Introduction
Inflammation is widely involved in the pathology of
acute pulmonary embolism (APE), in which the levels of tumor
necrosis factor (TNF)-α, interleukin (IL)-1β and IL-8 are
significantly increased (1). It
has been demonstrated that the use of aspirin may have protective
effects (2). However, it has not
yet been elucidated whether other important inflammatory factors
are involved in the inflammatory responses following APE.
The European Society of Cardiology has issued
‘Guidelines on the diagnosis and management of acute pulmonary
embolism’ (3), which suggested
that brain natriuretic peptide (BNP), troponin (TnT) and D-Dimer
may serve as prognostic markers of APE. A number of other studies
have shown that extracellular signal-regulated protein kinases
(ERK) (4–7) and phosphoinositide 3 kinase/protein
kinase B (PI3K/Akt) (8) are
important in the inflammatory response.
The ERK and PI3K/Akt signaling pathways have been
demonstrated to be important in the pathology of lung injuries
(4,5) and pulmonary arterial hypertension
(9,10). However, no study has yet
investigated the roles of these pathways in the pathology of
APE.
In the present study, the effects of aspirin on the
ERK and PI3K/Akt signaling pathways were studied in a rat model of
APE. Furthermore, the levels of BNP, TnT and D-Dimer were used to
predict the prognosis of the rats.
Materials and methods
Animals
A total of 108 Sprague Dawley rats bred in an
ultra-clean environment, with body weights of ~250±20 g were used
in this study. The animals were obtained from Huishan Jiangnan
Animal Center, Wuxi, China [animal certification no. SCXK (Su)
2009–0005]. The Medical Experimental Animal Management Committee of
Zhejiang, China, as well as the ethics committee of Zhejiang
Chinese Medical University (Hangzhou, China), approved this
study.
Drug
Aspirin enteric-coated capsules (W026) were obtained
from Yongxin Pharmaceuticals Inc. (Kunshan, China).
Materials
PI3K antibody (BS3678; Bioworld, Dublin, OH, USA),
Akt antibody (ab8805; Abcam, Cambridge, UK), ERK antibody (9102;
Cell Signaling Technology, Inc., Danvers, MA, USA), β-actin
antibody (sc-47778; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), goat anti-rabbit immunoglobulin G (IgG)-horseradish
peroxidase (HRP) (BS13278; Bioworld) and goat anti-mouse IgG-HRP
(BS12478; Bioworld) were used in this study. Cell culture plates
(96-well) were obtained from Fisher Scientific International, Inc.
(Hampton, NH, USA). The enzyme-labeling detection instrument
(SpectraMax Plus 384) was obtained from Molecular Devices
(Sunnyvale, CA, USA), while the electrophoresis
(Mini-Protean® Tetra system) and gel imaging (ChemiDoc™
XRS+) systems were purchased from Bio-Rad (Hercules, CA, USA). The
serum levels of BNP, TnT and D-Dimer were evaluated using
enzyme-linked immunosorbent assay (ELISA) using commercially
available kits (96T; Yifeng Biotechnology Co., Ltd., Shanghai,
China).
Experimental design
A total of 108 Sprague-Dawley rats were randomly
divided into six groups (n=18), based on their weight, by random
numbers generated in SPSS (SPSS, Inc., Chicago, IL, USA). The
groups included control, sham, model and low-, medium- and
high-dose aspirin (150, 300 and 600 mg/kg, respectively). Rats were
dosed once by gavage on day one and once again 40 min prior to
surgery. Having established the model, the rats in each group were
administered with the corresponding drugs by gavage at 6, 24 and 48
h. The rats in the control, sham and model groups were administered
with 2 ml normal saline (NS) and the same volume was given to the
aspirin dosing groups, once a day, for three consecutive days. In
addition, the rats accessed water and solid food ad libitum
throughout the experimental procedure.
Induction of the animal model
The rat model of APE was induced by the local
injection of an autologous thrombus (11). Briefly, blood was collected from
the orbital vein and the blood was allowed to clot at room
temperature for 4 h. The clots were trimmed to ~2 mm3,
prior to NS being added to suspend the clots. According to the body
weights of the rats, different doses of chloral hydrate were
injected for anesthetization. The right jugular vein was
subsequently separated and jugular venous catheterization was
performed. Following this, 0.5 ml of the thrombus suspension
(containing ~15–20 clots) was injected into the rat using a 1 ml
syringe. Rats in the sham group were administered with 0.5 ml NS
via the right jugular vein, while the rats in the control group did
not receive any intervention. The three aspirin groups were
administered with aspirin on the basis of the APE model.
Analysis of ERK, PI3K, Akt, BNP, TnT and
D-Dimer levels
Six rats were randomly selected at 6, 24 and 72 h
subsequent to the induction of the APE model, respectively. The
lungs and blood were then collected from the chloral
hydrate-anesthetized rats and western blot analyses were used to
evaluate the expression of ERK, PI3K and Akt. Briefly, the superior
lobe of the left lung was collected for protein extraction.
Following this, the protein concentration was determined using the
bicinchoninic acid method, by measuring the absorbance at 562 nm
and determining the protein concentration from a standard curve.
Polyacrylamide gel electrophoresis was subsequently performed and
the proteins were transferred to a polyvinylidene difluoride
membrane and blocked. The proteins were incubated with primary
antibody, prior to being washed and incubated with a secondary
antibody. Enhanced chemiluminescent imaging was then performed and
the optical density values of ERK, PI3K and Akt were compared with
the optical density value of β-actin using ImageJ software (Rasband
WS, National Institutes of Health, Bethseda, MD, USA). The levels
of BNP, TnT and D-Dimer were measured using commercially available
ELISA kits. Pulmonary pathology was also examined, as follows:
Subsequent to the examination of the gross pathology of the lung,
the lung tissue was fixed by 10% formalin for 24 h, prior to
paraffin-embedded sections being prepared and stained with
hematoxylin and eosin (H&E) reagents. Pathologists were asked
to examine the pathological changes visible in the lung
sections.
Statistical analysis
Experiments were performed independently at least
three times. Statistical analyses were performed using the SPSS
19.0 statistical software package (SPSS, Inc.). Data are expressed
as the arithmetic mean ± standard deviation. The results of the
ELISA were compared using multi-factorial analysis of variance
(ANOVA) and the western blot analyses were compared using one-way
ANOVA followed by least significant difference or
Student-Newman-Keuls tests. An α value of P<0.05 was considered
to indicate a statistically significant difference.
Results
Western blot analysis
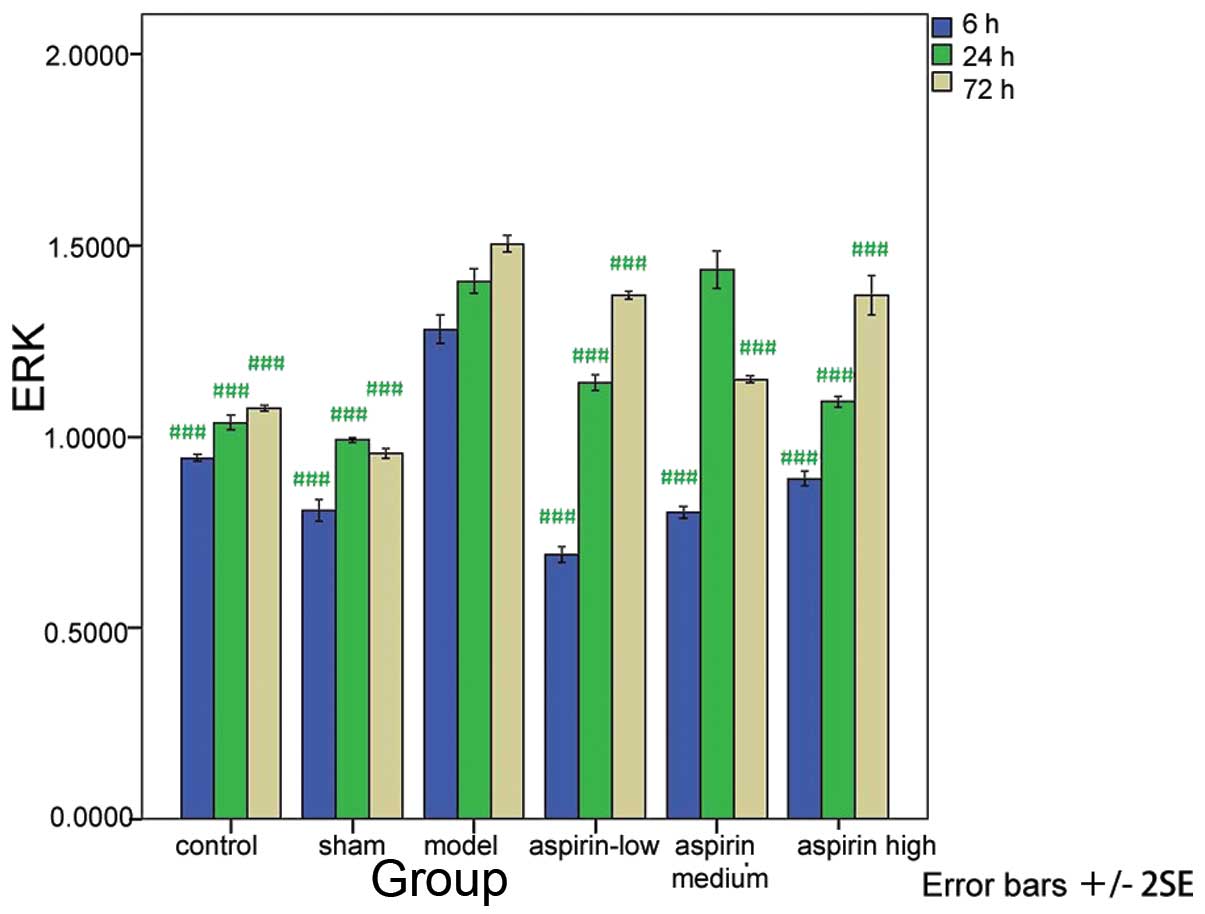
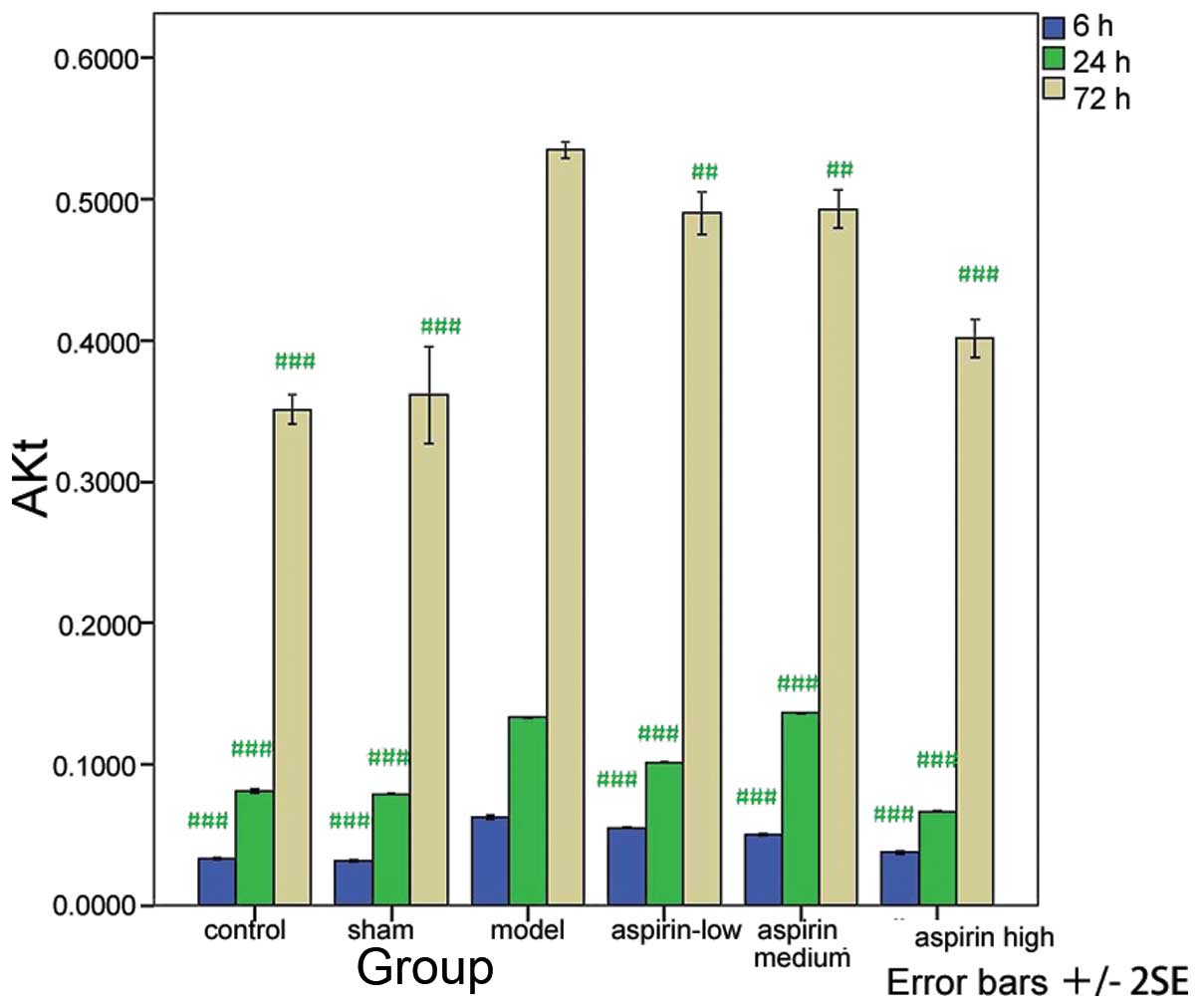
Compared with the model group, the levels of ERK,
PI3K and Akt were significantly decreased in the control, sham and
the three aspirin groups at the three time-points (P<0.001 or
P<0.01) with the exception of the levels measured at 24 h in the
medium-dose aspirin group (Tables
I–III; Figs. 1–3). The results of the western blot
analysis showed that the protein levels of ERK, PI3K and Akt were
markedly higher in the model group than in the other groups
(Fig. 4).
 | Table IERK1/2 level at different time-points
in the six groups. |
Table I
ERK1/2 level at different time-points
in the six groups.
| Group | 6 h | 24 h | 72 h |
|---|
| Control | 0.945±0.008a | 1.037±0.017a | 1.075±0.007a |
| Sham | 0.807±0.023a | 0.991±0.007a | 0.956±0.012a |
| Model | 1.281±0.033 | 1.407±0.027 | 1.505±0.019 |
| Low-dose aspirin | 0.692±0.018a | 1.140±0.017a | 1.371±0.009a |
| Medium-dose
aspirin | 0.803±0.013a | 1.437±0.043 | 1.149±0.008a |
| High-dose
aspirin | 0.890±0.016a | 1.091±0.012a | 1.371±0.045a |
| F-value | 304.455 | 199.379 | 294.028 |
| P-value | 0.000 | 0.000 | 0.000 |
 | Table IIIAkt levels at different time-points in
the six groups. |
Table III
Akt levels at different time-points in
the six groups.
| Group | 6 h | 24 h | 72 h |
|---|
| Control |
0.033±0.001a |
0.081±0.001a |
0.350±0.009a |
| Sham |
0.032±0.001a |
0.079±0.001a |
0.361±0.030a |
| Model | 0.063±0.001 | 0.133±0.001 | 0.534±0.005 |
| Low-dose
aspirin |
0.055±0.001a |
0.101±0.001a |
0.490±0.013b |
| Medium-dose
aspirin |
0.050±0.001a |
0.136±0.001a |
0.493±0.012b |
| High-dose
aspirin |
0.038±0.001a |
0.067±0.001a |
0.401±0.012a |
| F-value | 732.081 | 7443.823 | 74.888 |
| P-value | 0.000 | 0.000 | 0.000 |
ELISA analysis
The levels of BNP, TnT and D-Dimer were compared
with those of the model group at the corresponding time-points. The
results showed that the levels were significantly lower in the
control and sham groups than the model group at 6, 24 and 72 h
subsequent to the model induction.
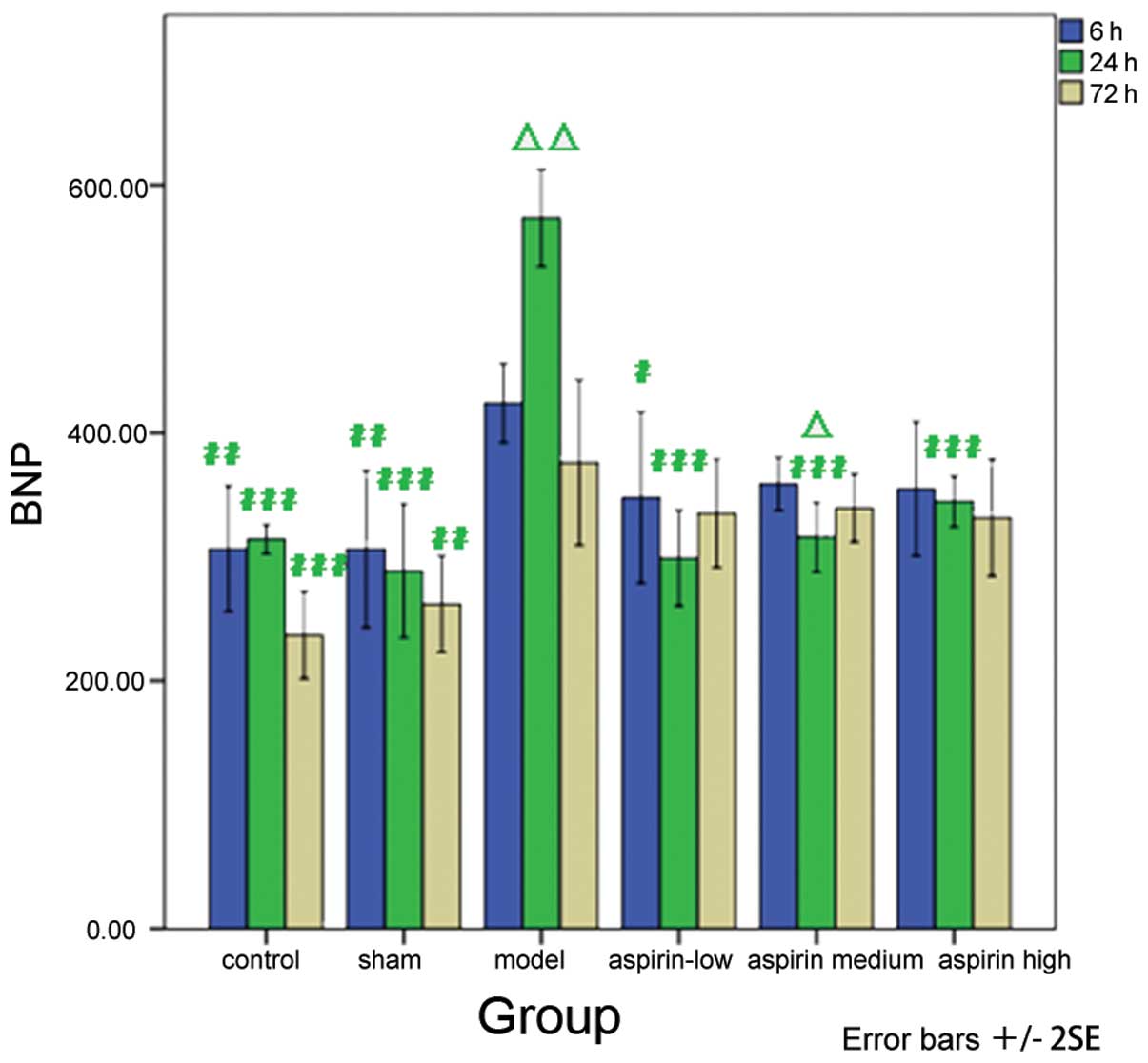
Decreased levels of BNP were observed in the
aspirin- treated groups compared with the model group at the
different time-points, with a significant decrease in BNP levels in
the low-dose aspirin group at 6 h subsequent to model induction
(P<0.05) and in the low-, medium- and high-dose aspirin groups
24 h subsequent to model induction (P<0.001). In addition, the
results at different time-points were compared with the result at 6
h subsequent to model induction within each group. The results
showed that at 24 h subsequent to model induction, the BNP levels
significantly increased in the model group (P<0.01). However,
the levels of BNP decreased significantly in the medium-dose
aspirin group (P<0.05; Table
IV and Fig. 5).
 | Table IVBNP level at different time-points in
the six groups. |
Table IV
BNP level at different time-points in
the six groups.
| Group | 6 h (pg/ml) | 24 h (pg/ml) | 72 h (pg/ml) |
|---|
| Control |
306.59±50.65b |
314.32±11.60c |
236.93±35.30c |
| Sham |
306.36±63.45b |
288.64±53.82c |
261.93±38.91b |
| Model | 423.98±31.92 |
573.41±38.95e | 376.02±66.67 |
| Low-dose
aspirin |
347.95±68.94a |
299.09±38.62c | 335.23±43.71 |
| Medium-dose
aspirin | 358.86±21.45 |
316.02±28.00c,d | 339.43±27.36 |
| High-dose
aspirin | 354.89±54.05 |
344.55±20.47c | 331.59±47.19 |
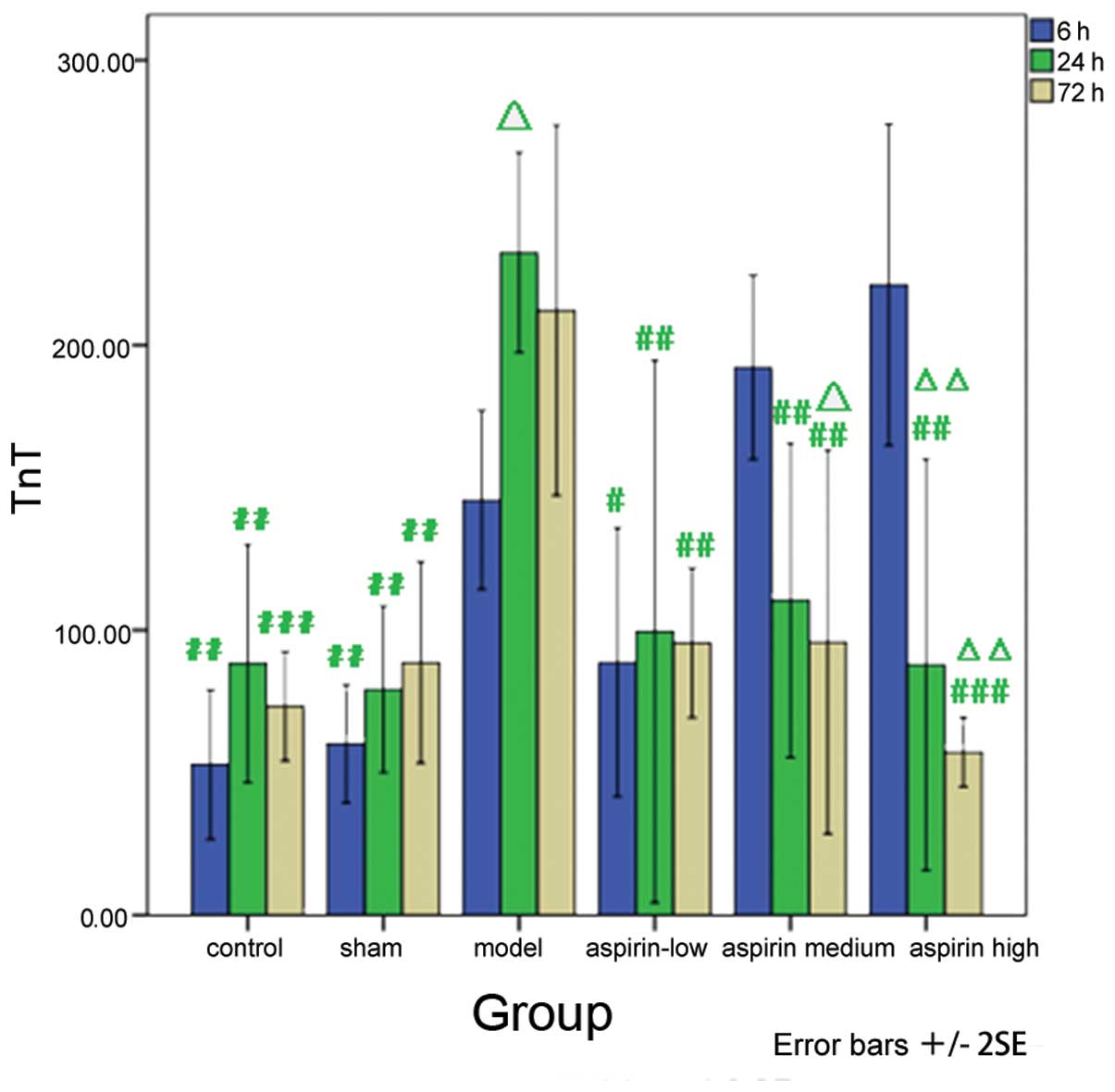
Similarly, as compared with the model group, the TnT
levels were markedly decreased in the low-dose aspirin group at 6 h
subsequent to model induction (P<0.05). A significant decrease
in TnT levels was also observed in the low-, medium- and high-dose
aspirin groups 24 h subsequent to model induction (P<0.01). In
addition, the results obtained at the different time-points were
compared with the observations made 6 h subsequent to model
induction within each group. The results showed that the levels of
TnT increased significantly in the model group 24 h subsequent to
model induction (P<0.05). However, a significant decrease in the
levels of TnT was observed in the medium (P<0.05) and high-dose
aspirin (P<0.01) groups at 72 h and in the high-dose aspirin
group at 24 h subsequent to model induction (P<0.01; Table V and Fig. 6).
 | Table VTnT level at different time-points in
the six groups. |
Table V
TnT level at different time-points in
the six groups.
| Group | 6 h (pg/ml) | 24 h (pg/ml) | 72 h (pg/ml) |
|---|
| Control |
52.79±26.27b |
88.27±41.75b |
73.27±19.17c |
| Sham |
60.19±20.75b |
79.23±29.21b |
88.63±35.33b |
| Model | 145.67±31.38 |
232.50±35.12d | 212.21±64.92 |
| Low-dose
aspirin |
88.75±47.13a |
99.52±95.19b |
95.48±26.23b |
| Medium-dose
aspirin | 192.21±32.29 |
110.48±55.13b |
95.87±67.31b,d |
| High-dose
aspirin | 221.15±56.37 |
87.88±72.17b,e |
57.21±12.15c,e |
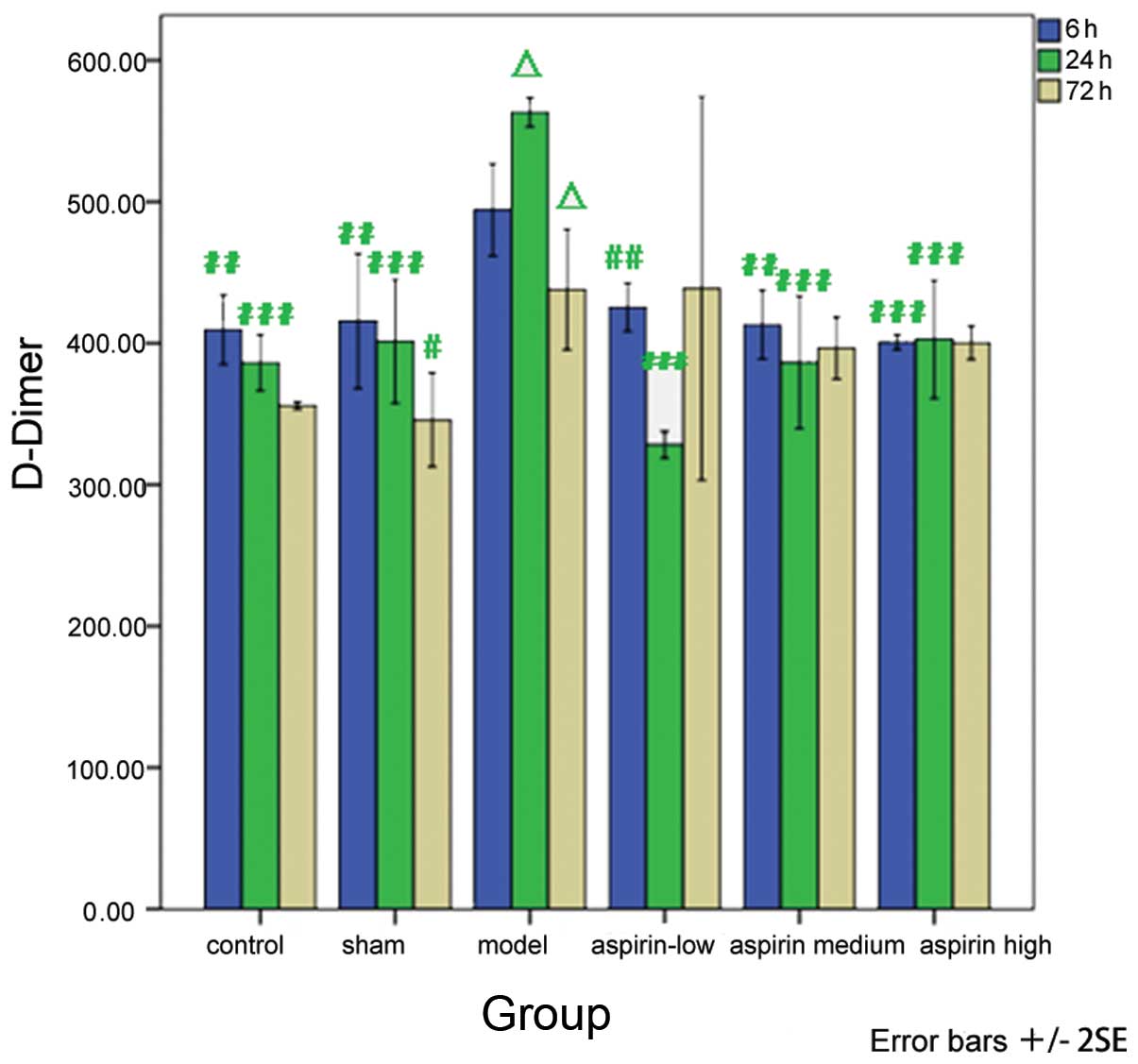
With regard to the D-Dimer levels, a significant
decrease was observed in the low-, medium- and high-dose aspirin
groups at 6 and 24 h susbequent to model induction as compared with
the model group (P<0.01 or P<0.001). When comparing the
results at different time-points with the observations made 6 h
subsequent to model induction within each group, it was revealed
that the levels of D-Dimer were significantly increased 24 h
subsequent to model induction (P<0.05) and significantly
decreased 72 h subsequent to model induction (P<0.05) in the
model group (Table VI and
Fig. 7).
 | Table VID-Dimer level at different
time-points in the six groups. |
Table VI
D-Dimer level at different
time-points in the six groups.
| Group | 6 h (ng/ml) | 24 h (ng/ml) | 72 h (ng/ml) |
|---|
| Control |
409.55±24.54b |
386.25±19.84c | 356.07±2.80 |
| Sham |
415.71±47.81b |
401.34±43.61c |
345.89±33.35a |
| Model | 494.38±32.69 |
563.30±10.36d |
438.13±42.70d |
| Low-dose
aspirin |
425.54±17.12b |
328.21±9.41c | 438.84±135.69 |
| Medium-dose
aspirin |
413.21±24.39b |
386.61±46.88c | 396.61±21.99 |
| High-dose
aspirin |
400.80±5.20c |
402.86±41.83c | 400.36±11.84 |
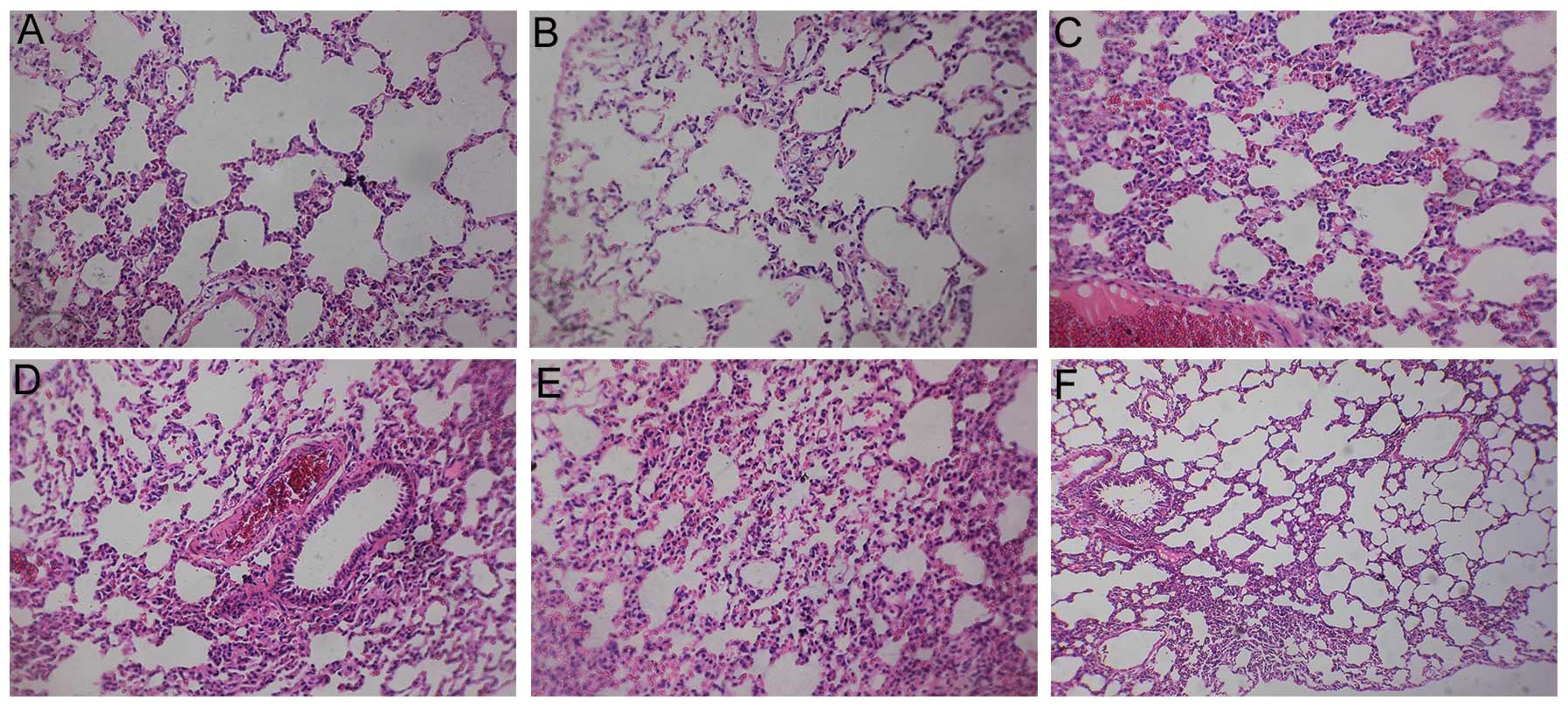
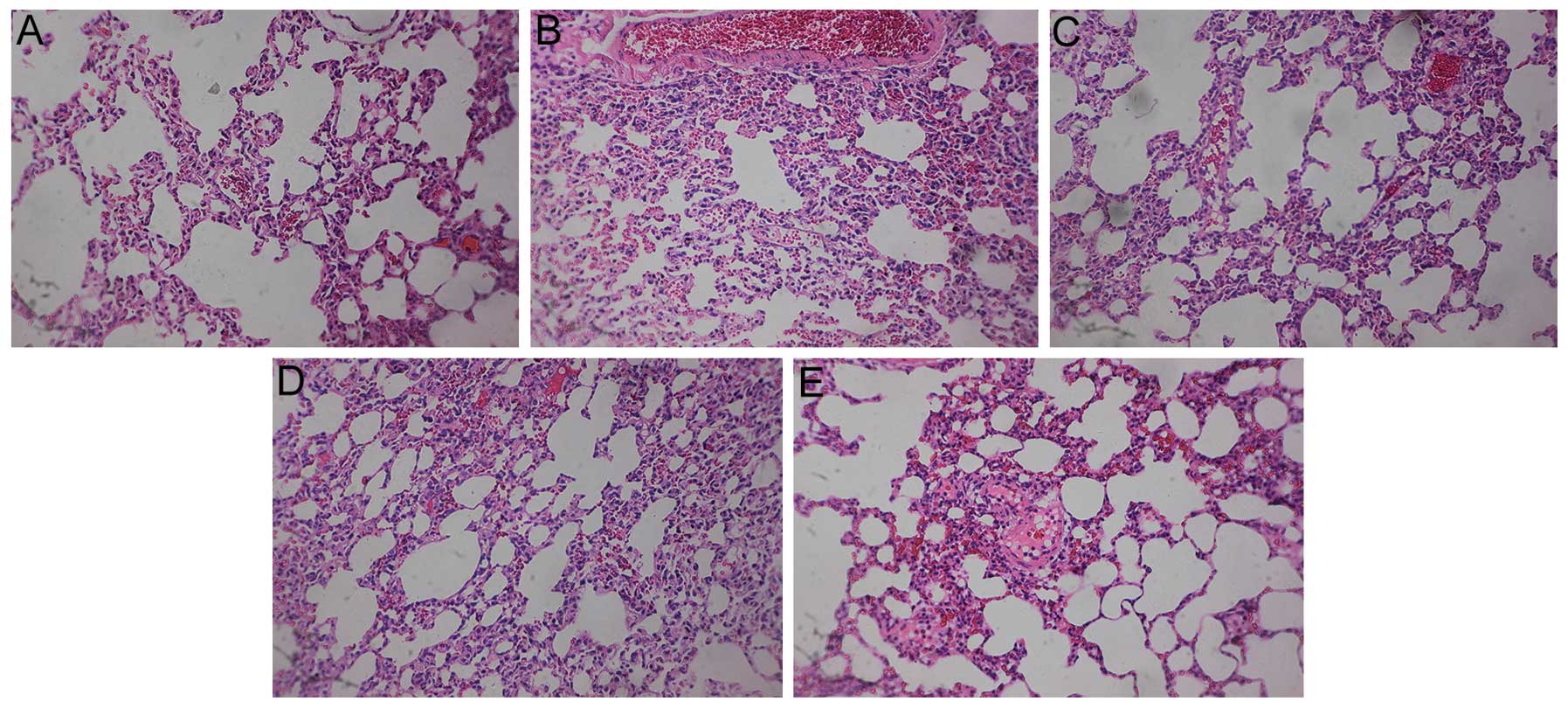
Pathological examination
In addition to the analysis of protein levels,
pathological examinations of the lungs were performed. The
structural integrity of the lung was maintained in the control and
sham groups. However, pulmonary embolism, alveolar wall necrosis
and alveolar hemorrhage were observed in the model group 6, 24 and
72 h subsequent to model induction. Indications of inflammatory
cell infiltration and alveolar wall thickening and congestion were
also observed. The congestion and inflammation were significantly
attenuated following treatment with aspirin (Figs. 8–10).
Discussion
APE is one of the most important global health
concerns, with a high incidence and mortality around the world. The
incidence of APE is ~0.5% in Western countries and the mortality
from APE is 25–30% for untreated patients. Studies have
demonstrated that there is widespread inflammation following APE
and the interaction between the blood clotting products and
inflammatory cells aggravates pathological reactions (1,2,12).
In the present study, the levels of factors involved
in inflammation, such as ERK, PI3K and Akt, were observed to
markedly change following APE. Mitogen-activated protein kinase
pathways are important intracellular signaling pathways, among
which the ERK signaling pathway is closely associated with cell
proliferation (13). There are two
closely related ERK isoforms, ERK1 and ERK2, which are often
referred to as ERK1/2. A number of factors, including growth
factors, irradiation and hydrogen peroxide, phosphorylate ERK1/2 to
P-ERK1/2, which is subsequently transported into the nucleus where
it interacts with transcription factors, such as nuclear factor-κB
(NF-κB), to regulate the transcription of target genes. The
activation of target genes, in turn, affects the function and
metabolic activity of the cells and affects cell proliferation and
the synthesis of protein and collagen (14).
The enhanced expression of P-ERK1/2 has been
demonstrated in the lung tissues of rats with acute lung injury
induced by lipopolysaccharide (LPS) (4), which suggests that ERK is critical in
the inflammatory reactions mediated by TNF-α and IL-12. In the
study by Shi et al(6),
these observations were further supported when it was shown that
LPS activated the ERK pathway and induced the expression of TNF-α.
The activation of the ERK pathway may be important in the synthesis
and secretion of inflammatory factors by macrophages (7), indicating that ERK is important in
the process of inflammatory reactions.
By contrast, the PI3K/Akt pathway is an
intracellular signaling pathway, which affects cell proliferation,
apoptosis and differentiation. PI3K is widely distributed in the
lung, heart and brain. PI3K/Akt consists of two subunits, p110 (a
110×103 kDa catalytic subunit) and p85 (an 85×1,000 kDa regulatory
subunit) (15). Akt, also known as
protein kinase B, is a serine/threonine protein kinase (16) and is the downstream target protein
of PI3K. The PI3K/Akt pathway may mediate the activation and
migration of immune/inflammatory cells and be important in the
pathology of asthma.
A previous study (8) demonstrated that there was a
significant upregulation of Akt in rats with experimental asthma
and that the expression of PI3K/Akt was also significantly higher
than that observed in the control group. In addition, a number of
other studies have shown that the PI3K/Akt pathway is involved in
the pathology of hypoxic pulmonary hypertension, while a PI3K/Akt
pathway inhibitor prevented pulmonary hypertension (9,10).
Furthermore, the PI3K/Akt pathway participates in inflammatory
responses and hypoxia. However, no study has yet investigated the
expression of ERK, PI3K and Akt following APE, and only very few
studies have explored the effects of aspirin on the expression of
ERK and PI3K/Akt (17,18).
In the present study, increased levels of ERK, PI3K
and Akt were observed in the lung tissues at 6, 24 and 72 h
subsequent to model induction. By contrast, treatment with
different doses of aspirin resulted in the decreased expression of
these proteins, suggesting that aspirin may downregulate the
expression of ERK, PI3K and Akt in rat lungs. Downregulation in
these signaling pathways may, in turn, inhibit the expression of
the inflammatory cytokines TNF-α, IL- 1β and IL-8 (1) and attenuate the inflammatory
responses following APE.
The characteristics of APE are alveolar wall
necrosis and hemorrhage. In the present study, the pathological
changes that were observed demonstrated that the model was induced
successfully and that aspirin was capable of ameliorating the
congestion and inflammation of the lung. This indicated that the
inhibition of ERK, PI3K and Akt was potentially the mechanism
involved in the protective effects of aspirin.
The ‘Guidelines on the diagnosis and management of
acute pulmonary embolism’ (3) have
recommended that the levels of prognostic markers, such as serum
BNP, TnT and D-Dimer, are evaluated, since this may be beneficial
for the prediction of the prognosis of patients with APE at an
early stage. Low levels of BNP, TnT and D-Dimer have been
correlated with a favorable prognosis, such as lower mortality and
positive outcomes. In patients with APE, the sharply increased
right ventricular pressure may compress the right coronary artery
and result in myocardial damage; the balance of oxygen
supply/demand in the right ventricle may also be disturbed, leading
to an increased expression of TnT.
BNP is always secreted by ventricular myocytes.
Ventricular dilatation is capable of stimulating the synthesis and
secretion of BNP, which is not always interrelated with the
function of the right ventricle. D-Dimer is a degradation product
of cross-linked fibrin. As a specific fibrinolytic marker, D-Dimer
is widely used in the diagnosis of APE. Although the specificity of
D-Dimer in diagnosing APE is relatively low, the sensitivity is
high. In the present study, significant increases were observed in
the serum levels of BNP, TnT and D-Dimer in the model group 6 and
24 h subsequent to model induction, particularly at 24 h. At the
72-h time-point, the serum levels of BNP, TnT and D-Dimer began to
decrease. However, treatment with aspirin significantly decreased
the levels of BNP, TnT and D-Dimer, suggesting that aspirin
treatment may result in a more favorable prognosis. Moreover, serum
levels of BNP, TnT and D-Dimer may be important in the protective
effects of aspirin.
In conclusion, treatment with aspirin reduced lung
damage in a rat model of APE and improved the prognosis of the
rats. Decreased expression levels of ERK, PI3K and Akt in the lungs
of rats with APE and reduced serum levels of BNP, TnT and D-Dimer
may be important in these effects.
Acknowledgements
This study was funded by the Zhejiang Provincial
Natural Science Foundation of China (grant no. LY12H29005).
References
|
1
|
Liu YC, Kao SJ, Chuang IC and Chen HI:
Nitric oxide modulates air embolism-induced lung injury in rats
with normotension and hypertension. Clin Exp Pharmacol Physiol.
34:1173–1180. 2007.PubMed/NCBI
|
|
2
|
Sheu JR, Hsiao G, Lee YM and Yen MH:
Antithrombotic effects of tetramethylpyrazine in in vivo
experiments. Int J Hematol. 73:393–398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torbicki A, Perrier A, Konstantinides S,
et al: Guidelines on the diagnosis and management of acute
pulmonary embolism: the task force for the diagnosis and management
of acute pulmonary Embolism of the European Society of Cardiology
(ESC). Eur Heart J. 29:2276–2315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu Z, Yang Z, Fu Y, et al: Protective
effect of gossypol on lipopolysaccharide-induced acute lung injury
in mice. Inflamm Res. 62:499–506. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiang JX, Zhang Y, Ji SH, Zhu P and Wang
ZG: Kinetics of mitogen-activated protein kinase family in
lipopolysaccharide-stimulated mouse Kupffer cells and their role in
cytokine production. Shock. 18:336–341. 2002. View Article : Google Scholar
|
|
6
|
Shi L, Kishore R, McMullen MR and Nagy LE:
Lipopolysaccharide stimulation of ERK1/2 increases TNF-α production
via Egr-1. Am J Physiol Cell Physiol. 282:C1205–C1211. 2002.
|
|
7
|
Jarrar D, Kuebler JF, Rue LW III, et al:
Alveolar macrophage activation after trauma-hemorrhage and sepsis
is dependent on NF-κB and MAPK/ERK mechanisms. Am J Physiol Lung
Cell Mol Physiol. 283:L799–L805. 2002.PubMed/NCBI
|
|
8
|
Yadav UC, Naura AS, Aguilera-Aguirre L, et
al: Aldose reductase inhibition prevents allergic airway remodeling
through PI3K/AKT/GSK3β pathway in mice. PLoS One.
8:e574422013.PubMed/NCBI
|
|
9
|
Banquet S, Delannoy E, Agouni A, et al:
Role of Gi/o-Src kinase-PI3K/Akt pathway and caveolin-1
in β2-adrenoceptor coupling to endothelial NO synthase
in mouse pulmonary artery. Cell Signal. 23:1136–1143. 2011.
|
|
10
|
Yang D, Liu Z, Zhang H and Luo Q: Ghrelin
protects human pulmonary artery endothelial cells against
hypoxia-induced injury via PI3-kinase/Akt. Peptides. 42:112–117.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diaz JA, Obi AT, Myers DD Jr, et al:
Critical review of mouse models of venous thrombosis. Arterioscler
Thromb Vasc Biol. 32:556–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smulders YM: Pathophysiology and treatment
of haemodynamic instability in acute pulmonary embolism: the
pivotal role of pulmonary vasoconstriction. Cardiovasc Res.
48:23–33. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu PJ, Ferrari G, Pirelli L, et al:
Vascular injury and modulation of MAPKs: a targeted approach to
therapy of restenosis. Cell Signal. 19:1359–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eblen ST, Slack JK, Weber MJ and Catling
AD: Rac-PAK signaling stimulates extracellular signal-regulated
kinase (ERK) activation by regulating formation of MEK1-ERK
complexes. Mol Cell Biol. 22:6023–6033. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Voigt P, Brock C, Nurnberg B and Schaefer
M: Assigning functional domains within the p101 regulatory subunit
of phosphoinositide 3-kinase γ. J Biol Chem. 280:5121–5127.
2005.PubMed/NCBI
|
|
16
|
Lin CH, Yeh SH, Lin CH, et al: A role for
the PI-3 kinase signaling pathway in fear conditioning and synaptic
plasticity in the amygdala. Neuron. 31:841–851. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Kebir D, Jozsef L, Khreiss T, et al:
Aspirin-triggered lipoxins override the apoptosis-delaying action
of serum amyloid A in human neutrophils: a novel mechanism for
resolution of inflammation. J Immunol. 179:616–622. 2007.PubMed/NCBI
|
|
18
|
Tsai KS, Yang RS and Liu SH:
Benzo[a]pyrene regulates osteoblast proliferation through an
estrogen receptor-related cyclooxygenase-2 pathway. Chem Res
Toxicol. 17:679–684. 2004.
|