Introduction
Post-intubation tracheal stenosis (PTS) is a
progressive and constant narrowing of the tracheal lumen with
fibrous scars, and is related to the use of intubation tubes
(1,2). PTS is a life-threatening pathological
modification of the upper segment of the trachea. The absolute
number of PTS cases, which is regarded as the most common cause of
benign tracheostenosis, is rising due to the increased use of
assisted ventilation in intensive care units. Tracheal stenosis of
patients is often caused by prolonged tracheal intubation, which
may require surgery to remove the narrowed portion of the airway or
endoscopic interventional therapy, to which close attention has
been paid in recent years, but these methods can only achieve
short-term effects. To date, the mechanism of PTS remains unclear.
Certain studies suggest that the nature of scar formation in the
tracheal lumen resulting in stenosis shows that PTS is a
fibroproliferative disease, which is caused by aberrant repair
after tissue injury (3,4). Its pathological hallmark is the
overexpression of extracellular matrix (ECM) presenting collagen
type I, collagen type III and fibronectin, while elastic fibers was
significantly reduced. Fibroblasts, as the main cells synthesizing
ECM, play an important role in granulation formation, wound
contraction and scar formation. The lethality and increasing
morbidity of PTS have led us to investigate the precise processes
underlying pathological stenosis in order to develop clinical tools
to inhibit scar formation.
Hypoxia may be an important factor in the induction
of post-intubation tracheal hypertrophic scars. Studies of tracheal
stenosis showed that the pressure exerted by the cuff on the
tracheal mucosa is the main cause of mucosal ischemia and anoxia,
followed by tracheal mucosal erosion, ulceration and chondritis
(5,6). Fibrous scar formation is a
compensatory mechanism aimed at healing the circumferential damage.
Hypoxia-inducible factor (HIF)-1 has been investigated in recent
years, as HIF-1 is a DNA-binding protective nuclear transcription
factor under hypoxia in mammalian and human cells, and is
associated with specific nuclear cofactors to transactivate genes
in order to adapt to the compromised oxygen tension. HIF-1 is a
heterodimer comprising an active α-subunit (HIF-1α) and a
constitutive β-subunit, which indicates that the biological
activities are determined by the expression of HIF-1α (7). Under normoxic conditions, HIF-1α is
hydroxylated on proline residues by prolyl-hydroxylase domain
(PHDs)-containing enzymes, then recognized by the β-subunit, and
subsequently ubiquitylated and degraded by the 26S proteasome
(8–10). With lowering oxygen tension in the
microenvironment, HIF-1α is abrogated from hydroxylation as PHDs
require oxygen for catalytic activity, and therefore HIF-1α escapes
recognition by ubiquitin ligase, which results in the accumulation
of HIF-1α in the nucleus. Upregulation of HIF-1α subsequently
activates a myriad of downstream genes that protect cells in
response to hypoxia. The responses that HIF-1α mediates in hypoxic
conditions include increasing oxygen delivery by the formation of
new blood vessels and efficient utilization (11) by the activation of genes, including
angiogenesis-related genes, such as vascular endothelial growth
factor (VEGF), and genes related to cell proliferation and
differentiation, such as transforming growth factor (TGF) and basic
fibroblast growth factor (bFGF).
In recent years, increasing evidence has suggested
that HIF-1α acts as a potential contributor to facilitate
fibrogenesis by activating fibroblast transdifferentiation into
myofibroblasts or enhancing epithelial-to-mesenchymal transition in
hypoxia (12–15). HIF-1α is being focally targeted as
a novel cancer therapy, as it allows sustained angiogenesis and
tissue metastasis of cancer (16).
Scheid et al(17) showed
that the upregulation of HIF-1α in adults may represent a pathway
in the pathogenesis of scarring. Based on all of the above
findings, we speculate that HIF-1α may be involved in the process
of PTS by activating angiogenesis and cell proliferation. The aim
of the present study was to identify the expression of HIF-lα and
its target genes in tracheal scars of PTS, analyze their
correlation in order to explore new hypotheses in the development
of PTS, and further provide a theoretical basis for the theory that
HIF-1 inhibitors are a preventive method for PTS.
Materials and methods
Ethics approval
The investigation and protocols were approved by the
Bioethical Committee of the Second Hospital of Hebei Medical
University (Shijiazhuang, Hebei, China). All procedures were
strictly performed according to the Declaration of Helsinki revised
in 1983.
Experiment one
Patients and methods
All intubated patients in the intensive care units
of the Second Hospital of Hebei Medical University (Shijiazhuang,
Hebei, China) between March 2010 and June 2012, had the tracheal
mucosal pressure exerted by the cuff measured through indirect
methods. The detailed steps used are as follows: i) the appropriate
endotracheal tube was selected and in vitro intracuff
pressure was measured after injection of 1–15 ml of gas; ii) after
endotracheal intubation, the cuff was inflated to the smallest
value at which no air leaked, the in vivo intracuff pressure
was measured and recorded at end-expiration, and the volume of
inflated air was recorded. The pressure on the tracheal mucosa was
calculated by subtracting the in vitro intracuff pressure
from the in vivo intracuff pressure of equal volumes of gas.
Intracuff pressure in vivo was measured twice per day until
extubation. Pressures were measured using cuff pressure gauges (VBM
Medizintechnik GmbH, Einsteinstr.1, 72172 Sulz a.N., Germany). The
duration of intubation was also recorded. The recorded number of
patients was 1,384 cases, and they were followed for six months or
until being diagnosed with PTS. Eighteen patients with dyspnea were
demonstrated to have tracheal stenosis by neck computed tomography,
and the same number of patients without PTS was randomly selected
from the recorded cases. We studied the effects of mucosal pressure
exerted by the cuff of the tracheal tube and the duration of
intubation on scar formation of patients with PTS, and measured
distance from the vocal cord to the stenosed site in order to
locate tracheal stenosis.
Experiment two
Patients and samples
We selected specimens from patients according to the
following inclusion and exclusion criteria. Inclusion criteria: i)
patients with PTS observed and verified by bronchoscopy; ii)
tracheal stenosis was related to previous intubation. Exclusion
criteria: i) patients who were <18 years old; ii) concomitant
disease in the region of tracheal stenosis and; iii) patients who
were smokers. Normal control tissue samples were not obtained, as
normal tissue barely expresses HIF-1α according to the literature
(16,18), which was verified by our
preliminary experiment (data not shown).
The selected 24 patients, showing different degrees
of dyspnea within six months of endotracheal intubation removal,
were admitted to the Second Hospital of Hebei Medical University
(Shijiazhuang, Hebei, China) for bronchoscopic interventional
therapy. All patients were Chinese and each patient in the two
experiments signed a written informed consent form. The age of the
patients varied between 18 and 65 years (15 males and 9
females).
According to endoscopic results, patients were
divided into three groups (per group, n=8), namely, granulation
phase group (GRA), the proliferative phase group (PRO) and mature
phase group (MAT). The specimens were obtained by biopsy forceps
through bronchoscopy from the hyperplastic tissue in the tracheal
lumen, rinsed in saline, cut into ~1×1 mm sections using a scalpel,
frozen immediately in liquid nitrogen and stored at −80°C for
western blotting.
Western blot analysis
Western blot analyses were performed as follows.
Firstly, total protein extraction was performed. The specimen was
ground in a container with liquid nitrogen, and put on ice with 1
ml of hypotonic buffer A for 1 h. Then, 80 ml of 10% Nonidet P-40
(NP-40) solution was added to the homogenates, and the mixture was
centrifuged at 14,000 × g for 15 min. The protein concentration was
determined by a micro bicinchoninic acid assay (Pierce Chemical
Co., Rockford, IL, USA), xSDS buffer [0.2 M Tris-HCl (pH 6.8), 4%
sodium dodecyl sulphate (SDS), 0.18% glycerol, 0.02%
β-mercaptoethanol and bromophenol blue] was added to the
supernatant samples and boiled for 5 min. Proteins were separated
electrophoretically by 10% SDS-PAGE and then transferred onto a
PVDF membrane. After blocking membranes with Odyssey Blocking
buffer (LI-COR Biosciences, Inc., Lincoln, NE, USA), immunoblot
analysis was performed by incubation with 1:500 diluted primary
antibodies against HIF-1α (Novus Biologicals, LLC, Littleton, CO,
USA), VEGF, bFGF and β-actin (these three reagents were obtained
from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), α-SMA
(Sigma-Aldrich, St. Louis, MO, USA), and antibodies against TGF-β,
type I and type III collagen and GAPDH (these four reagents were
obtained from Meilian Bioengineering Institute, Shanghai, China) at
4°C overnight. The membranes were washed and incubated for 1 h with
horseradish peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.) at room temperature. Proteins were visualized
with an enhanced chemiluminescence kit (Santa Cruz Biotechnology,
Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
(version, 13.0; SPSS Inc., Chicago, IL, USA). The results are
presented as the mean values with standard deviation (SD).
Comparisons were made by the two-tailed Student’s t-test for
independent samples or one-way analysis of variance (ANOVA)
followed by the least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Results of experiment one
Effects of pressure exerted by the
cuff on tracheal mucosa and duration of intubation on tracheal
stenosis
There was no difference in age between the two
groups. The tracheal mucosal pressure exerted by the endotracheal
tube cuff in the PTS group was significantly higher than that in
the group without PTS (NPTS; P<0.05; Table I). The duration of intubation was
increased in the PTS group compared with the NPTS group (P<0.05;
Table I). The location of tracheal
stenosis determined by distance from the vocal cord to the stenosed
site was 2.30±0.22 cm, which was consistent with the site where the
cuff exerted pressure on the tracheal wall.
 | Table IEffects of pressure exerted by the
cuff on tracheal mucosa and duration of intubation on scar
formation in PTS (n=18). |
Table I
Effects of pressure exerted by the
cuff on tracheal mucosa and duration of intubation on scar
formation in PTS (n=18).
| Group | Age (years) | Pressure on mucosa
(mmHg) | Duration of
intubation (days) |
|---|
| PTS | 55.00±12.26 | 6.39±2.23a | 20.06±9.04a |
| NPTS | 53.44±11.69 | 20.33±1.97 | 12.22±3.95 |
Results of experiment two
Using western blot analysis, the present study
evaluated the protein expression profiles of HIF-1α and its target
genes VEGF, bFGF and TGF-β, respectively, in different periods of
scar formation in 24 patients with PTS. We also evaluated the ECM
in the hyperplasia tissue.
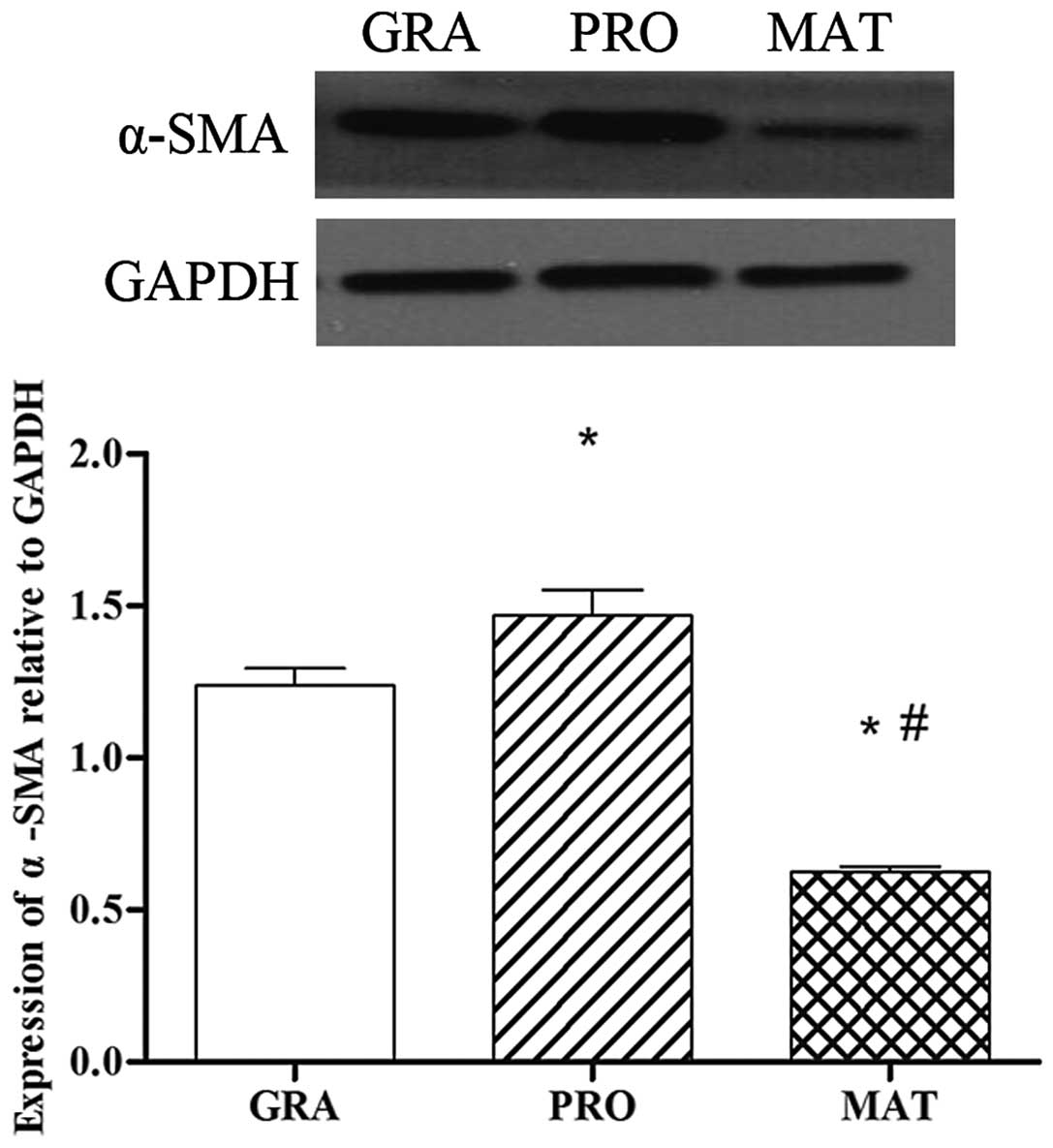
α-smooth muscle actin (α-SMA) protein
expression in different phases of scar formation in PTS
α-SMA is a symbol of fibroblast activation. As shown
in Fig. 1, the protein expression
of α-SMA in different phases of tracheal scar formation were
significantly different. α-SMA was detected in the granulation
phase, and markedly increased further in the proliferative phase
compared with that of the granulation phase (P<0.05). The mature
phase showed the lowest levels of expression among the three groups
(P<0.05).
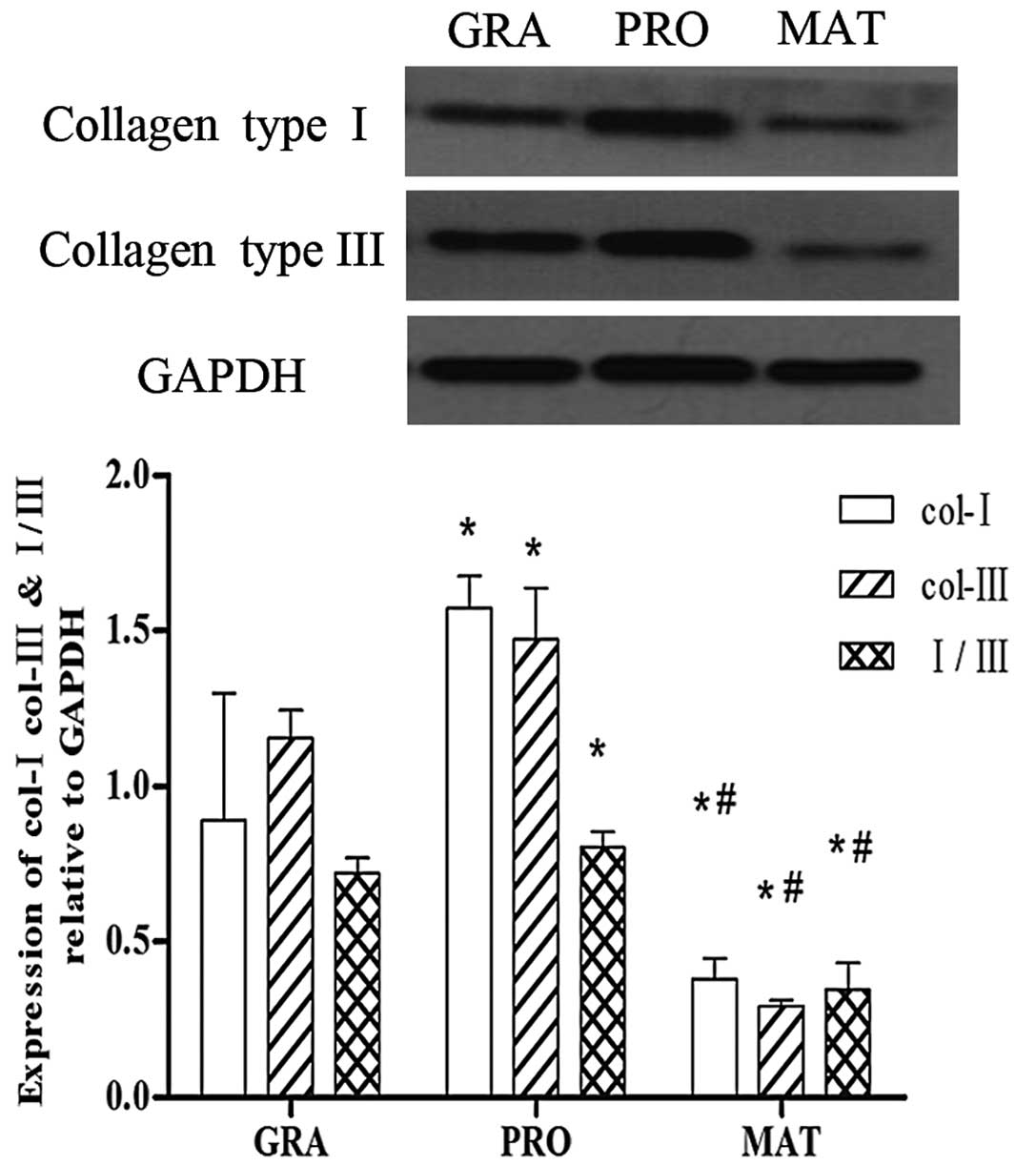
ECM expression in different phases of
tracheal scar formation in PTS
Type I and III collagen are the main components of
the ECM during scar formation, and are synthesized and secreted by
myofibroblasts. As shown in Fig.
2, the expression of type I and III collagen in different
phases of tracheal scar formation was detected by western blot
analysis. Type I and III collagen were increased markedly in the
proliferative phase compared with the granulation phase (P<0.05)
and levels were significantly decreased in the mature phase
(P<0.05). The mature phase showed the lowest levels of the three
groups. The same trend was observed in the ratio of type I collagen
vs. type III collagen.
Protein expression of HIF-1α and its
target genes in different phases of scar formation in PTS
To determine whether the HIF-1α signaling pathway is
involved in the formation of PTS, we investigated protein
expression of HIF-1α and its target genes by western blot analysis.
Protein expression of HIF-1α in the granulation period was the
highest of the three groups, and then gradually reduced, so that in
the mature stage the levels were significantly reduced compared
with the other two stages (P<0.05) (Fig. 3A and D). Similar trends were
observed for protein expression of VEGF (Fig. 3A and B) and bFGF (Fig. 3A and C); whereas for TGF-β protein
expression, the level of TGF-β was highest in the proliferative
phase, and there was a statistically significant difference
compared with the other two groups (P<0.05). The mature stage
showed the lowest levels of the three groups (Fig. 3A and E).
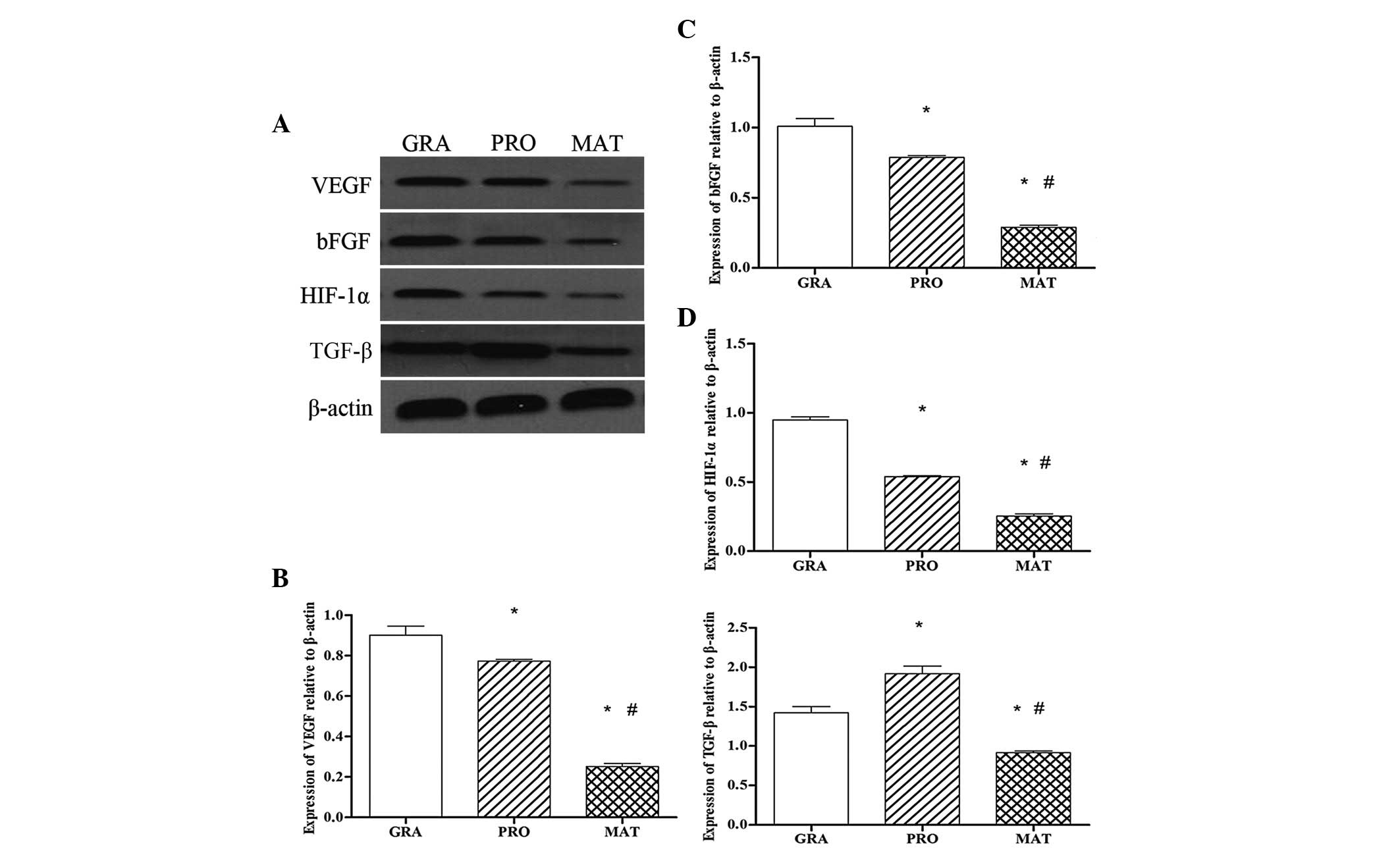 | Figure 3Protein expression of
hypoxia-inducible factor-1α (HIF-1α) and its target genes in
different phases of scar formation in PTS. Protein expression was
detected by western blot analysis. The bands correspond to vascular
endothelial growth factor (VEGF), basic fibroblast growth factor
(bFGF), HIF-1α, TGF-β and β-actin in the GRA, PRO and MAT groups.
Bar graph shows densitometry of VEGF, bFGF, HIF-1α and TGF-β,
normalized to that of β-actin. Values are the means ± standard
deviations, n=8/group. *P<0.05 vs. GRA;
#P<0.05 vs. PRO. GRA, granulation phase; PRO,
proliferative phase; MAT, mature phase; PTS, post-intubation
tracheal stenosis; TGF, transforming growth factor. |
Discussion
The present study was performed with the objective
of identifying whether molecular pathways induced by hypoxia
contribute to the activation of tracheal fibroblasts in PTS. We
found in experiment one that the tracheal mucosal pressure exerted
by the cuff and the duration of intubation had an effects on PTS.
In experiment two, the HIF-1α protein was markedly expressed in
granulation formation phase of PTS, and sustained a high level in
the proliferative phase, while it was scarcely detected in the
mature phase scars. HIF-1α target genes related to formation of
fibrous scars, such as VEGF and bFGF, were also expressed, whereas
the protein expression of TGF-β showed a different trend. The ECM
proteins showed higher expression in the granulation and
proliferative phases compared with that of the mature phase. These
results demonstrated that hypoxia contributed to the initiation and
progression of fibrosis in PTS and therefore provided a novel
hypothesis for the pathogenesis of PTS.
The pathological changes of patients with PTS are
similar to those of patients with skin scars, including, but not
limited to, fibroblast activation, proliferation, contraction and
remodeling of ECM. α-SMA, a marker of myofibroblasts, is
overexpressed after fibroblasts are activated to form
myofibroblasts according to experiments in vivo and in
vitro. The activated fibroblasts produce ECM. The two types of
collagen, I and III, comprising the main structural element of the
ECM during the healing process, play the largest role in wound
repair and correlate with the sustained presence of myofibroblasts
(19,20), as they present a significant
capacity of tension-resistance to form a protective belt following
injury. In this study, we observed that α-SMA expression in the
proliferative group markedly increased (Fig. 1), which suggested transformation of
fibroblasts to myofibroblasts, compared with the granulation and
mature groups. In turn, myofibroblasts produce large amounts of
collagen, therefore collagen type I and type III were observed to
be markedly accumulated in the proliferative and granulation phases
of the tracheal pathological hyperplasia, particularly in the
proliferative phase, and significantly decreased in the mature
phase. The trend of their ratios was the same (Fig. 2). Our results revealed that
excessive proliferation and active function of myofibroblasts were
involved in the repair of tracheal mucosal damage and tracheal
stenosis formation in PTS. Our results are in line with previous
studies (21,22), in which the investigators reported
that tracheal damage promoted production of myofibroblasts and
regulated the homeostasis of ECM components.
The clinical investigations revealed that vascular
proliferation occurs in the early stage of scar formation. VEGF, as
the most important angiogenesis-stimulating factor, plays a pivotal
role in the angiogenesis of scars. Le et al(23) revealed a large quantity of VEGF in
fibroblasts located in the papillary dermis, and consistent
elevation in the homogenate lysate of keloid tissues compared with
normal skin controls. Certain studies on airway stenosis have also
reported that granulation tissue, as the initial change that occurs
in airway stenosis, exhibited an increase in angiogenesis (24–26).
Our results were in agreement with these findings. We found that
VEGF protein expression in the granulation and proliferative phases
significantly increased compared with the mature phase,
particularly in the granulation phase (Fig. 3A and B), which suggested the
important role of VEGF in tracheal cicatrization. Besides
angiogenesis, Wu et al(27)
have also indicated that VEGF may alter ECM homeostasis and lead to
a state of excessive accumulation and impaired degradation in
keloid formation, and Yang et al(28) have shown that VEGF also controlled
the dynamics and expression of dermal fibroblasts in wound healing.
Taken together, the data suggest that VEGF is important in
granulation during tissue repair.
Basic FGF, a member of the fibroblast growth factor
family, has been confirmed by an increasing number of studies to
promote wound healing by increasing fibroblast migration,
proliferation and angiogenic function, and in particular, to play a
role in granulation tissue formation (29–31).
Accordingly, in the present study we observed that protein
expression of bFGF in the granulation phase was the highest of the
three phases, and it gradually decreased in the proliferative phase
(Fig. 3A and C), and there was a
marked reduction in the mature phase, which suggests the importance
of bFGF in the formation of tracheal stenosis found in PTS.
The emerging experiments showed stabilization and
accumulation of HIF-1α in hypoxia, since its degradation was
impeded (32,33). Accumulated HIF-1α translocates into
the nucleus leading to the activation of its target genes related
to scar formation, including VEGF and bFGF (35–37).
Previous experiments found that HIF-1α induced myofibroblast
differentiation (38) and hypoxia
contributes directly to ECM accumulation in patients with systemic
sclerosis (39), which suggests
that hypoxia may contribute to tracheal fibrosis based on the
duration of hypoxia. In experiment one, we found that the pressure
exerted on tracheal mucosa by the endotracheal tube cuff was
significantly higher in the PTS group than in the NPTS group and
the duration of intubation was longer in the PTS group compared
with the NPTS group. These results correlated with the conclusions
in the studies of Leigh and Maynard (40) and Lewis et al(41), which showed that high tracheal wall
pressure that exceeds 20 mmHg, which is approximately the mean
capillary perfusion pressure, may damage the tracheal mucosa
leading to topical hypoxia, particularly when accompanied by
prolonged tracheal intubation, and this was the cause of PTS. In
the process of scar formation, ECM protein accumulation worsens the
hypoxia of interstitial fibroblasts by further increasing the
distance to blood vessels, resulting in a vicious circle of ECM
accumulation and hypoxia, and therefore the hypoxic
microenvironment is sustained. To determine the molecular mechanism
involved in the process of PTS, in experiment two, we detected
protein expression of HIF-1α. As the HIF-1α subunit is rapidly
degraded and scarcely detected in normoxia (42,43),
we studied the pathological state in PTS patients. We found that
the protein expression level of HIF-1α in the granulation phase was
the highest among the three groups. HIF-1α expression was sustained
in the proliferative phase, and significantly decreased in the
mature scars of patients (Fig. 3A and
D). We also observed that the variation of VEGF and bFGF, as
two main target genes of HIF-1α, was consistent with that of
HIF-1α.
TGF-β is well known as a traditional profibrotic
factor, and has been identified as the major stimulating factor to
promote scar formation. A large number of experiments have shown
that sustained expression of TGF-β plays an important role in
tissue repair, remodelling and hypertrophic scar formation
(44–46), as well as in the formation of
stent-related airway stenosis (47). Previous studies found that TGF-β,
as one of the HIF-1α target genes, was regulated by HIF-1α
(48,49). In this experiment, we observed that
in tracheal scars of PTS patients, HIF-1α and TGF-β protein
expression in the granulation phase and proliferative phase were
significantly higher than the mature phase, but the peak TGF-β
levels occurred in the proliferative phase (Fig. 3A and E), which was not in
accordance with HIF-1α. The possible reasons leading to this
discrepancy may be based on cell type, extent and duration of
hypoxic state (11) or some other
uncertain cause. This result indicates the mechanism of PTS is more
complex and involves a network in vivo. Other pathways or
genes (50) may act on the TGF-β
pathway of fibration besides HIF-1α regulation.
Based on our results, we concluded that ischemia and
hypoxia of the tracheal wall on which the cuff exerted the pressure
leads to stabilization and an increase of HIF-1α levels. HIF-1α
subsequently induced overexpression of its target genes related to
fibroplastic events. Corresponding to these target genes,
fibroblasts were activated in impaired trachea resulting in the
persistent and abundant proliferation of myofibroblasts. This study
serves as the first part of efforts to examine HIF-1α as a possible
leading regulator in the process of PTS. Our group will perform
further in vitro and animal studies in order to explore the
cellular and molecular mechanisms involved and to identify whether
a HIF-1α inhibitor could be used as an adjuvant medicine for the
treatment of PTS.
Acknowledgements
This study was supported by grants from the Medical
Research Foundation of the Hebei Province (no. 20120064).
References
|
1
|
Freitag L, Ernst A, Unger M, Kovitz K and
Marquette CH: A proposed classification system of central airway
stenosis. Eur Respir J. 30:7–12. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008. View Article : Google Scholar
|
|
3
|
Dohar JE, Klein EC, Betsch JL, et al:
Acquired subglottic stenosis-depth and not extent of the insult is
key. Int J Pediatr Otorhinolaryngol. 46:159–170. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Corrêa Reis JG, Takiya CM, Lima Carvalho
A, et al: Myofibroblast persistence and collagen type I
accumulation in the human stenotictrachea. Head Neck. 34:1283–1293.
2012.PubMed/NCBI
|
|
5
|
Knowlson GT and Bassett HF: The pressures
exerted on the trachea by endotracheal inflatable cuffs. Br J
Anaesth. 42:834–837. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cooper JD and Grillo HC: The evolution of
tracheal injury due to ventilatory assistance through cuffed tubes:
a pathologic study. Ann Surg. 169:334–348. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang BH, Zheng JZ, Leung SW, Roe R and
Semenza GL: Transactivation and inhibitory domains of
hypoxia-inducible factor 1α. Modulation of transcriptional activity
by oxygen tension. J Biol Chem. 272:19253–19260. 1997.
|
|
8
|
Ohh M, Park CW, Ivan M, et al:
Ubiquitination of hypoxia-inducible factor requires direct binding
to the β-domain of the von Hippel-Lindau protein. Nat Cell Biol.
2:423–427. 2000.PubMed/NCBI
|
|
9
|
Maxwell PH, Wiesener MS, Chang GW, et al:
The tumour suppressor protein VHL targets hypoxia-inducible factors
for oxygen-dependent proteolysis. Nature. 399:271–275. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Masson N, Willam C, Maxwell PH, Pugh CW
and Ratcliffe PJ: Independent function of two destruction domains
in hypoxia-inducible factor-α chains activated by prolyl
hydroxylation. EMBO J. 20:5197–5206. 2001.
|
|
11
|
Semenza GL: Regulation of cancer cell
metabolism by hypoxia-inducible factor 1. Semin Cancer Biol.
19:12–16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Norman JT, Clark IM and Garcia PL: Hypoxia
promotes fibrogenesis in human renal fibroblasts. Kidney Int.
58:2351–2366. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Basu RK, Hubchak S, Hayashida T, Runyan
CE, Schumacker PT and Schnaper HW: Interdependence of HIF-1α and
TGF-β/Smad3 signaling in normoxic and hypoxic renal epithelial cell
collagen expression. Am J Physiol Renal Physiol. 300:F898–F905.
2011.
|
|
14
|
Clancy RM, Zheng P, O’Mahony M, et al:
Role of hypoxia and cAMP in the transdifferentiation of human fetal
cardiac fibroblasts: implications for progression to scarring in
autoimmune-associated congenital heart block. Arthritis Rheum.
56:4120–4131. 2007. View Article : Google Scholar
|
|
15
|
Higgins DF, Kimura K, Bernhardt WM, et al:
Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of
epithelial-to-mesenchymal transition. J Clin Invest. 117:3810–3820.
2007.PubMed/NCBI
|
|
16
|
Nordgren IK and Tavassoli A: Targeting
tumour angiogenesis with small molecule inhibitors of hypoxia
inducible factor. Chem Soc Rev. 40:4307–4317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scheid A, Wenger RH, Christina H, et al:
Hypoxia-regulated gene expression in fetal wound regeneration and
adult wound repair. Pediatr Surg Int. 16:232–236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiroi M, Mori K, Sakaeda Y, et al: STAT1
represses hypoxia-inducible factor-1-mediated transcription.
Biochem Biophys Res Commun. 387:806–810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sidgwick GP and Bayat A: Extracellular
matrix molecules implicated in hypertrophic and keloid scarring. J
Eur Acad Dermatol Venereol. 26:141–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Friedman DW, Boyd CD, Mackenzie JW, Norton
P, Olson RM and Deak SB: Regulation of collagen gene expression in
keloids and hypertrophic scars. J Surg Res. 55:214–222. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bellon G, Moreau M, Cam YJ, et al:
Modifications of collagens in the course of inflammatory tracheal
stenoses. Ann Otol Rhinol Laryngol. 94:403–408. 1985.PubMed/NCBI
|
|
22
|
Doolin EJ, Tsuno K, Strande LF and Santos
MC: Pharmacologic inhibition of collagen in an experimental model
of subglottic stenosis. Ann Otol Rhinol Laryngol. 107:275–279.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le AD, Zhang Q, Wu Y, et al: Elevated
vascular endothelial growth factor in keloids: relevance to tissue
fibrosis. Cells Tissues Organs. 176:87–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Chen JC, Holinger LD and
Gonzalez-Crussi F: Histopathologic fundamentals of acquired
laryngeal stenosis. Pediatr Pathol Lab Med. 15:655–677. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pokharel RP, Maeda K, Yamamoto T, et al:
Expression of vascular endothelial growth factor in exuberant
tracheal granulation tissue in children. J Pathol. 188:82–86. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rahbar R, Brown LF, Folkman J, et al: Role
of vascular endothelial growth factor A in children with acquired
airway stenosis. Ann Otol Rhinol Laryngol. 116:430–435. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Y, Zhang Q, Ann DK, et al: Increased
vascular endothelial growth factor may account for elevated level
of plasminogen activator inhibitor-1 via activating ERK1/2 in
keloid fibroblasts. Am J Physiol Cell Physiol. 286:C905–C912. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang GP, Lim IJ, Phan TT, Lorenz HP and
Longaker MT: From scarless fetal wounds to keloids: molecular
studies in wound healing. Wound Repair Regen. 11:411–418. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suehiro A, Hirano S, Kishimoto Y, Tateya
I, Rousseau B and Ito J: Effects of basic fibroblast growth factor
on rat vocal fold fibroblasts. Ann Otol Rhinol Laryngol.
119:690–696. 2010.PubMed/NCBI
|
|
30
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Powers CJ, McLeskey SW and Wellstein A:
Fibroblast growth factors, their receptors and signaling. Endocr
Relat Cancer. 7:165–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagatsuma A, Kotake N and Yamada S:
Spatial and temporal expression of hypoxia-inducible factor-1α
during myogenesis in vivo and in vitro. Mol Cell Biochem.
347:145–155. 2011.
|
|
33
|
Distler JH, Jüngel A, Pileckyte M, et al:
Hypoxia-induced increase in the production of extracellular matri-x
proteins in systemic sclerosis. Arthritis Rheum. 56:4203–4215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sogabe Y, Abe M, Yokoyama Y and Ishikawa
O: Basic fibroblast growth factor stimulates human keratinocyte
motility by Rac activation. Wound Repair Regen. 14:457–462. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brahimi-Horn C and Pouyssegur J: The role
of the hypoxia-inducible factor in tumor metabolism growth and
invasion. Bull Cancer. 93:E73–E80. 2006.PubMed/NCBI
|
|
36
|
Chang JH, Han KY and Azar DT: Wound
healing fibroblasts modulate corneal angiogenic privilege:
interplay of basic fibroblast growth factor and matrix
metalloproteinases in corneal angiogenesis. Jpn J Ophthalmol.
54:199–205. 2010. View Article : Google Scholar
|
|
37
|
Bos R, van Diest PJ, de Jong JS, van der
Groep P, van der Valk P and van der Wall E: Hypoxia-inducible
factor-1α is associated with angiogenesis, and expression of bFGF,
PDGF-BB, and EGFR in invasive breast cancer. Histopathology.
46:31–36. 2005.
|
|
38
|
Kottmann RM, Kulkarni AA, Smolnycki KA, et
al: Lactic acid is elevated in idiopathic pulmonary fibrosis and
induces myofibroblast differentiation via pH-dependent activation
of transforming growth factor-β. Am J Respir Crit Care Med.
186:740–751. 2012.PubMed/NCBI
|
|
39
|
Distler JH, Jungel A, Pileckyte M, et al:
Hypoxia-induced increase in the production of extracellular matrix
proteins in systemic sclerosis. Arthritis Rheum. 56:4203–4215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leigh JM and Maynard JP: Pressure on the
tracheal mucosa from cuffed tubes. Br Med J. 1:1173–1174. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lewis FR Jr, Schiobohm RM and Thomas AN:
Prevention of complications from prolonged tracheal intubation. Am
J Surg. 135:452–457. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)α: its protein stability and
biological functions. Exp Mol Med. 36:1–12. 2004.
|
|
43
|
Powis G and Kirkpatrick L: Hypoxia
inducible factor-1α as a cancer drug target. Mol Cancer Ther.
3:647–654. 2004.
|
|
44
|
Leask A and Abraham DJ: TGF-β signaling
and the fibrotic response. FASEB J. 18:816–827. 2004.
|
|
45
|
Liu W, Wang DR and Cao YL: TGF-β: a
fibrotic factor in wound scarring and a potential target for
anti-scarring gene therapy. Curr Gene Ther. 4:123–136. 2004.
|
|
46
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor β in human disease. N Engl J Med.
342:1350–1358. 2000.
|
|
47
|
Karagiannidis C, Velehorschi V,
Obertrifter B, Macha HN, Linder A and Freitag L: High-level
expression of matrix-associated transforming growth factor-β1 in
benign airway stenosis. Chest. 129:1298–1304. 2006.
|
|
48
|
Higgins DF, Kimura K, Iwano M and Haase
VH: Hypoxia-inducible factor signaling in the development of tissue
fibrosis. Cell Cycle. 7:1128–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Copple BL: Hypoxia stimulates hepatocyte
epithelial to mesenchymal transition by hypoxia-inducible factor
and transforming growth factor-β-dependent mechanisms. Liver Int.
30:669–682. 2010.PubMed/NCBI
|
|
50
|
Chen Z, Zhang D, Yue F, Zheng M, Kovacevic
Z and Richardson DR: The iron chelators Dp44mT and DFO inhibit
TGF-β-induced epithelial-mesenchymal transition via up-regulation
of N-Myc downstream-regulated gene 1 (NDRG1). J Biol Chem.
287:17016–17028. 2012.PubMed/NCBI
|