Introduction
Gender differences in behavior and cognitive
performance involve cultural and biological factors. Numerous
parameters of brain function and structure differ between males and
females (1–3); however, the exact mechanisms involved
and how they affect each cognitive domain remain unclear. The
catechol-O-methyltransferase (COMT) enzyme has been implicated as a
potential biological candidate involved in this gender dimorphism
(4–6). To the best of our knowledge, there
are a limited number of studies on this matter, the results of
which are controversial.
Gender may impact cognitive function. Generally,
females outperform males in their verbal abilities, while males
outperform females in visuospatial tasks. Herlitz et
al(7) investigated memory
function and identified gender differences in the episodic memory
that were in favor of females. Among individuals aged ≥85 years,
females have demonstrated superior scores in cognitive speed and
memory tasks, regardless of their often lower level of formal
education (8). Halari et
al(9) investigated whether
sexually dimorphic cognitive performance in males and females was
associated with sex hormones. Significant gender differences were
observed, favoring males in the spatial and inhibition tasks, and
favoring females in the verbal task (category fluency). However, no
significant correlation was demonstrated between gender hormones
and cognitive performance. van Hooren et al(10) analyzed the effects of age,
education and gender on cognitive speed, verbal memory, executive
function and verbal fluency in healthy older adults. A marked,
age-related decrease in these tasks was identified. Education had a
substantial effect on cognitive function; participants with a
middle or high level of education demonstrated a superior
performance in the cognitive tests (10). Additionally, females outperformed
males in the verbal memory tasks (10).
The prefrontal cortex (PFC) is an important region
of the brain for cognition, and is strongly influenced by dopamine
(DA). COMT is regarded as an important regulator of PFC DA levels,
whereas the DA transporter (DAT) is not as widely found in the PFC
as it is in the striatum (11,12).
COMT is a key enzyme that is specifically involved in the metabolic
degradation of extraneuronal DA (13). With regard to gender differences,
one study identified a 17% increase in COMT enzyme activity in
healthy males compared with that in healthy females (11). However, certain studies have
demonstrated similar COMT levels and expression in both genders
(11,14,15),
while others have revealed higher levels in females (16). Males and females also appear to
differentially regulate the abundance of novel mRNA (17) or protein (18) isoforms of COMT, which may alter the
enzyme activity without affecting the total level of COMT
transcripts or proteins (4). These
results are concordant with earlier findings demonstrating a 30%
increase in the activity of the enzyme in males compared with that
in females (19). A similar
difference was described in studies using human erythrocytes
(20–22); however, one study was not
concordant (23).
Genetic studies have suggested that COMT enzyme
activity may also vary considerably according to COMT single
nucleotide polymorphisms (SNPs). The COMT gene SNP, rs4680, also
known as Val158Met, reduces the activity of the enzyme
in Met carriers (Met+ individuals) (24). This, in turn, lowers the enzyme
activity and presumably increases the PFC DA levels (24,25).
COMT exerts a significant regulatory effect on cognitive function
(26–29), which involves the well-established
effects of PFC DA levels on working memory and executive function
(11,30–32).
It has been suggested that the COMT rs4680 SNP may exert a direct
effect on cognition in schizophrenic patients and healthy controls
(26–29). The COMT SNP rs4680 Met allele has
been demonstrated to be correlated with higher PFC DA levels
(24,25), and with superior performance in
working memory, intelligence and executive function in numerous
studies (11,28,33–36).
However, a number of studies did not reveal similar cognitive
results (37–39). Furthermore, the COMT Met allele has
been demonstrated to modulate cognitive dysfunction across mood
episodes of bipolar disorder (40).
Results of previous COMT studies specifically
concerned with the effects of gender/genotype on cognition require
further clarification. Only a limited number of studies have
demonstrated a gender-by-genotype effect on cognition (4–6). Two
studies addressing the association between gender/genotype and
cognition have focused on children and elderly individuals
(5,6). Barnett et al(5) performed a range of cognitive tests in
>5000 children (age, 8–10 years), including tests for IQ,
attention span and working memory. Subjects were genotyped for the
COMT Val158Met SNP, and the Met allele was found to be
associated with improved function in several domains selectively
among males. O'Hara et al(6) evaluated 163 older healthy adults and
revealed that only Val/Val males performed superiorly in a test of
delayed verbal recall, while a worse performance was observed in
younger individuals.
Insufficient (hypodopaminergic) and excessive
(hyperdopaminergic) DA receptor 1 (D1) stimulation has been
demonstrated to impair PFC function in animal studies, resulting in
cognitive dysfunction (41–43);
however, the extension of these findings to healthy subjects
remains speculative. Therefore, PFC cognition is hypothesized to
depend on a specific level of DA in order to achieve optimal
cognitive function (31,32). Given the limited data available on
whether gender effects are associated with the COMT genotype
interaction with cognition, the aim of the present study was to
investigate this potential association in a homogeneous sample of
healthy young adults. Based on studies demonstrating lower COMT
activity in female COMT Met carriers, we hypothesized that females
carrying the Met allele would have a lower cognitive performance
due to altered PFC DA regulation.
Materials and methods
Subjects
Seventy-six healthy individuals (37 females and 39
males), aged 18–35 years (mean age, 23.2±3.26 years) with a mean
schooling duration of 14.1±2.32 years and a mean IQ of
115.49±12.25, were recruited at the University of São Paulo (São
Paulo, Brazil). Study participants were predominantly medical
students. None of the subjects had a past history of or were
currently suffering with a psychiatric disorder, according to the
evaluation conducted by psychiatrists using the Mini International
Neuropsychiatric Interview (MINI) (44). In addition, all subjects had no
family history (amongst first-degree relatives) of mood or
psychotic disorders, and had not used psychotropic substances or
indulged in substance abuse within the last three months. Only
females with a regular menstrual cycle, taking oral contraceptives,
were included.
Neurocognitive assessments
A range of neurocognitive assessments were designed
to assess the following domains: i) Attention, by the Wechsler
Adult Intelligence Scale III [WAIS-III, including the Digit Span
(DS) subtest] and the Trail Making Test, part A (TMT-A); ii)
memory, using the immediate and delayed Logical Memory subtests of
the Wechsler Memory Scale (WMS-LM1 and -LM2, respectively); iii)
verbal fluency, by the Controlled Oral Word Association Test (FAS);
and iv) executive function, assessed by the TMT-B (45–48).
Experienced clinical neuropsychologists performed the
neurocognitive assessments. Raw scores, corrected for demographic
factors, were used given the absence of standardized scores for the
Brazilian population.
Genotyping
DNA was extracted from the peripheral blood
according to the salting out procedure (49), and was then genotyped for COMT
rs4680 using quantitative (q)PCR allelic discrimination. PCR
amplification for rs4680 was performed in 5-μl reactions with 5 ng
template DNA, 1X TaqMan Universal Master mix (Applied Biosystems,
Inc., Foster City, CA, USA), each primer and probe assay, and
H2O. Thermal cycling consisted of initial denaturation
for 10 min at 95°C, followed by 40 cycles of denaturation at 95°C
for 15 sec, and annealing at 60°C for 1 min. The allele detection
process and allelic discrimination were performed for 1 min at 60°C
on a 7500 Real-Time PCR system (Applied Biosystems, Inc.). Quality
control of qPCR results was achieved by direct sequencing using an
ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Inc.).
Statistical analyses
Cognitive tests were stratified as a function of
COMT rs4680 genotype (Met/Met, Val/Met and Val/Val) and functional
allele [Met+ (Met/Met or Met/Val) or Met−
(Val/Val)]. The cognitive test results had a normal distribution,
and parametric tests were used to analyze the data. The
multivariate analysis of the covariance test was performed using
the cognitive test results as factors, and age, gender, education,
genotype and Met allele as covariates. COMT genotype and gender, as
well as COMT Met allele and gender, interactions were also
analyzed. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using the PASW statistical software, version 18.0 (SPSS Inc.,
Chicago, IL, USA). Correction for multiple comparisons was
performed using the Bonferroni test.
Ethics
The research ethics board of the Clinics Hospital of
the University of São Paulo approved this study. Written informed
consent was obtained from all subjects.
Results
No statistically significant differences in the
sociodemographic data (age, gender and educational level) were
observed among the COMT genotypes (Table I). Moreover, there were no
differences in gender, age, education or IQ (Table I). The COMT genotype distributions
in the experimental samples of males and females were identified to
be in accordance with the Hardy-Weinberg equilibrium
(χ2=0.65), thus indicating representative samples. The
allelic frequency of rs4680 was 51.3% for the Met allele and 48.7%
for the Val allele.
 | Table ISociodemographic and clinical
variables by COMT genotype. |
Table I
Sociodemographic and clinical
variables by COMT genotype.
| Variable | Met+
(n=57) | Met−
(n=19) | P-valuea |
|---|
| Age (years, mean ±
SD) | 23.37±3.40 | 23.53±3.20 | 0.85b |
| Gender
(male/female) | 26/31 | 13/6 | 0.11c |
| Years of education
(mean ± SD) | 14.19±2.31 | 13.82±2.43 | 0.99b |
A multivariate general linear test model, using age,
educational level, gender, COMT rs4680 genotype and the interaction
between COMT genotype and gender as covariates, was
implemented.
Age negatively influenced cognitive performance on
the WAIS-DS-FW (P=0.02, partial η2, 7.66%), TMT-A
(P<0.001, partial η2, 17.4%) and TMT-B (P=0.02,
partial η2, 7.57%) (Table
II). Gender influenced WAIS-DS (P=0.01, partial η2,
9.1%) (Table II). Females
performed better than males in both tests. The COMT genotype had no
influence on cognitive performance after Bonferroni correction.
Gender and COMT genotype interaction influenced the FAS total score
(P=0.03, partial η2, 6.67%) and FAS letter S (P=0.01,
partial η2, 8.0%) (Table
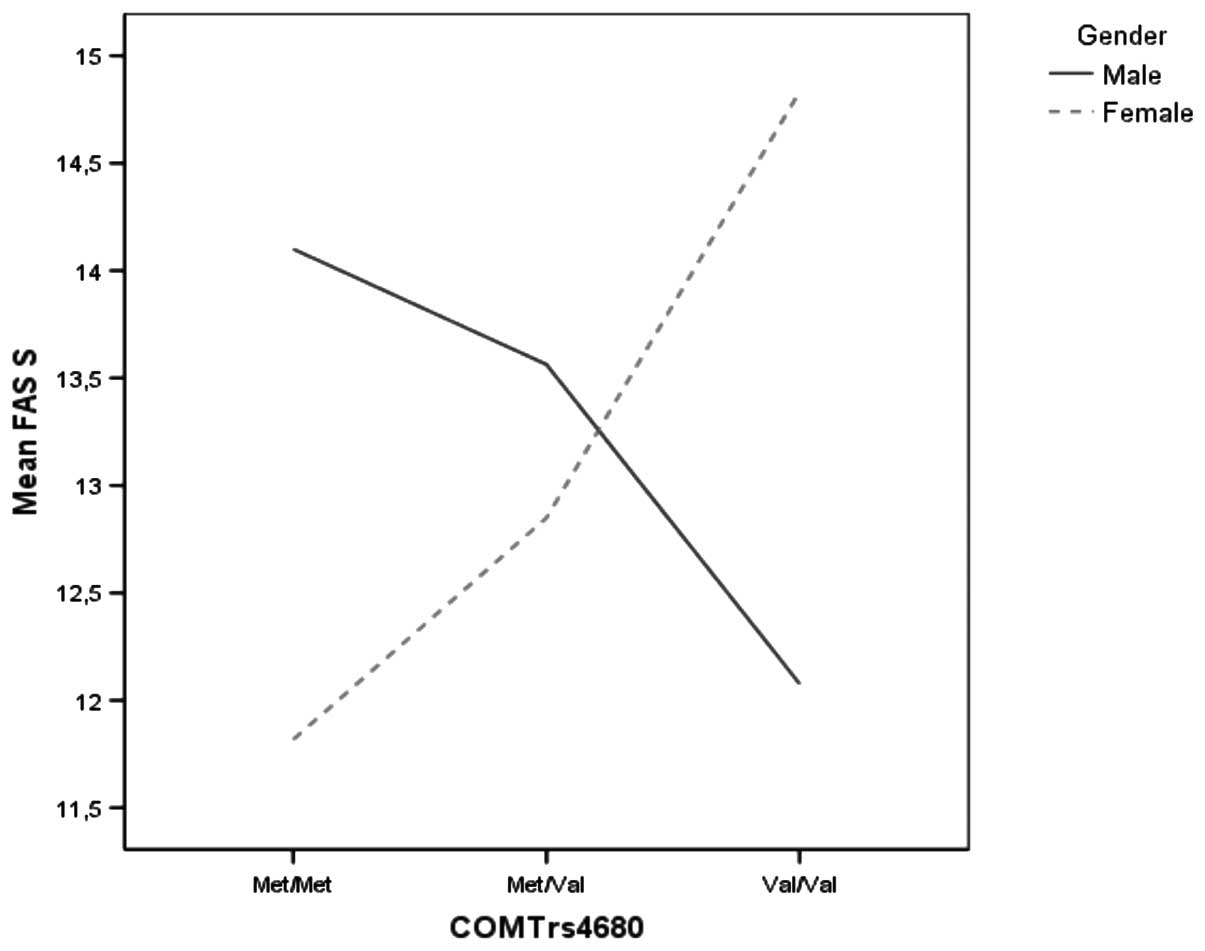
III). Among males, Met homozygotes had the highest scores, Met
heterozygotes had intermediate performance while Val homozygotes
had the lowest scores. Among females, Val homozygotes had the
highest scores, while Met homozygotes had the lowest scores
(Fig 1).
 | Table IIMultivariate analysis of cognitive
tests, with age, education, gender, COMT allele Met and gender*COMT
allele Met interaction as the cofactors. |
Table II
Multivariate analysis of cognitive
tests, with age, education, gender, COMT allele Met and gender*COMT
allele Met interaction as the cofactors.
| Source | Dependent
variable | B | F | P-value | Partial
η2 (%) | Observed power (a)
(%) |
|---|
| Age |
WAIS-DS-FW | −0.19 | 5.638 | 0.020 | 7.66 | 64.84 |
| WAIS-DS-BK | −0.09 | 0.971 | 0.328 | 1.41 | 16.3 |
| WAIS-DS | −0.23 | 2.475 | 0.120 | 3.51 | 34.16 |
| WMS-LM1 | −0.10 | 0.179 | 0.673 | 0.26 | 7.02 |
| WMS-LM2 | −0.11 | 0.222 | 0.639 | 0.33 | 7.51 |
| RCFT-COPY | −0.02 | 0.198 | 0.658 | 0.29 | 7.24 |
| RCFT-RECALL | −0.26 | 1.899 | 0.173 | 2.72 | 27.42 |
| TMT-A | 0.98 | 14.393 | 0.000 | 17.47 | 96.24 |
| TMT-B | 2.50 | 5.565 | 0.021 | 7.57 | 64.27 |
| FAS TOTAL | −0.38 | 1.983 | 0.164 | 2.83 | 28.42 |
| FAS F | −0.10 | 0.567 | 0.454 | 0.83 | 11.51 |
| FAS A | −0.16 | 1.805 | 0.184 | 2.59 | 26.31 |
| FAS S | −0.17 | 2.583 | 0.113 | 3.66 | 35.38 |
| Education | WAIS-DS-FW | 0.13 | 1.219 | 0.273 | 1.76 | 19.29 |
| WAIS-DS-BK | 0.14 | 1.214 | 0.274 | 1.75 | 19.24 |
| WAIS-DS | 0.26 | 1.605 | 0.209 | 2.31 | 23.93 |
| WMS-LM1 | −0.17 | 0.281 | 0.598 | 0.41 | 8.18 |
| WMS-LM2 | −0.23 | 0.498 | 0.483 | 0.73 | 10.70 |
| RCFT-COPY | 0.06 | 1.123 | 0.293 | 1.62 | 18.14 |
|
RCFT-RECALL | 0.56 | 4.367 | 0.040 | 6.03 | 53.99 |
| TMT-A | −0.28 | 0.590 | 0.445 | 0.86 | 11.79 |
| TMT-B | 2.03 | 1.862 | 0.177 | 2.67 | 26.99 |
| FAS TOTAL | 0.26 | 0.491 | 0.486 | 0.72 | 10.63 |
| FAS F | 0.10 | 0.323 | 0.572 | 0.47 | 8.67 |
| FAS A | 0.13 | 0.590 | 0.445 | 0.86 | 11.79 |
| FAS S | −0.03 | 0.055 | 0.816 | 0.08 | 5.61 |
| Gender | WAIS-DS-FW | 0.10 | 0.122 | 0.727 | 0.18 | 6.37 |
| WAIS-DS-BK | 0.78 | 1.415 | 0.238 | 2.04 | 21.64 |
| WAIS-DS | 0.98 | 6.885 | 0.011 | 9.19 | 73.46 |
| WMS-LM1 | −2.00 | 0.468 | 0.496 | 0.68 | 10.35 |
| WMS-LM2 | −3.78 | 0.318 | 0.575 | 0.47 | 8.61 |
|
RCFT-COPY | 0.25 | 4.328 | 0.041 | 5.98 | 53.63 |
| RCFT-RECALL | −0.84 | 0.965 | 0.329 | 1.40 | 16.25 |
| TMT-A | 1.42 | 1.336 | 0.252 | 1.93 | 20.70 |
| TMT-B | −0.34 | 3.315 | 0.073 | 4.65 | 43.45 |
| FAS TOTAL | 2.77 | 0.729 | 0.396 | 1.06 | 13.43 |
| FAS F | 0.19 | 0.639 | 0.427 | 0.93 | 12.36 |
| FAS A | 0.99 | 0.166 | 0.685 | 0.24 | 6.86 |
| FAS S | 1.11 | 1.092 | 0.300 | 1.58 | 17.76 |
| COMT allele
Met | WAIS-DS-FW | −0.50 | 0.990 | 0.323 | 1.44 | 16.55 |
| WAIS-DS-BK | 0.14 | 0.021 | 0.884 | 0.03 | 5.24 |
| WAIS-DS | 3.62 | 2.076 | 0.154 | 2.96 | 29.51 |
| WMS-LM1 | 7.35 | 5.424 | 0.023 | 7.39 | 63.16 |
| WMS-LM2 | 5.74 | 2.799 | 0.099 | 3.95 | 37.82 |
| RCFT-COPY | 0.46 | 0.018 | 0.893 | 0.03 | 5.20 |
| RCFT-RECALL | 2.66 | 0.068 | 0.796 | 0.10 | 5.76 |
| TMT-A | −6.14 | 1.511 | 0.223 | 2.17 | 22.79 |
| TMT-B | −32.86 | 5.121 | 0.027 | 7.00 | 60.68 |
| FAS TOTAL | −4.78 | 0.014 | 0.907 | 0.02 | 5.15 |
| FAS F | −1.20 | 0.052 | 0.820 | 0.08 | 5.58 |
| FAS A | −0.95 | 0.200 | 0.656 | 0.29 | 7.25 |
| FAS S | −2.31 | 0.201 | 0.655 | 0.29 | 7.27 |
| Gender*COMT
interaction | WAIS-DS-FW | −0.23 | 0.035 | 0.852 | 0.05 | 5.39 |
| WAIS-DS-BK | −0.08 | 0.004 | 0.953 | 0.01 | 5.04 |
| WAIS-DS | −4.00 | 3.129 | 0.081 | 4.40 | 41.45 |
| WMS-LM1 | −6.44 | 3.283 | 0.074 | 4.61 | 43.12 |
| WMS-LM2 | −5.56 | 2.448 | 0.122 | 3.48 | 33.84 |
| RCFT-COPY | −0.84 | 1.722 | 0.194 | 2.47 | 25.32 |
| RCFT-RECALL | −4.56 | 2.429 | 0.124 | 3.45 | 33.63 |
| TMT-A | 7.43 | 3.527 | 0.065 | 4.93 | 45.68 |
| TMT-B | 29.03 | 3.184 | 0.079 | 4.47 | 42.05 |
| FAS
TOTAL | 9.07 | 4.862 | 0.031 | 6.67 | 58.46 |
| FAS F | 1.96 | 0.986 | 0.324 | 1.43 | 16.50 |
| FAS A | 2.74 | 2.167 | 0.146 | 3.09 | 30.58 |
| FAS S | 3.90 | 5.932 | 0.017 | 8.02 | 67.04 |
 | Table IIIMultivariate analysis of cognitive
tests, with age, education, gender, COMT rs4680 genotype and
gender*COMT rs4680 genotype interaction as the cofactors. |
Table III
Multivariate analysis of cognitive
tests, with age, education, gender, COMT rs4680 genotype and
gender*COMT rs4680 genotype interaction as the cofactors.
| Source | Dependent
variable | F | P-value | Partial
η2 (%) | Observed power (a)
(%) |
|---|
| COMT rs4680
genotype | WAIS-DS-FW | 1.043 | 0.358 | 3.06 | 22.50 |
| WAIS-DS-BK | 0.251 | 0.779 | 0.75 | 8.79 |
| WAIS-DS | 0.965 | 0.386 | 2.84 | 21.07 |
| WMS-LM1 | 2.776 | 0.070 | 7.76 | 52.91 |
| WMS-LM2 | 1.322 | 0.273 | 3.85 | 27.62 |
| RCFT-COPY | 0.952 | 0.391 | 2.80 | 20.85 |
| RCFT-RECALL | 1.882 | 0.160 | 5.39 | 37.80 |
| TMT-A | 0.699 | 0.501 | 2.07 | 16.31 |
| TMT-B | 2.579 | 0.083 | 7.25 | 49.76 |
| FAS TOTAL | 0.272 | 0.763 | 0.82 | 9.13 |
| FAS F | 2.008 | 0.142 | 5.73 | 40.04 |
| FAS A | 0.225 | 0.799 | 0.68 | 8.39 |
| FAS S | 0.123 | 0.885 | 0.37 | 6.81 |
| Gender*COMT rs4680
genotype | WAIS-DS-FW | 0.310 | 0.735 | 0.93 | 9.73 |
| WAIS-DS-BK | 0.614 | 0.544 | 1.83 | 14.84 |
| WAIS-DS | 2.105 | 0.130 | 6.00 | 41.76 |
| WMS-LM1 | 1.554 | 0.219 | 4.50 | 31.86 |
| WMS-LM2 | 1.436 | 0.245 | 4.17 | 29.70 |
| RCFT-COPY | 2.200 | 0.119 | 6.25 | 43.39 |
| RCFT-RECALL | 2.482 | 0.091 | 7.00 | 48.17 |
| TMT-A | 2.569 | 0.084 | 7.22 | 49.59 |
| TMT-B | 2.784 | 0.069 | 7.78 | 53.03 |
| FAS TOTAL | 3.021 | 0.056 | 8.39 | 56.65 |
| FAS F | 1.552 | 0.219 | 4.49 | 31.83 |
| FAS A | 1.141 | 0.326 | 3.34 | 24.29 |
| FAS S | 3.442 | 0.038 | 9.44 | 62.63 |
Discussion
To the best of our knowledge, this is the first
study to investigate the effect of the interaction between gender
and the COMT rs4680 Met allele on verbal fluency, with opposite
results identified for each gender. Female carriers of the Met
allele had a lower performance than males in a verbal fluency test.
In addition, subjects that were heterozygous (Val/Met) for COMT
rs4680 had an intermediate performance in the verbal fluency
test.
The results suggested a specific gender-dependent
effect of Met+ on verbal fluency, reinforcing the
hypothesis that there is a distinct optimal DA activity/level for
different components of cognition (28,50).
Furthermore, the COMT Met allele negatively influenced cognitive
performance (verbal fluency) in females compared with that in males
(Fig. 1). Given that females have
a lower COMT activity than males, per se(11,19),
one possible explanation of our findings is that females carrying
the low activity Met allele are likely to exceed the optimal PFC DA
levels, thus receiving no benefit from its excessively high
levels.
The effects of DA on neurocognition have been
previously described. Several studies have demonstrated that lower
doses of D1 agonists enhance working memory and attention span
(31,51), while higher levels of DA impair PFC
function (52). Low doses of
psychostimulants in hyperkinetic children have been demonstrated to
be associated with significant improvements in short-term memory,
whereas higher doses worsen cognitive performance (53). Similar dose-dependent effects were
also observed in healthy volunteers using dextroamphetamine, a drug
that potentiates dopaminergic activity (52). Studies on COMT functional SNPs and
the differential effects of D1 and D2 receptor binding have also
demonstrated cognitive decline to be associated with COMT activity.
Healthy subjects carrying the Met+ allele (rs4680)
exhibited lower phasic and higher subcortical tonic DA
transmission, which was proposed to be associated with an increase
in central DA levels (28,54).
COMT enzyme activity appears to have a
gender-specific effect; molecular postmortem studies have revealed
that COMT enzyme activity in the PFC was 17% higher in males
compared with that in females (11). These results are concordant with
earlier findings demonstrating a 30% higher enzyme activity in
males compared with females (19).
A similar difference was described in studies using human
erythrocytes (20–22); however, one study did not find
differences between genders (23).
Notably, 17-b-estradiol (E2) administration decreased COMT activity
in the rat liver (55,56). Similarly, Xie et al(1999)
demonstrated that there are two estrogen response elements in the
COMT promoter, and that, at physiological concentrations, E2
inhibited COMT mRNA expression in cells expressing estrogen
receptors, but not in cells that did not express these receptors.
An estrogen-mediated decrease in COMT mRNA was also accompanied by
a proportional decrease in COMT immunoreactivity and activity
(55,56). In another study, the PFC DA levels
were affected in male, but not female, COMT knockout mice (13). One possible explanation for this
gender-distinct function is the bidirectional association between
COMT and estrogen-related compounds, whereby COMT activity varies
inversely according to estrogen levels (29). Moreover, estrogen levels affect the
striatal dopaminergic system (57,58),
and affect cognition (41–43,59,60).
Overall, these findings may aid in the clarification of the role of
gender-specific effects on COMT-modulated cognition.
Studies investigating the cognitive effects of DA in
the PFC have mainly focused on the D1 receptor, the predominant
type of receptor in the PFC. Hypodopaminergic and hyperdopaminergic
D1 receptor stimulation have been demonstrated to impair PFC
function (31,32,41–43),
resulting in cognitive dysfunction. Therefore, PFC cognition may
depend on an optimal level of DA to achieve normal function
(31,32,51,52).
These kinetics have been described by an inverted-U response
function model in pharmacological studies (31,51,52).
In this model, the effect of amphetamine and other drugs on
cognition is described as an inverted-U shape, in which the peak is
the threshold for maximum cognitive performance, with subsequent
decline thereafter. We propose that the inverted-U shape model,
which states that an optimal level of DA is required to benefit
cognitive function, represents a plausible explanation as to why
female Met allele carriers exhibit a worse performance in verbal
fluency tests. The basis for this explanation is that females have
lower COMT activity per se, which may be due to the effect
of estrogen on this enzyme (29).
Consequently, at least theoretically, male and female
Met+ subjects have different baseline DA levels.
The FAS is also considered to be a test of executive
functions, including cognitive organization, initiation,
maintenance of effort and the ability to conduct a non-routine
search for words based on a specific first letter, rather than the
lexical definition (61–63). This interpretation is consistent
with studies demonstrating a worse performance in individuals with
frontal lobe lesions (63,64), and sensitivity to cognitive
dysfunction in disorders that affect executive functions (65). Effects of demographic variables are
important to consider when interpreting FAS results. Age effects
have not been identified in numerous studies (64,66,67);
however, a number of studies have demonstrated modest age effects,
with higher age predicting a worse performance (68). A higher level of education has been
associated with improved FAS performance in several studies
(64,67,69).
In the present study, neither age nor education influenced FAS.
However, with regard to gender, a superior FAS performance was
observed in females. Previous studies have demonstrated
controversial results; a number of studies identified superior FAS
performance in females compared with males (66,70),
while other studies did not observe a difference between the two
genders (69,71).
Limitations of this study included the absence of
information with regard to the menstrual cycle of participants.
Furthermore, the use of oral contraceptives may have affected COMT
activity.
To the best of our knowledge, this is the first
study to identify a gender-specific effect of COMT SNP rs4680 on
verbal fluency in young healthy subjects, with opposite effects on
performance in each gender. The presence of the Met allele in
female subjects was associated with worse verbal fluency; while in
males, it was correlated with an improvement in this particular
cognitive function. Our results suggested that DA activity affects
cognitive function in different ways, according to gender, most
likely due to COMT gender differences.
Acknowledgements
The authors would like to thank the Institute of
Psychiatry at the University of São Paulo, particularly the members
of the Mood Disorders Unit (GRUDA) and the Laboratory of
Neuroscience (LIM27), for their dedication and hard work, as well
as the volunteers, for their collaboration. The São Paulo Research
Foundation [Fundo de Apoio a Pesquisa do Estado de São Paulo
(FAPESP), 2010/06230-0)] and the Association Beneficente Alzira
Denise Hertzog Da Silva (ABADHS), provided financial support for
this study.
References
|
1
|
Murphy DG, DeCarli C, McIntosh AR, Daly E,
Mentis MJ, Pietrini P, et al: Sex differences in human brain
morphometry and metabolism: an in vivo quantitative magnetic
resonance imaging and positron emission tomography study on the
effect of aging. Arch Gen Psychiatry. 53:585–594. 1996. View Article : Google Scholar
|
|
2
|
Goldstein JM, Seidman LJ, Horton NJ,
Makris N, Kennedy DN, Caviness VS Jr, et al: Normal sexual
dimorphism of the adult human brain assessed by in vivo magnetic
resonance imaging. Cereb Cortex. 11:490–497. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
De Vries GJ: Minireview: Sex differences
in adult and developing brains: compensation, compensation,
compensation. Endocrinology. 145:1063–1068. 2004.PubMed/NCBI
|
|
4
|
Harrison PJ and Tunbridge EM:
Catechol-O-methyltransferase (COMT): a gene contributing to sex
differences in brain function, and to sexual dimorphism in the
predisposition to psychiatric disorders. Neuropsychopharmacology.
33:3037–3045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barnett JH, Heron J, Ring SM, Golding J,
Goldman D, Xu K and Jones PB: Gender-specific effects of the
catechol-O-methyltransferase Val108/158Met polymorphism on
cognitive function in children. Am J Psychiatry. 164:142–149.
2007.PubMed/NCBI
|
|
6
|
O'Hara R, Miller E, Liao CP, Way N, Lin X
and Hallmayer J: COMT genotype, gender and cognition in
community-dwelling, older adults. Neurosci Lett. 409:205–209. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herlitz A, Nilsson LG and Bäckman L:
Gender differences in episodic memory. Mem Cognit. 25:801–811.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Exel E, Gussekloo J, de Craen AJ,
Bootsma-van der Wiel A, Houx P, Knook DL and Westendorp RG:
Cognitive function in the oldest old: women perform better than
men. J Neurol Neurosurg Psychiatry. 71:29–32. 2001.
|
|
9
|
Halari R, Hines M, Kumari V, Mehrotra R,
Wheeler M, Ng V and Sharma T: Sex differences and individual
differences in cognitive performance and their relationship to
endogenous gonadal hormones and gonadotropins. Behav Neurosci.
119:104–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Hooren SA, Valentijn AM, Bosma H,
Ponds RW, van Boxtel MP and Jolles J: Cognitive functioning in
healthy older adults aged 64–81: a cohort study into the effects of
age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol
Cogn. 14:40–54. 2007.
|
|
11
|
Chen J, Lipska BK, Halim N, Ma QD,
Matsumoto M, Melhem S, et al: Functional analysis of genetic
variation in catechol-O-methyltransferase (COMT): effects on mRNA,
protein, and enzyme activity in postmortem human brain. Am J Hum
Genet. 75:807–821. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wayment HK, Schenk JO and Sorg BA:
Characterization of extracellular dopamine clearance in the medial
prefrontal cortex: role of monoamine uptake and monoamine oxidase
inhibition. J Neurosci. 21:35–44. 2001.PubMed/NCBI
|
|
13
|
Gogos JA, Morgan M, Luine V, Santha M,
Ogawa S, Pfaff D and Karayioegou M:
Catechol-O-methyltransferase-deficient mice exhibit sexually
dimorphic changes in catecholamine levels and behavior. Proc Natl
Acad Sci USA. 95:9991–9996. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bray NJ, Buckland PR, Williams NM,
Williams HJ, Norton N, Owen MJ and O'Donovan MC: A haplotype
implicated in schizophrenia susceptibility is associated with
reduced COMT expression in human brain. Am J Hum Genet. 73:152–161.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tunbridge E, Burnet PW, Sodhi MS and
Harrison PJ: Catechol-o-methyltransferase (COMT) and proline
dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex
in schizophrenia, bipolar disorder, and major depression. Synapse.
51:112–118. 2004. View Article : Google Scholar
|
|
16
|
Dempster EL, Mill J, Craig IW and Collier
DA: The quantification of COMT mRNA in post mortem cerebellum
tissue: diagnosis, genotype, methylation and expression. BMC Med
Genet. 7:102006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tunbridge EM, Lane TA and Harrison PJ:
Expression of multiple catechol-o-methyltransferase (COMT) mRNA
variants in human brain. Am J Med Genet B Neuropsychiatr Genet.
144B:834–839. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tunbridge EM, Harrison PJ and Weinberger
DR: Catechol-o-methyltransferase, cognition, and psychosis:
Val158Met and beyond. Biol Psychiatry. 60:141–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boudíková B, Szumlanski C, Maidak B and
Weinshilboum R: Human liver catechol-O-methyltransferase
pharmacogenetics. Clin Pharmacol Ther. 48:381–389. 1990.
|
|
20
|
Fähndrich E, Coper H, Christ W, Helmchen
H, Müller-Oerlinghausen B and Pietzcker A: Erythrocyte
COMT-activity in patients with affective disorders. Acta Psychiatr
Scand. 61:427–437. 1980.
|
|
21
|
Floderus Y and Wetterberg L: The
inheritance of human erythrocyte catechol-O-methyltransferase
activity. Clin Genet. 19:392–395. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Philippu G, Hoo JJ, Milech U, Argarwall
DP, Schrappe O and Goedde HW: Catechol-O-methyltransferase of
erythrocytes in patients with endogenous psychoses. Psychiatry Res.
4:139–146. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fitzgerald GA, Hamilton CA, Jones DH and
Reid JL: Erythrocytes catechol-O-methyltransferase activity and
indices of sympathetic activity in man. Clin Sci (Lond).
58:423–425. 1980.PubMed/NCBI
|
|
24
|
Lachman HM, Papolos DF, Saito T, Yu YM,
Szumlanski CL and Weinshilboum RM: Human
catechol-O-methyltransferase pharmacogenetics: description of a
functional polymorphism and its potential application to
neuropsychiatric disorders. Pharmacogenetics. 6:243–250. 1996.
View Article : Google Scholar
|
|
25
|
Weinshilboum RM, Otterness DM and
Szumlanski CL: Methylation pharmacogenetics: catechol
O-methyltransferase, thiopurine methyltransferase, and histamine
N-methyltransferase. Annu Rev Pharmacol Toxicol. 39:19–52. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Egan MF, Goldberg TE, Kolachana BS,
Callicott JH, Mazzanti CM, Straub RE, et al: Effect of COMT
Val108/158 Met genotype on frontal lobe function and risk for
schizophrenia. Proc Natl Acad Sci USA. 98:6917–6922. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joober R, Gauthier J, Lal S, Bloom D,
Lalonde P, Rouleau G, et al: Catechol-O-methyltransferase
Val-108/158-Met gene variants associated with performance on the
Wisconsin Card Sorting Test. Arch Gen Psychiatry. 59:662–663. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bilder RM, Volavka J, Lachman HM and Grace
AA: The catechol-O-methyltransferase polymorphism: relations to the
tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes.
Neuropsychopharmacology. 29:1943–1961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Diamond A, Briand L, Fossella J and
Gehlbach L: Genetic and neurochemical modulation of prefrontal
cognitive functions in children. Am J Psychiatry. 161:125–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Meyer-Lindenberg A, Nichols T, Callicott
JH, Ding J, Kolachana B, Buckholtz J, et al: Impact of complex
genetic variation in COMT on human brain function. Mol Psychiatry.
11:867–877. 7972006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mehta MA, Owen AM, Sahakian BJ, Mavaddat
N, Pickard JD and Robbins TW: Methylphenidate enhances working
memory by modulating discrete frontal and parietal lobe regions in
the human brain. J Neurosci. 20:RC652000.PubMed/NCBI
|
|
32
|
Goldman-Rakic PS, Castner SA, Svensson TH,
Siever LJ and Williams GV: Targeting the dopamine D1 receptor in
schizophrenia: insights for cognitive dysfunction.
Psychopharmacology (Berl). 174:3–16. 2004.PubMed/NCBI
|
|
33
|
Caldú X, Vendrell P, Bartrés-Faz D,
Clemente I, Bargalló N, Jurado MA, et al: Impact of the COMT
Val108/158 Met and DAT genotypes on prefrontal function in healthy
subjects. Neuroimage. 37:1437–1444. 2007.PubMed/NCBI
|
|
34
|
Bruder GE, Keilp JG, Xu H, Shikhman M,
Schori E, Gorman JM and Gilliam TC: Catechol-O-methyltransferase
(COMT) genotypes and working memory: associations with differing
cognitive operations. Biol Psychiatry. 58:901–907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Frias CM, Annerbrink K, Westberg L,
Eriksson E, Adolfsson R and Nilsson LG: COMT gene polymorphism is
associated with declarative memory in adulthood and old age. Behav
Genet. 34:533–539. 2004.PubMed/NCBI
|
|
36
|
de Frias CM, Annerbrink K, Westberg L,
Eriksson E, Adolfsson R and Nilsson LG: Catechol
O-methyltransferase Val158Met polymorphism is associated with
cognitive performance in nondemented adults. J Cogn Neurosci.
17:1018–1025. 2005.PubMed/NCBI
|
|
37
|
Barnett JH, Scoriels L and Munafò MR:
Meta-analysis of the cognitive effects of the
catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol
Psychiatry. 64:137–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dennis NA, Need AC, LaBar KS,
Waters-Metenier S, Cirulli ET, Kragel J, et al: COMT val108/158 met
genotype affects neural but not cognitive processing in healthy
individuals. Cereb Cortex. 20:672–683. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Solís-Ortiz S, Pérez-Luque E,
Morado-Crespo L and Gutiérrez-Muñoz M: Executive functions and
selective attention are favored in middle-aged healthy women
carriers of the Val/Val genotype of the
catechol-o-methyltransferase gene: a behavioral genetic study.
Behav Brain Funct. 6:672010.PubMed/NCBI
|
|
40
|
Soeiro de Souza MG, Machado-Vieira R,
Soares Bio D, Do Prado CM and Moreno RA: COMT polymorphisms as
predictors of cognitive dysfunction during manic and mixed episodes
in bipolar I disorder. Bipolar Disord. 14:554–564. 2012.PubMed/NCBI
|
|
41
|
Zahrt J, Taylor JR, Mathew RG and Arnsten
AF: Supranormal stimulation of D1 dopamine receptors in the rodent
prefrontal cortex impairs spatial working memory performance. J
Neurosci. 17:8528–8535. 1997.PubMed/NCBI
|
|
42
|
Granon S, Passetti F, Thomas KL, Dalley
JW, Everitt BJ and Robbins TW: Enhanced and impaired attentional
performance after infusion of D1 dopaminergic receptor agents into
rat prefrontal cortex. J Neurosci. 20:1208–1215. 2000.PubMed/NCBI
|
|
43
|
Arnsten AFT and Li BM: Neurobiology of
executive functions: catecholamine influences on prefrontal
cortical functions. Biol Psychiatry. 57:1377–1384. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sheehan DV, Lecrubier Y, Sheehan KH,
Amorim P, Janavs J, Weiller E, et al: The Mini-International
Neuropsychiatric Interview (M.I.N.I.): the development and
validation of a structured diagnostic psychiatric interview for
DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20): 22–33; quiz
34–57. 1998.PubMed/NCBI
|
|
45
|
Strauss E, Sherman EMS and Spreen O: A
Compendium of Neuropsychological Tests: Administration, Norms and
Commentary. 3rd edition. Oxford University Press, Inc; New York,
NY: 2006
|
|
46
|
Wechsler D: Wechsler Abbreviated Scale of
Intelligence. The Psychological Corporation: Harcourt Brace and
Company; New York, NY: 1999
|
|
47
|
Wechsler D: Wechsler Adult Intelligence
Scale-Revised. The Psychological Corporation; San Antonio, TX:
1981
|
|
48
|
Lezak MD: Neuropsychological Assessment.
Oxford University Press, Inc; New York, NY: 2004
|
|
49
|
Laitinen J, Samarut J and Hölttä E: A
nontoxic and versatile protein salting-out method for isolation of
DNA. Biotechniques. 17:316318320–322. 1994.PubMed/NCBI
|
|
50
|
Clark L, Cools R and Robbins TW: The
neuropsychology of ventral prefrontal cortex: decision-making and
reversal learning. Brain Cogn. 55:41–53. 2004. View Article : Google Scholar
|
|
51
|
Kimberg DY, D'Esposito M and Farah MJ:
Effects of bromocriptine on human subjects depend on working memory
capacity. Neuroreport. 8:3581–3585. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mattay VS, Goldberg TE, Fera F, Hariri AR,
Tessitore A, Egan F, et al: Catechol O-methyltransferase val158-met
genotype and individual variation in the brain response to
amphetamine. Proc Natl Acad Sci USA. 100:6186–6191. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sprague RL and Sleator EK: Methylphenidate
in hyperkinetic children: differences in dose effects on learning
and social behavior. Science. 198:1274–1276. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cousins DA, Butts K and Young AH: The role
of dopamine in bipolar disorder. Bipolar Disord. 11:787–806. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang H, Xie T, Ramsden DB and Ho SL:
Human catechol-O-methyltransferase down-regulation by estradiol.
Neuropharmacology. 45:1011–1018. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cohn CK and Axelrod J: The effect of
estradiol on catechol-O-methyltransferase activity in rat liver.
Life Sci I. 10:1351–1354. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Lindamer LA, Lohr JB, Harris MJ and Jeste
DV: Gender, estrogen, and schizophrenia. Psychopharmacol Bull.
33:221–228. 1997.PubMed/NCBI
|
|
58
|
Halbreich U: Role of estrogen in
postmenopausal depression. Neurology. 48(Suppl 7): S16–S19. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Colzato LS, Hertsig G, van den Wildenberg
WP and Hommel B: Estrogen modulates inhibitory control in healthy
human females: evidence from the stop-signal paradigm.
Neuroscience. 167:709–715. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gasbarri A, Pompili A, d'Onofrio A,
Cifariello A, Tavares MC and Tomaz C: Working memory for emotional
facial expressions: role of the estrogen in young women.
Psychoneuroendocrinology. 33:964–972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Andrewes D: Neuropsychology From Theory to
Practice. Psychology Press; New York, NY: 2001
|
|
62
|
Devinsky O and D'Esposito M: Neurology of
Cognitive and Behavioral Disorders. Oxford University Press; New
York, NY: 2004
|
|
63
|
Darby D and Walsh KW: Walsh's
Neuropsychology: A Clinical Approach. 5th edition. Elsevier
Churchill Livingstone; Edinburgh: 2005
|
|
64
|
Ruff RM, Light RH, Parker SB and Levin HS:
Benton Controlled Oral Word Association Test: reliability and
updated norms. Arch Clin Neuropsychol. 11:329–338. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Henry JD and Beatty WW: Verbal fluency
deficits in multiple sclerosis. Neuropsychologia. 44:1166–1174.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bolla KI, Lindgren KN, Bonaccorsy C and
Bleecker ML: Predictors of verbal fluency (FAS) in the healthy
elderly. J Clin Psychol. 46:623–628. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Selnes OA, Jacobson L, Machado AM, Becker
JT, Wesch J, Miller EN, et al: Normative data for a brief
neuropsychological screening battery. Multicenter AIDS Cohort
Study. Percept Mot Skills. 73:539–550. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Libon D, David J, Glosser G, Malamut BL,
Kaplan E, Goldberg E, et al: Age, executive functions, and
visuospatial functioning in healthy older adults. Neuropsychology.
8:38–43. 1994. View Article : Google Scholar
|
|
69
|
Tombaugh TN, Kozak J and Rees L: Normative
data stratified by age and education for two measures of verbal
fluency: FAS and animal naming. Arch Clin Neuropsychol. 14:167–177.
1999.PubMed/NCBI
|
|
70
|
Ruff RM, Allen CC, Farrow CE, Niemann H
and Wylie T: Figural fluency: differential impairment in patients
with left versus right frontal lobe lesions. Arch Clin
Neuropsychol. 9:41–55. 1994. View Article : Google Scholar
|
|
71
|
Miller BL and Cummings JL: The Human
Frontal Lobes: Functions and Disorders. 2nd edition. The Guilford
Press; New York, NY: 2007
|