclin E is expressed
intermittently; it is accumulated in the late G1 phase and rapidly
degraded in the early S phase. Thus, interfering with cyclin E
degradation may affect cell cycle progression (17). The Skp2 gene is also periodically
expressed throughout the cell cycle. It is low in the G0 and mid-G1
phases, then the Skp2 level increases in late G1 and remains high
during the S phase (18,19). Both Skp2 mRNA and protein levels
are regulated during the cell cycle.
It is now recognized that the addition of
O-linked N-acetylglucosamine (O-GlcNAc) to target
proteins may modulate cellular functions, including nuclear
transport, transcription, cell signaling, apoptosis and cell shape
(20–22). In this context, it has been shown
that glucosamine inhibits ICAM-1 and MCP-1 expression by
interfering with p38-MAPK and nuclear factor (NF)-κB specific site
phosphorylation (4,5). The turnover of cyclin E is controlled
by SCFFbw7, and specific amino acid phosphorylation is
important for its degradation level (23,24).
Alanine point mutation studies have shown that Thr380 and Thr62
prevent cyclin E degradation through the SCFFbw7 pathway
(25). In the present study,
protein O-GlcNAc modification levels in lung cancer cell lines
incubated with glucosamine were investigated, and the results
obtained were consistent with expectations (data not shown).
Glucosamine was able to modulate protein phosphorylation by
O-GlcNAc modification of serine and threonine residues (26–28).
Thus, we observed the specific phosphorylation site associated with
cyclin E and p27Kip1 degradation in the presence or
absence of glucosamine. Data showed that glucosamine inhibited
cyclin E Thr62 and p27Kip1 Thr187 phosphorylation, and
simultaneously reduced degradation. These results indicated that
glucosamine inhibited G1/S cell cycle progression through affecting
cyclin E degradation. Results obtained from flow cytometric
analysis also demonstrated that glucosamine treatment increased the
number of cells arrested in the G1 phase.
In conclusion, glucosamine may inhibit lung cancer
cell proliferation by blocking cell cycle progression. The
expression levels of cyclin E and Skp2, two key G1/S phase
regulators, at a transcriptional (data not shown), translational
and post-translational level were found to be suppressed by
glucosamine. In conclusion, negative cell cycle control by cyclin E
and Skp2 may constitute one of the potential underlying mechanisms
via which glucosamine inhibits lung cancer cell proliferation.
Further studies are required for an in-depth evaluation of the
anticancer effects of glucosamine in vivo.
Acknowledgements
This study was supported by a grant from the
Department of Science and Technology of Liaoning Province
(20091105).
References
|
1
|
Crolle G and D’Este E: Glucosamine
sulphate for the management of arthrosis: a controlled clinical
investigation. Curr Med Res Opin. 7:104–109. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Meininger CJ, Kelly KA, Li H, Haynes TE
and Wu G: Glucosamine inhibits inducible nitric oxide synthesis.
Biochem Biophys Res Commun. 279:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hua J, Sakamoto K and Nagaoka I:
Inhibitory actions of glucosamine, a therapeutic agent for
osteoarthritis, on the functions of neutrophils. J Leukoc Biol.
71:632–640. 2002.PubMed/NCBI
|
|
4
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Glucosamine, a naturally occurring amino monosaccharide
modulates LL-37-induced endothelial cell activation. Int J Mol Med.
22:657–662. 2008.PubMed/NCBI
|
|
5
|
Ju Y, Hua J, Sakamoto K, Ogawa H and
Nagaoka I: Modulation of TNF-α-induced endothelial cell activation
by glucosamine, a naturally occurring amino monosaccharide. Int J
Mol Med. 22:809–815. 2008.
|
|
6
|
Brasky TM, Lampe JW, Slatore CG and White
E: Use of glucosamine and chondroitin and lung cancer risk in the
VITamins And Lifestyle (VITAL) cohort. Cancer Causes Control.
22:1333–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hwang MS and Baek WK: Glucosamine induces
autophagic cell death through the stimulation of ER stress in human
glioma cancer cells. Biochem Biophys Res Commun. 399:111–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Liang R, Huang GS, Piao Y, Zhang
YQ, Wang AQ, Dong BX, Feng JL, Yang GR and Guo Y: Glucosamine
sulfate-induced apoptosis in chronic myelogenous leukemia K562
cells is associated with translocation of cathepsin D and
downregulation of Bcl-xL. Apoptosis. 11:1851–1860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oh HJ, Lee JS, Song DK, Shin DH, Jang BC,
Suh SI, Park JW, Suh MH and Baek WK: D-glucosamine inhibits
proliferation of human cancer cells through inhibition of p70S6K.
Biochem Biophis Res Commun. 360:840–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Welcker M and Clurman BE: FBW7 ubiquitin
ligase: a tumour suppressor at the crossroads of cell division,
growth and differentiation. Nat Rev Cancer. 8:83–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nam EJ and Kim YT: Alteration of
cell-cycle regulation in epithelial ovarian cancer. Int J Gynecol
Cancer. 18:1169–1182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tammali R, Saxena A, Srivastava SK and
Ramana KV: Aldose reductase regulates vascular smooth muscle cell
proliferation by modulating G1/S phase transition of cell cycle.
Endocrinology. 151:2140–2150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mittendorf EA, Liu Y, Tucker SL, McKenzie
T, Qiao N, Akli S, Biernacka A, Liu Y, Meijer L, Keyomarsi K and
Hunt KK: A novel interaction between HER2/neu and cyclin E in
breast cancer. Oncogene. 29:3896–3907. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kapur P, Lotan Y, King E, Kabbani W, Mitra
AP, Mosbah A, Abol-Enein H, Ghoneim M and Youssef RF: Primary
adenocarcinoma of the urinary bladder: value of cell cycle
biomarkers. Am J Clin Pathol. 135:822–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsieh HY, Shieh JJ, Chen CJ, Pan MY, Yang
SY, Lin SC, Chang JS, Lee AY and Chang CC: Prodigiosin
down-regulates SKP2 to induce p27(KIP1) stabilization and
antiproliferation in human lung adenocarcinoma cells. Br J
Pharmacol. 166:2095–2108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang G, Chan CH, Gao Y and Lin HK: Novel
roles of Skp2 E3 ligase in cellular senescence, cancer progression,
and metastasis. Chin J Cancer. 31:169–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lunn CL, Chrivia JC and Baldassare JJ:
Activation of Cdk2/Cyclin E complexes is dependent on the origin of
replication licensing factor Cdc6 in mammalian cells. Cell Cycle.
9:4533–4541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei Z, Jiang X, Liu F, Qiao H, Zhou B,
Zhai B, Zhang L, Zhang X, Han L, Jiang H, Krissansen GW and Sun X:
Downregulation of Skp2 inhibits the growth and metastasis of
gastric cancer cells in vitro and in vivo. Tumour Biol. 34:181–192.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin E, Kim SH, Jeong HY, Jang JJ and Lee
K: Nuclear expression of S-phase kinase-associated protein 2
predicts poor prognosis of hepatocellular carcinoma. APMIS.
120:349–357. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guinez C, Filhoulaud G, Rayah-Benhamed F,
Marmier S, Dubuquoy C, Dentin R, Moldes M, Burnol AF, Yang X,
Lefebvre T, Girard J and Postic C: O-GlcNAcylation increases
ChREBP protein content and transcriptional activity in the liver.
Diabetes. 60:1399–1413. 2011. View Article : Google Scholar
|
|
21
|
Carrillo LD, Froemming JA and Mahal LK:
Targeted in vivo O-GlcNAc sensors reveal discrete
compartment-specific dynamics during signal transduction. J Biol
Chem. 286:6650–6658. 2011.PubMed/NCBI
|
|
22
|
Butt AM, Khan IB, Hussain M, Idress M, Lu
J and Tong Y: Role of post translational modifications and novel
crosstalk between phosphorylation and O-beta-GlcNAc
modifications in human claudin-1, -3 and -4. Mol Biol Rep.
39:1359–1369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schülein C, Eilers M and Popov N:
PI3K-dependent phosphorylation of Fbw7 modulates substrate
degradation and activity. FEBS Lett. 585:2151–2157. 2011.PubMed/NCBI
|
|
24
|
Lerner M, Lundgren J, Akhoondi S, Jahn A,
Ng HF, Akbari Moqadam FA, Oude Vrielink JA, Agami R, Den Boer ML,
Grandér D and Sangfelt O: MiRNA-27a controls FBW7/hCDC4-dependent
cyclin E degradation and cell cycle progression. Cell Cycle.
10:2172–2183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brandt Y, Mitchell T, Wu Y and Hartley RS:
Developmental downregulation of Xenopus cyclin E is
phosphorylation and nuclear import dependent and is mediated by
ubiquitination. Dev Biol. 355:65–76. 2011.
|
|
26
|
Yang WH, Kim JE, Nam HW, Ju JW, Kim HS,
Kim YS and Cho JW: Modification of p53 with O-linked
N-acetylglucosamine regulates p53 activity and stability.
Nat Cell Biol. 8:1074–1083. 2006.
|
|
27
|
Smet-Nocca C, Broncel M, Wieruszeski JM,
Tokarski C, Hanoulle X, Leroy A, Landrieu I, Rolando C, Lippens G
and Hackenberger CP: Identification of O-GlcNAc sites within
peptides of the Tau protein and their impact on phosphorylation.
Mol Biosyst. 7:1420–1429. 2011.
|
|
28
|
Mishra S, Ande SR and Salter NW:
O-GlcNAc modification: why so intimately associated with
phosphorylation? Cell Commun Signal. 9:12011. View Article : Google Scholar
|