Introduction
Cancer-testis antigens (CTAs) are a group of
antigens that are exclusively expressed in germline cells, such as
testis and placental cells under normal physiological conditions.
CTAs are also expressed in numerous human tumor cells of various
histological origins (1). The
first CTA identified in 1991 is termed melanoma-associated antigen
1 (MAGE-A1) (2). In 1994,
Weber et al(3) demonstrated
that decitabine, a demethylation agent, upregulates MAGE-A1
expression in melanoma cell lines. In 1996, the activation of
MAGE-A1 in tumor cells was correlated with genome-wide
demethylation (4). Subsequent to
this, more CTAs were identified, including PRAME(5), NY-ESO-1(6) and SSX family antigens
(7). Numerous studies concerning
the immunogenicity of CTAs and the asssociation between CTAs and
demethylation have been conducted (8–11).
For example, in acute myeloid leukemia (AML) cell lines, NY-ESO-1
was upregulated by decitabine and thus decitabine-treated AML cells
become susceptible to NY-ESO-1-specific T-cell cytotoxicity
(8). Those results have suggested
CTA to be a potential target for tumor immunotherapy. However, the
majority of studies concerning CTAs and decitabine were limited to
in vitro investigations and few CTAs have been demonstrated
to be activated by decitabine in vivo by clinical decitabine
treatment.
The nuclear RNA export factor 2 (NXF2) human
gene was first identified in spermatogonia (12). Early studies on NXF2 were
focused on its function as an mRNA exporter (13) and its involvement in male
infertility (14–16). NXF2 has been found to be positive
in 1.8% of invasive ductal carcinomas of the breast (17). Dubovsky et al(18) observed its involvement in chronic
lymphocytic leukemia (CLL). Following screening of 22 CLL patients,
two were observed to be positive for NXF2-specific IgG antibodies.
The presence of these antibodies supported the hypothesis that NXF2
exhibits high enough immunogenicity to induce tumor-specific immune
responses (18). However, no
studies have been conducted concerning the expression pattern,
regulation mechanism and clinical significance of NFX2 in
acute leukemia. The aim of the current study was to investigate the
expression and epigenetic regulation mechanism of NXF2 in
acute leukemia cells, in vitro and in vivo.
Materials and methods
Cell lines and patient samples
Bone marrow and peripheral blood samples were
collected from healthy donors and leukemia patients in the
Hematology Department of the Chinese People’s Liberation Army
General Hospital, (Beijing, China). Samples of testis were provided
by the laboratory of the Urology Department, Peking University
Third Hospital (Beijing, China). Written informed consent was
obtained from all donors and patients. All experiments were
approved by the ethics committee of the Chinese People’s Liberation
Army General Hospital. Raji, Z-138, Hut-78, Jurkat, Molt-4,
Kasumi-1, NB4, THP-1, U937 and K562 acute leukemia cell lines were
obtained from the cell culture center of Peking Union Medical
College (Beijing, China). Primary cells were separated from bone
marrow or peripheral blood samples using the Ficoll-Paque method
(19). Cell lines and primary
acute leukemia cells were maintained in RPMI-1640 supplemented with
10% fetal calf serum, 1% penicillin/streptomycin and 1% glutamine.
Cultures were maintained in a 5% CO2-humidified
incubator at 37°C. Decitabine (5-aza-2′-deoxycytidine, Dacogen,
DAC) was purchased from Xi’an Jansson Pharmaceutical, Ltd. (Xi’an,
China). For demethylation treatment, decitabine was added to the
in vitro culture system at a concentration of 1 μmol/l for 3
days for leukemia cell lines and 5 μmol/l for 3 days for primary
leukemia samples (18). For
clinical treatment, decitabine was administered alone at a dose of
20 mg/m2/day for 5 consecutive days (20).
RNA isolation, reverse transcription and
semi-quantitative reverse transcription-polymerase chain reaction
(RT-PCR)
Total RNA was extracted from samples or cell lines
with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), according to the manufacturer’s instructions. Total RNA was
reverse transcribed to complementary DNA (cDNA) as previously
described (21). Reverse
transcription and semi-quantitative RT-PCR were conducted on a
Veriti® Thermal Cycler (Applied Biosystems, Inc., Foster
City, CA, USA). Primers used for semi-quantitative RT-PCR were:
Forward: 5′-TGA AAC CCT GCA AGG AAA AC-3′ and reverse: 5′-GCA CTG
AGG GAG TCC ACA AT-3′ for NXF2; and forward: 3′-GAG TCA ACG
GAT TTG GTC GT-5′ and reverse: 3′-TTG ATT TTG GAG GGA TCT CG-5′ for
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The 25 μl
PCR mixture was prepared with 12.5 μl 2X GoTaq Green Master mix
(Promega Corporation, Fitchburg, WI, USA), cDNA, primers and
nuclease-free water. The amplification procedure was as follows: 35
cycles of denaturation for 30 sec at 95°C, annealing for 50 sec at
60°C and extension for 50 sec at 72°C. PCR amplification products
were analyzed on 1.5% agarose gels stained with ethidium
bromide.
qPCR
qPCR was performed to quantify target genes in bone
marrow samples using the Stratagene Mx3000P real-time qPCR system
(Stratagene, La Jolla, CA, USA). Primers and probes used were:
Forward: 3′-GAAGCCAGGCCAAAT GGA-5′, reverse:
3′-AGTCTGGGTCAAAGCGGAGAT-5′ and probe:
3′-FAM-ATGAACAAACGGTACAATGTCTCC CA-TAMRA-5′ for NFX2; and
forward: CATACCAGGAAA TGAGCTTGACAA, reverse: CATACCAGGAAATGAGCT
TGACAA and probe: 3′-FAM-CTCCTCTGACTTCAA CAGCGACACCCA-TAMRA-5′.
Each qPCR reaction was conducted in 20 μl reaction volume with
TaqMan universal master mix (Applied Biosystems, Inc.), 0.25
μM primers and probe and 20 ng cDNA. The amplification procedure
was as follows: 40 cycles of denaturation for 15 sec at 95°C and
annealing for 60 sec at 60°C. NXF2 mRNA expression was
determined by 2−ΔΔCT relative to GAPDH.
Western blot analysis
Protein extracts from U937 and Raji cells were
isolated using immunoprecipitation assay buffer (Sigma-Aldrich, St.
Louis, MO, USA) and quantified to ensure equivalent protein
loading. Polyacrylamide gel electrophoresis, tank-based transfer to
Immobilon Hybond-C membranes (Amersham Pharmacia Biotech,
Piscataway, NJ, USA) and immunodetection were performed as
described (22). β-actin antibody
was purchased from Abcam (Cambridge, MA, USA) and NXF2 antibody was
purchased from Sigma-Aldrich. Signals were visualized using
Immobilon Western Chemiluminescent horseradish peroxidase substrate
(Millipore Corporation, Billerica, MA, USA) by exposure to films
(Kodak, Rochester, NY, USA).
Firefly luciferase reporter
constructs
Five promoter regions of the wild-type human
NXF2 gene were amplified from one testis sample by PCR using
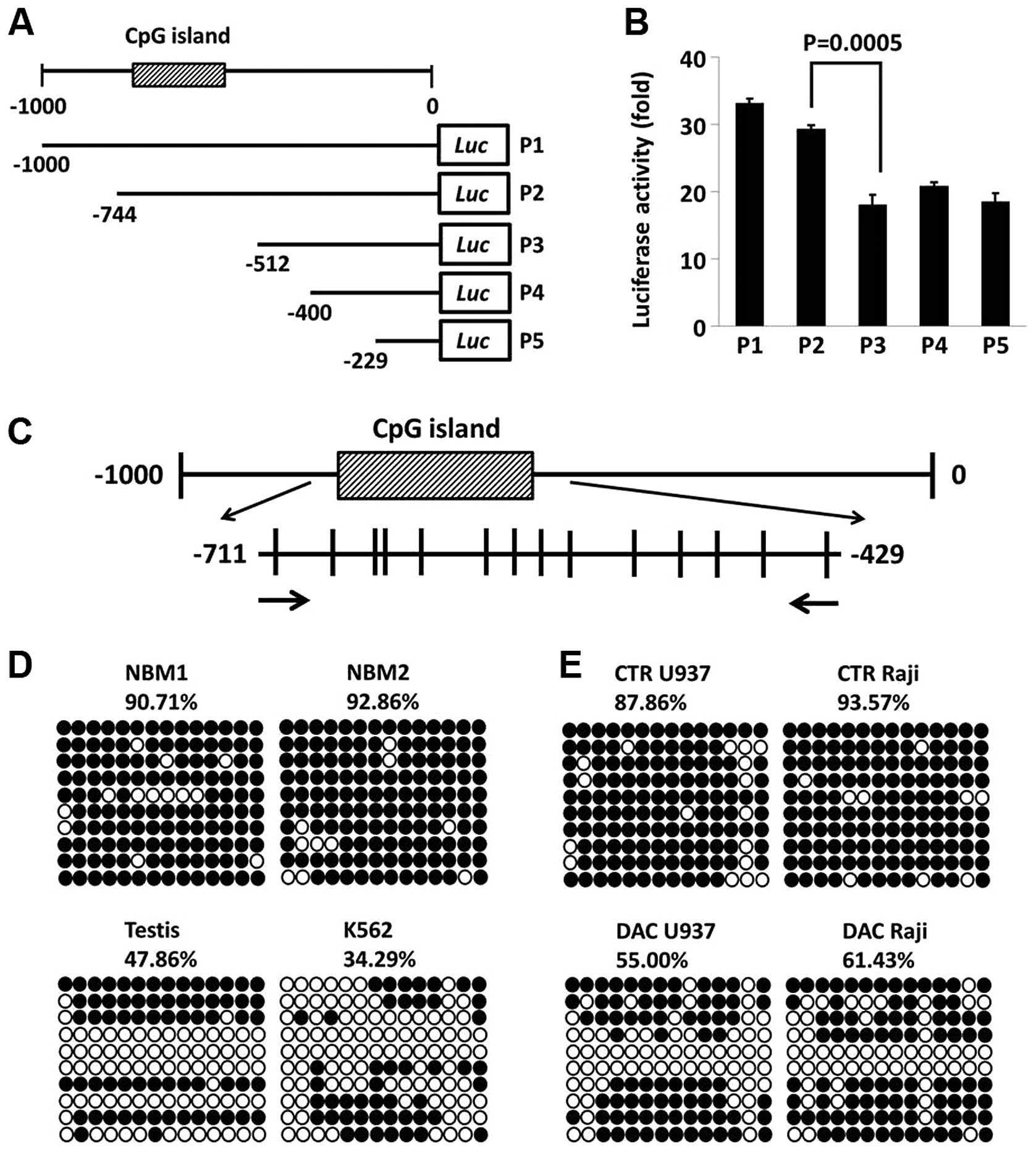
specific primers (Fig. 3A).
Primers were designed to contain KpnI and HindIII
restriction enzyme sites. Sense primers used were: 5′-CGGGGTACCAGT
GTGGGCTGAGGGTTG GA-3′, −1,000 to +28 bp for P1;
5′-CGGGGTACCGTAAGCATCCCCTGCTACACG-3′, −744 to +28 bp for P2;
5′-CGGGGTACCCAGGTGCCTGTAATG CCAGCT-3′, −512 to +28 bp for P3;
5′-CGGGGTACCCAG AGCGAGACGCCGTCT-3′, −400 to +28 bp for P4; 5′-CGG
GGTACCGGCAGGCTTATAATCAGAACACCC-3′, −229 to +28 bp for P5; and the
antisense primer was 5′-CCCAAG CTTCAAAGCAGTGGGGAGAGGAC-3′. The
amplified promoter regions were cloned downstream of the firefly
luciferase-coding region between KpnI and HindIII of
a modified pGL3-control plasmid. Successful construction was
confirmed by sequencing.
Transfection and luciferase assays
293T cells were plated in 24-well plates at
5×104 cells/well and grown overnight. Firefly luciferase
reporter vector (500 ng) containing different promoter regions of
NXF2 and 10 ng control vector pRL-TK (Promega Corporation)
containing Renilla luciferase were cotransfected to 293 T
cells in a final volume of 0.35 ml using SuperFect (Qiagen, Hilden,
German). Cells were collected 48 h following transfection and
luciferase activity was measured using a dual-reporter luciferase
assay system (Promega Corporation).
Bisulfite modification and genomic
sequencing
Genomic DNA was extracted from cells using the
Wizard Genomic DNA Purification kit (Promega Corporation). DNA (1
μg) was modified with sodium bisulfite using the EpiTect Bisulfite
kit (Qiagen). Primers for bisulfite-sequencing analysis were
designed by MethPrimer software (San Francisco, CA, USA) (23) using the bisulfite-treated DNA as a
template (Forward: 5′-GAGTTTTTAATTGTTTGTGTTGAG-3′ and reverse:
5′-GGTAGAGGTTGTATGAGATGGG-3′). PCR products were gel-purified and
cloned into a pGEM-T vector (Promega Corporation). The inserted PCR
fragments of individual clones were sequenced using an ABI PRISM
DNA sequencer (Applied Biosystems, Inc.).
Results
Activation of NXF2 expression following
decitabine treatment in acute leukemia cell lines
NXF2 expression was screened in two normal
bone marrow (NBM) samples, two normal peripheral blood samples and
two normal testis samples. The results showed that NXF2 was
only positively expressed in the testis samples (Fig. 1A). NXF2 expression was
screened in 10 acute leukemia cell lines, including five AML cell
lines (Kasumi-1, NB4, THP-1, U937 and K562 cell lines; K562 cells
are cells of the blastic transformation of chronic myeloid
leukemia) and five acute lymphocytic leukemia (ALL) cell lines
(Raji, Z-138, HuT-78, Jurkat and Molt-4), prior to and following
decitabine treatment. The results showed that only K562 cells
expressed NXF2 originally. However, following decitabine
treatment, eight of the remaining nine acute leukemia cell lines
demonstrated activation of NXF2 expression, with the
exception of NB4 cells (Fig. 1B).
This upregulation was confirmed in one AML cell line (U937) and one
ALL cell line (Raji) by western blot analysis (Fig. 1C). Raji cells were then analyzed
for the effect of different doses of decitabine on the activation
of NXF2. The results showed that NXF2 activation was
dose-dependent. Following treatment with 1 μM decitabine for 3
days, it was noted that NXF2 remained positive 28 days
subsequent to the cessation of decitabine treatment (Fig. 1D).
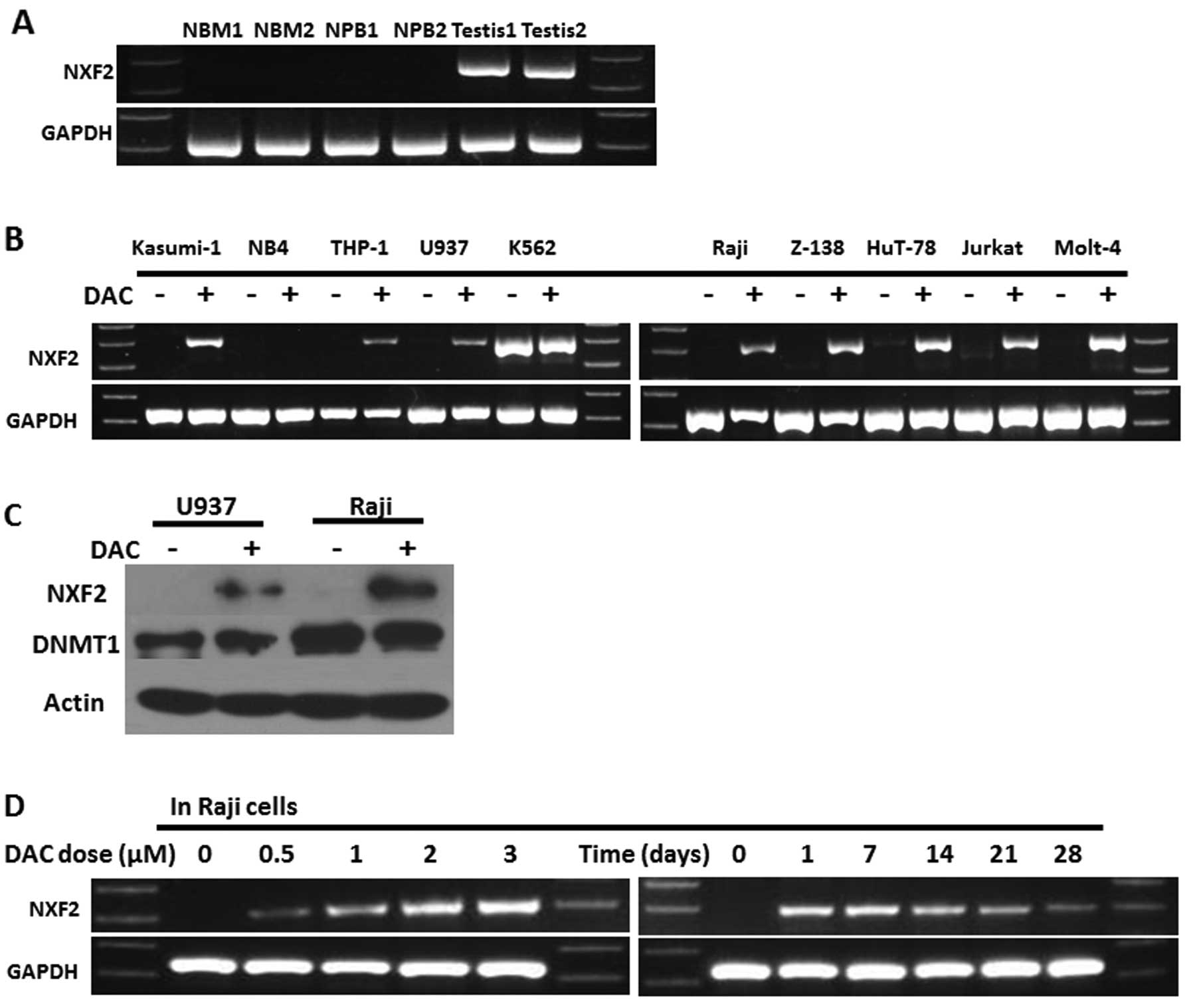 | Figure 1Decitabine activated NXF2
expression in acute leukemia cell lines in vitro. (A) RT-PCR
showed that NXF2 was originally expressed only in testis and
not in bone marrow or peripheral blood samples from healthy donors.
(B) Acute leukemia cell lines, including 5 acute myeloid leukemia
cell lines (Kasumi-1, NB4, THP-1, U937 and K562) and 5 acute
lymphocytic leukemia cell lines (Raji, Z-138, HuT-78, Jurkat and
Molt-4) were screened for NXF2 expression prior to and
following 1 μM decitabine treatment for 3 days in vitro.
NXF2 expression was positive in K562 cells and was activated in
the majority of other acute leukemia cell lines except NB4
following decitabine treatment. (C) In Raji and U937 cells,
upregulation of NXF2 expression following decitabine treatment was
confirmed by western blot analysis. β-actin was used as the
positive control. (D) In Raji cells, NXF2 activation was
decitabine dose-dependent and its expression lasted ≥28 days
following the cessation of decitabine treatment. DAC,
decitabine-treated cells; NFX2, nuclear RNA export factor
2. |
Activation of NXF2 expression following
decitabine treatment in primary acute leukemia cells in vitro and
in vivo
Primary cells from 11 bone marrow samples, including
four AML patients, four ALL patients and three healthy donors were
collected. The primary cells were treated with 5 μM decitabine for
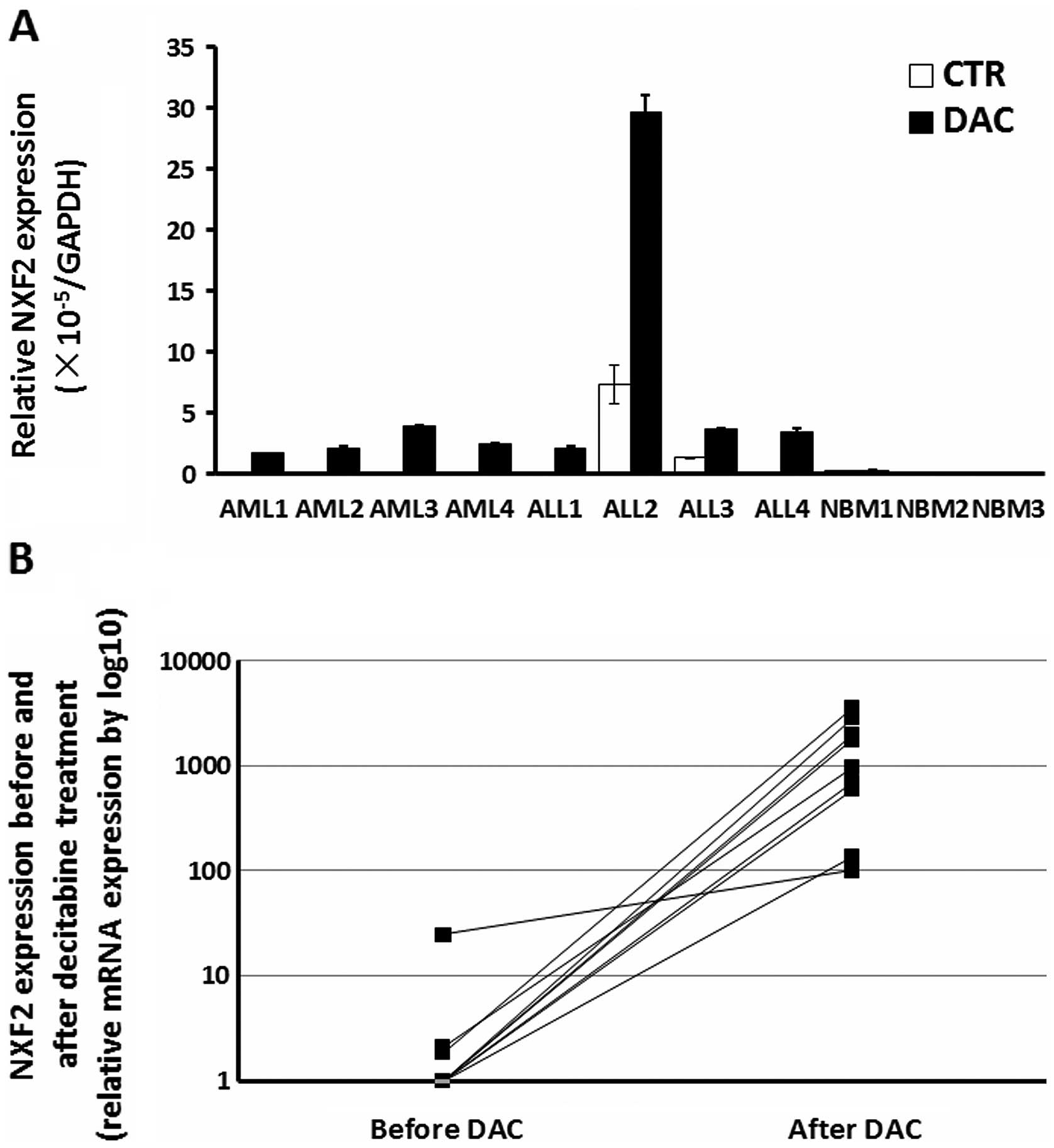
3 days in vitro. Relative NXF2 expression (Fig. 2A) was low in the majority of the
samples. Following decitabine treatment, NXF2 mRNA
expression in all acute leukemia samples was significantly
upregulated. However, this upregulation was not observed in healthy
donor samples. The results suggested that decitabine treatment
activated NXF2 expression in primary acute leukemia cells
in vitro.
In addition, another nine patients with AML or
myelodysplastic syndrome, who received decitabine treatment
clinically were selected. The samples were collected prior to and
following the first cycle of decitabine treatment. The
characteristics of these patients are listed in Table I. Samples were obtained from the
bone marrow, with the exception of patient 6, which was a
peripheral blood sample as the patient had hyperleukocytosis.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Patient | Age (years) | Gender | Chromosome | Gene
abnormalities | Diagnosis | Response | DAC cycles | Prognosis |
|---|
| 1 | 61 | M | 46,XY |
MLL-PTD(+) | MDS-RAEB | Failure | 3 | Progressed to AML
after 3 cycles |
| 2 | 75 | M | 46,XY,12p-inc[10]/,
hypodiploid(43–45) |
NUP98/HOXA9(+) | AML (MDS
transformed) | SD | 8 | Still in DAC
maintenance |
| 3 | 38 | M | 46,XY | - | AML (MDS
transformed) | SD | 5 | Quit DAC treatment
after the 5th cycle |
| 4 | 62 | F | 46,XX | - | AML (MDS
transformed) | PD | 3 | Quit DAC treatment
after the 3rd cycle |
| 5 | 53 | F | 46,XX |
GIT2/PDGFRB(+) | AML (CMML
transformed) | PD | 3 | This patient
received TKI treatment first. DAC was added in combination with TKI
when her CMML progressed to AML. She quit DAC treatment after the
3rd cycle and received allo-HSCT |
| 6 | 49 | M | - | - | AML-M6 | SD | 7 | Quit DAC treatment
and received allo-HSCT after the 7th cycle |
| 7 | 59 | F | 46,XY | - | AML (MDS
transformed) | CR | 14 | CR after the 7th
cycle, but relapsed after the 14th cycle, quit DAC treatment after
relapse |
| 8 | 64 | M | 46,XY | P15
hyper-methylated | AML (MDS
transformed) | CR | 12 | CR after the 5th
cycle, but relapsed after the 10th cycle, quit DAC treatment after
relapse. |
| 9 | 82 | F | 46,XX | - | AML (MDS | PR | 10 | PR after the 4th
cycle, but transformed) turned to AML after the 7th cycle, another
3 cycles of DAC after progression did not work |
As the nine patients did not respond to the first
cycle of decitabine, leukemia cells were observed in all of the
samples. qPCR results showed that NXF2 expression was
upregulated to varying degrees in all nine patients following
decitabine treatment (Fig. 2B).
This result suggests that decitabine treatment activated
NXF2 expression in primary acute leukemia cells in
vivo.
NXF2 expression correlates with CpG
island hypomethylation in its promoter region
There was a typical CpG island located in the
NXF2 promoter region spanning 282 bp and containing 14 CpG
sites (Fig. 3A and C). By
luciferase reporter construction, it was demonstrated that the
promoter region containing the CpG island was essential for
NXF2 expression (Fig. 3B).
The methylation status of this CpG island in two NBM samples
without NXF2 expression in one testis sample and in K562
cells with NXF2 expression was detected. The CpG island was
densely-methylated in the two NBM samples (>90%), but only
partially methylated in the testis sample and in the K562 cells
(Fig. 3D). Raji and U937 cell
lines were originally NXF2-negative and were analyzed for
the methylation status of the CpG island prior to and following
decitabine treatment. The methylation level of the CpG island
decreased following decitabine treatment, concurrent with
NXF2 activation (Fig. 3E).
These results suggest that NXF2 expression was silenced by
CpG island hypermethylation; thus, decitabine activated NXF2
expression by the demethylation mechanism.
Discussion
NXF2 is a CTA gene, however, there is limited
information available concerning its expression pattern and the
mechanism of NXF2 regulation. The present study showed that
NXF2 expression was activated by a demethylating agent,
decitabine, in acute leukemia cell lines and primary acute leukemia
samples in vitro and in vivo. This study also showed
that NXF2 expression is regulated by CpG island
hypermethylation in its promoter region. To the best of our
knowledge, this is the first study to demonstrate the mechanism of
NXF2 regulation in acute leukemia cells.
Currently, certain CTAs have been demonstrated to be
potential targets for immunotherapy against cancer due to their
tumor specificity and strong immunogenicity. For example, Hunder
et al(9) expanded
autologous CD4+ T-cell clones specific for NY-ESO-1 (a
CTA). Specific T cells were infused into a patient with refractory
metastatic melanoma and a durable clinical remission was observed.
In another study, Quintarelli et al(24) showed that cytotoxic T lymphocytes
specific for PRAME, another type of CTA, target leukemic and
leukemic-precursor cells. It was also demonstrated that NXF2
exhibits a high enough level of immunogenicity to induce immune
responses in CLL patients (18).
However, the application of CTA-specific immunotherapy is limited
due to a relatively low expression in acute leukemia. It was
suggested that this limitation was able to be overcome by
demethylation treatment. Yan et al(25) demonstrated that, following
demethylation treatment, the expression of PRAME in the ALL cell
line (Raji cells) was upregulated. These Raji cells with greater
PRAME expression showed increased sensitivity to killing by
formerly low avidity PRAME-specific T-cell clones. However, the
majority of similar studies are performed in vitro and few
studies have observed that CTAs were activated in malignant cells
in vivo by clinical decitabine treatment. In the present
study it was demonstrated that NXF2 was activated in acute
leukemia cells not only in vitro, but also in vivo by
clinical decitabine treatment. It was hypothesized that NXF2 served
as a novel target for immunotherapy against acute leukemia
following clinical demethylation treatment. However, as no epitope
sequence of NXF2 has yet been identified, it was difficult to
determine the details of NXF2-specific immune responses following
decitabine treatment. Thus, future studies are required to
investigate the epitope of NXF2.
In conclusion, to the best of our knowledge, this
was the first study to demonstrate that NXF2 is activated by
decitabine in acute leukemia cells in vitro and in
vivo; this activation was due to demethylation of the CpG
island in the NXF2 promoter region. According to these
results, it was hypothesized that NXF2 may serve as a novel
clinical target for immunotherapy against acute leukemia.
Acknowledgements
This study was supported by grants from the National
Basic Research Program of China (grant no. 2005CB522400); the
National Natural Science Foundation of China (grant nos. 90919044,
30971297 and 81000221); the Capital Medical Development Scientific
Research Fund (grant no. 2007–2040); the National Public Health
Grand Research Foundation (grant no. 201202017); and the capital of
the Public Health Project (grant no. Z111107067311070).
References
|
1
|
Fratta E, Coral S, Covre A, et al: The
biology of cancer testis antigens: putative function, regulation
and therapeutic potential. Mol Oncol. 5:164–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Bruggen P, Traversari C, Chomez P,
et al: A gene encoding an antigen recognized by cytolytic T
lymphocytes on a human melanoma. Science. 254:1643–1647. 1991.
|
|
3
|
Weber J, Salgaller M, Samid D, et al:
Expression of the MAGE-1 tumor antigen is up-regulated by the
demethylating agent 5-aza-2′-deoxycytidine. Cancer Res.
54:1766–1771. 1994.PubMed/NCBI
|
|
4
|
De Smet C, De Backer O, Faraoni I, Lurquin
C, Brasseur F and Boon T: The activation of human gene MAGE-1 in
tumor cells is correlated with genome-wide demethylation. Proc Natl
Acad Sci USA. 93:7149–7153. 1996.PubMed/NCBI
|
|
5
|
van Baren N, Chambost H, Ferrant A, et al:
PRAME, a gene encoding an antigen recognized on a human melanoma by
cytolytic T cells, is expressed in acute leukaemia cells. Br J
Haematol. 102:1376–1379. 1998.PubMed/NCBI
|
|
6
|
Chen YT, Boyer AD, Viars CS, Tsang S, Old
LJ and Arden KC: Genomic cloning and localization of CTAG, a gene
encoding an autoimmunogenic cancer-testis antigen NY-ESO-1, to
human chromosome Xq28. Cytogenet Cell Genet. 79:237–240. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gure AO, Türeci O, Sahin U, et al: SSX: a
multigene family with several members transcribed in normal testis
and human cancer. Int J Cancer. 72:965–971. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Almstedt M, Blagitko-Dorfs N, Duque-Afonso
J, et al: The DNA demethylating agent 5-aza-2′-deoxycytidine
induces expression of NY-ESO-1 and other cancer/testis antigens in
myeloid leukemia cells. Leuk Res. 34:899–905. 2010.
|
|
9
|
Hunder NN, Wallen H, Cao J, et al:
Treatment of metastatic melanoma with autologous CD4+ T
cells against NY-ESO-1. N Engl J Med. 358:2698–2703. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fradet Y, Picard V, Bergeron A and LaRue
H: Cancer-testis antigen expression in bladder cancer. Prog Urol.
15(Suppl 1): 1303–1313. 2005.PubMed/NCBI
|
|
11
|
Calabrò L, Fonsatti E, Altomonte M, et al:
Methylation-regulated expression of cancer testis antigens in
primary effusion lymphoma: immunotherapeutic implications. J Cell
Physiol. 202:474–477. 2005.PubMed/NCBI
|
|
12
|
Wang PJ, McCarrey JR, Yang F and Page DC:
An abundance of X-linked genes expressed in spermatogonia. Nat
Genet. 27:422–426. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takano K, Miki T, Katahira J and Yoneda Y:
NXF2 is involved in cytoplasmic mRNA dynamics through interactions
with motor proteins. Nucleic Acids Res. 35:2513–2521. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CP, Su YN, Lin HH, et al: De novo
duplication of Xq22.1→q24 with a disruption of the NXF gene cluster
in a mentally retarded woman with short stature and premature
ovarian failure. Taiwan J Obstet Gynecol. 50:339–344.
2011.PubMed/NCBI
|
|
15
|
Pan J, Eckardt S, Leu NA, et al:
Inactivation of Nxf2 causes defects in male meiosis and
age-dependent depletion of spermatogonia. Dev Biol. 330:167–174.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stouffs K, Tournaye H, Van der Elst J,
Liebaers I and Lissens W: Is there a role for the nuclear export
factor 2 gene in male infertility? Fertil Steril. 90:1787–1791.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YT, Ross DS, Chiu R, et al: Multiple
cancer/testis antigens are preferentially expressed in
hormone-receptor negative and high-grade breast cancers. PLoS One.
6:e178762011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dubovsky JA, McNeel DG, Powers JJ, Gordon
J, Sotomayor EM and Pinilla-Ibarz JA: Treatment of chronic
lymphocytic leukemia with a hypomethylating agent induces
expression of NXF2, an immunogenic cancer testis antigen. Clin
Cancer Res. 15:3406–3415. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jaatinen T and Laine J: Isolation of
mononuclear cells from human cord blood by Ficoll-Paque density
gradient. Curr Protoc Stem Cell Biol. Chapter 2(Unit
2A.1)2007.PubMed/NCBI
|
|
20
|
Lübbert M, Ruter BH, Claus R, et al: A
multicenter phase II trial of decitabine as first-line treatment
for older patients with acute myeloid leukemia judged unfit for
induction chemotherapy. Haematologica. 97:393–401. 2012.PubMed/NCBI
|
|
21
|
van Dongen JJ, Macintyre EA, Gabert JA, et
al: Standardized RT-PCR analysis of fusion gene transcripts from
chromosome aberrations in acute leukemia for detection of minimal
residual disease. Report of the BIOMED-1 Concerted Action:
investigation of minimal residual disease in acute leukemia.
Leukemia. 13:1901–1928. 1999.
|
|
22
|
Li CS, Chen C, Zheng P and Liu Y:
Transgenic expression of P1A induced thymic tumor: a role for
onco-fetal antigens in tumorigenesis. PLoS One. 5:e134392010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li LC and Dahiya R: MethPrimer: designing
primers for methylation PCRs. Bioinformatics. 18:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quintarelli C, Dotti G, Hasan ST, et al:
High-avidity cytotoxic T lymphocytes specific for a new
PRAME-derived peptide can target leukemic and leukemic-precursor
cells. Blood. 117:3353–3362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan M, Himoudi N, Basu BP, et al:
Increased PRAME antigen-specific killing of malignant cell lines by
low avidity CTL clones, following treatment with
5-aza-2′-deoxycytidine. Cancer Immunol Immunother. 60:1243–1255.
2011.PubMed/NCBI
|