Introduction
Ovarian cancer is one of the most common types of
malignancy in females worldwide, with an estimated 225,500 novel
cases and 140,200 cancer-related mortalities occurring annually
(1). Although chemotherapy for
ovarian cancer has been well established throughout the last three
decades, the 5-year survival rate remains <50%, the lowest of
all gynecological malignancies. The cause for this is that ovarian
cancer tends to have distantly metastasized by the time the
majority of cases are diagnosed (1). Local invasion is an initiating step
in cell migration and commonly occurs prior to distant metastasis;
therefore, decreasing invasion activity of cancer cells may improve
the outcome of ovarian cancer patients.
In healthy individuals, malignant cells are under
the surveillance of immune cells to resist invasion and metastasis,
which is an essential aspect of anticancer immunity. Dendritic
cells (DCs) are known to be the most specialized antigen-presenting
cells and are important in the development of anticancer immune
responses as they control the initiation and polarization of
adaptive immunity (2). A previous
clinical trial showed promising results achieved by DC vaccination
in advanced ovarian cancer patients, with 90% overall survival
during 36 months of observation (3). Fulfilling this function, however, is
dependent upon the migration of DCs to T-cell-rich lymph organs
following the uptake of the vaccination and the presentation of
tumor antigens. Inhibition of DC invasion activity facilitates
tumor escape from host immune surveillance. A previous study
indicated that intratumoral injection of DCs generated in
vitro, fails to initiate systemic antitumor immunity as the DCs
migrated into regional lymph nodes less efficiently (4). Thus, a complementary therapy, which
decreases the motility of cancer cells without markedly affecting
DC invasion, may be beneficial in preventing ovarian cancer
metastasis.
Cell invasion results from cross-talk between cells
and the extracellular matrix (ECM). Osteopontin (OPN), a
phosphorylated glycoprotein with pleiotropic properties, is
expressed on the ECM and on the surface of a number of cells,
including malignant cells, lymphocytes and vascular smooth muscle
cells. OPN promotes cell adhesion and invasion in various
physiological or pathological conditions (5–7).
These functions are primarily achieved by its interaction with
receptors on the cell surface, including the α5β3 integrin and CD44
(8), and the initiation of
downstream signaling events, for example, matrix metallopeptidase-9
(MMP-9) expression (9–11). A previous study suggested that OPN
decisively enhanced the migration of DCs into draining lymph nodes
(12). In ovarian cancer, OPN
serves as one of the biomarkers for early detection and predictors
of prognosis (13,14), indicating its involvement in cancer
development and progression. However, the specific mechanisms of
OPN in ovarian cancer invasion remain unknown.
Among chemotherapeutic agents against cancer,
natural bioactive molecules possess a unique advantage of milder
side-effects. Curcumin, a polyphenolic pigment extracted from
turmeric (Curcuma longa), is a prime example, due to its low
toxicity and high anticancer potency. The application of curcumin
as a complementary therapy for ovarian cancer appears promising as
it induces apoptosis (15) and
sensitivity to cisplatin (16) in
ovarian carcinoma, without decreasing quality of life (17). Moreover, the enhancement of
adaptive immunity was involved in curcumin-mediated tumor growth
retardation (18,19). However, to the best of our
knowledge, no systematic analysis of curcumin on the invasion of
ovarian cancer cells and DCs has been reported. Sweet basil
(Ocimum basilicum) is commonly used in Chinese traditional
medicine for detumescence, anti-inflammation and promoting
circulation, and previous studies have shown its cytotoxic effects
in cancer cells (20). In the
current study, a polysaccharide extraction was obtained from
Ocimum basilicum (basil polysaccharide, BPS) and was
compared with curcumin for its ability to effect the regulation of
invasion of SKOV3 ovarian cancer cells and DCs. The underlying
mechanisms were investigated. The results indicated that curcumin
and BPS significantly inhibit the invasion of SKOV3 cells, while
curcumin prevented the invasion of DCs to a greater extent compared
with BPS. This diversity was achieved, at least partly, by
distinctly regulating OPN, CD44 and matrix metallopeptidase-9
(MMP-9) expression.
Materials and methods
Materials
For flow cytometry, the following monoclonal
antibodies: anti-CD14, anti-CD1a, anti-CD80, anti-CD86, anti-CD83,
anti-human leukocyte antigen (HLA)-DR, anti-CD209, anti-CD44 and
their isotype antibodies were purchased from BD-Pharmingen
(Heidelberg, Germany). Recombinant human interleukin-4 (IL-4) and
recombinant human granulocyte-macrophage colony-stimulating factor
(GM-CSF) were purchased from R&D Systems (Minneapolis, MN,
USA). Lipolysaccharide (LPS) purified from Escherichia coli
and Eosin Y were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rabbit anti-human OPN polyclonal antibody, anti-mouse and
anti-rabbit horseradish peroxidase conjugated-immunoglobulin G were
purchased from Abcam (Cambridge, MA, USA). Endotoxin levels in all
agents were low (<1.0 EU/ml). The sources of other reagents are
indicated in the text.
Preparation of curcumin and BPS
Curcumin was purchased from Enzo Life Sciences
(Shanghai, China) and dissolved in ethyl alcohol. BPS was prepared
in the Department of Pathology, Shandong University of Traditional
Chinese Medicine (21) and
dissolved in normal saline. The potential contamination of
endotoxin in curcumin and BPS was detected using
QCL-1000® Chromogenic LAL end-point assay (Lonza
Walkersville, Inc., Walkersville, MD, USA), according to the
manufacturer’s instructions. The detection limit of the kit was 0.1
EU/ml. The endotoxin level of 50 μM curcumin and 100 μg/ml BPS
preparations was <0.1 EU/ml.
Cell culture
The SKOV3 human ovarian cancer cell line was
purchased from the Chinese Academy of Medical Sciences (Beijing,
China). Cells were cultured in RPMI-1640 (Thermo Scientific
HyClone, Logan, UT, USA), supplemented with 10% heat inactivated
fetal bovine serum (FBS) (Thermo Scientific HyClone), 100 U/ml
penicillin and 100 μg/ml streptomycin, at 37°C in a 5%
CO2 humidified atmosphere. Curcumin (50 μM) or BPS (100
μg/ml) were added to the medium 24 h prior to experiments.
Generation of human monocyte-derived
DCs
The use of human peripheral blood mononuclear cells
(PBMCs) from healthy donors was approved by the Institutional
Review Board of Shandong University (Jinan, China) and informed
consent was obtained from the DC donors. Monocyte-derived DCs were
prepared as previously described (22). Briefly, CD14+ cells
separated from PBMCs were enriched with a bead-labeled anti-CD14
monoclonal antibody using the magnetic antibody cell sorting system
(Miltenyi Biotec, Bergisch-Gladbach, Germany). The purity of
CD14+ monocytes was >93%. CD14+ monocytes
were cultured for 5 days in complete RPMI medium containing
granulocyte macrophage colony-stimulating factor (GM-CSF; 1,000
U/ml), IL-4 (500 U/ml) and curcumin (50 μM) or BPS (100 μg/ml).
Cells were identified as immature DCs based on the positive
expression of CD1a and CD209, the lack of CD14 and CD83 (purity
>93%) and low expression of human leukocyte antigen (HLA)-DR,
CD80 and CD86. To induce maturation, LPS (1 μg/ml) was added on day
five and cells were cultured for a subsequent two days. Cell
morphology and viability were determined by light microscopy
(CKX31, Olympus, Tokyo, Japan) and flow cytometry (FACSCalibur;
Becton Dickinson, San Jose, CA, USA). Cells were defined as mature
DCs based on positive expression of CD1a, CD209, HLA-DR, CD83 and
CD86 and a lack of CD14 (purity >93%).
Invasion assay
In preparation for the assay, a 24-well Transwell
chamber with 8.0 μm pore size (CoStar, Cambridge, MA, USA) was
pre-coated with 30 μg Matrigel (Becton Dickinson) diluted in
phosphate-buffered saline. Cell suspensions (1×105
cells/well in serum-free growth media + 0.1% bovine serum albumin)
were treated and added to the upper compartment of the insert.
Media containing a chemoattractant (10% FBS) was added to the
bottom chamber of the Transwell plates. Following incubation at
37°C for 12 h, non-invaded cells (which remained on the upper
surface of the filter) were removed and invaded cells (on the lower
surface of the filter) were stained with Eosin Y and counted by
light microscopy (Olympus CKX31, Tokyo, Japan). The number of
migrating cells was adjusted by the cell viability assay to correct
for possible toxic effects of curcumin or BPS treatment using the
following equation: corrected migrating cell number = counted
migrating cell number/percentage of viable cells.
Quantitative polymerase chain reaction
(qPCR)
Total mRNA was extracted from cells by an RNeasy
mini kit and purified by RNeasy mini spin columns (Qiagen Inc.,
Valencia, CA, USA), according to the manufacturer’s instructions.
cDNA synthesis was performed with oligo dT16 primers and Moloney
murine leukemia virus reverse transcriptase according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). qPCR was performed on the LightCycler 2.0
instrument (Roche Diagnostics GmbH, Penzberg, Germany). Primer
sequences were listed as follows: Forward:
5′-GGACAGCCAGGACTCCATTG-3′ and reverse: 5′-TGTGGGGACAACTGGAGTGAA-3′
for OPN; forward: 5′-CAGAGATGCGTGGAGAGTCG-3′ and reverse:
5′-CAAAGGCGTCGTCAATCACC-3′ for MMP-9, and, forward:
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse: 5′-GGGCACGAAGGCTCATCATT-3′
for β-actin. Gene-specific amplifications were demonstrated by
melting-curve data.
Western blot analysis
A total of 20 μg protein (cell lysates) was
subjected to electrophoresis in 10% sodium dodecyl
sulfate-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes (Immobilon™; Millipore, Bedford, MA, USA).
Following transfer, gels were blocked with 5% skimmed milk in
Tris-buffered saline with 0.05% Tween 20 (TBST) for 1 h, followed
by overnight blotting with primary antibodies at 4°C. Primary
antibodies included a rabbit anti-human OPN polyclonal antibody
(1:1,000) and a mouse anti-human β-actin mAb (1:1,000). Membranes
were washed with TBST prior to incubation with secondary antibodies
conjugated with horseradish peroxidase (1:2,000). An enhanced
chemiluminescence system was used to detect horseradish peroxidase
enzyme activity. Briefly, membranes were washed three times with
buffer after the incubation with secondary antibodies and treated
with enhanced chemilumescent substrates according to the
manufacturer’s instructions (Pierce Biotechnology, Inc., Rockford,
IL, USA). The immunobands were visualized using the Kodak Digital
Image Station IS2000 (Kodak, Rochester, NY, USA) and subsequently
analyzed using densitometry with AlphaEaseFC software (version
4.0.0, Alpha Innotech Corp., Santa Clara, CA, USA).
Enzyme-linked immunosorbent assay
(ELISA)
OPN and MMP-9 in the cell supernatant were measured
using ELISA kits (R&D Systems, Weisbaden, Germany), according
to the manufacturer’s instructions. Marker concentration was
calculated from the standard curve. A subset of samples was assayed
five times in every ELISA plate for quality control.
Flow cytometry
Surface receptor expression on SKOV-3 and DCs in the
respective groups was detected using a FACSCalibur flow cytometer
(Becton Dickinson). Two- or three-color immunofluorescence was
performed using the following panel of fluorescein isothiocyanate
(FITC)-, phycoerythrin (PE)- or PE-carbocyanin (Cy) 5 labeled
monoclonal antibodies against CD14, CD1a, CD83, CD80, CD86, HLA-DR,
CD209 and CD44. Isotype controls were run in parallel. Cell debris
was eliminated from the analysis by forward and side scatter
gating. For viability assays, cells were stained with Annexin V and
propidium iodide, according to the manufacturer’s instructions
(Bender MedSystems, Vienna, Austria). The mean fluorescence
intensities were determined by CellQuest software (Becton
Dickinson).
Statistical analysis
The SPSS software package (version 13.0; SPSS Inc.,
Chicago, IL, USA) was used for all statistical analysis. Data is
expressed as the mean ± SD from at least three independent
experiments. Statistical analysis was performed using a t-test or
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Distinct regulation of curcumin and BPS
on invasion of SKOV3 cells and DCs
The regulation of BPS on the invasion of SKOV3 cells
and DCs was investigated and compared with that of curcumin, as a
number of previous studies have indicated that curcumin inhibited
the motility of ovarian cancer cells and DCs (23–25).
Results of the invasion assay showed that curcumin and BPS
inhibited the invasion of SKOV3 cells and no difference was
identified between the inhibitory efficiency of these two
substances (Fig. 1). Curcumin
significantly inhibited the invasion of immature and mature DCs,
whereas the inhibitory effect was not exerted by BPS on immature or
mature DCs (Fig. 1). These results
indicated that BPS possesses the same inhibitory efficiency on the
invasion of ovarian cancer cells as curcumin, but exerts minimal
inhibition on DCs.
Curcumin and BPS alter the expression of
OPN in SKOV3 cells and DCs
Previous studies have suggested that OPN promotes
the motility of ovarian cancer cells and DCs (12,14,26).
To investigate the underlying mechanisms of curcumin- and
BPS-regulated cell invasion, mRNA and protein levels of OPN were
measured. qPCR analysis showed that curcumin inhibited OPN mRNA
expression in SKOV3 cells and immature and mature DCs, whereas BPS
decreased OPN mRNA levels in SKOV3 cells, but not in immature or
mature DCs (Fig. 2A). Consistent
with its mRNA level, the protein expression and secretion of OPN
regulated by curcumin in SKOV3 cells and DCs were significantly
decreased; however, this decrease was only observed in BPS-treated
SKOV3 cells and not in DCs (Fig. 2B
and C). These results indicated that OPN expression was closely
correlated with the curcumin- and BPS-regulated motility of ovarian
cancer cells and DCs.
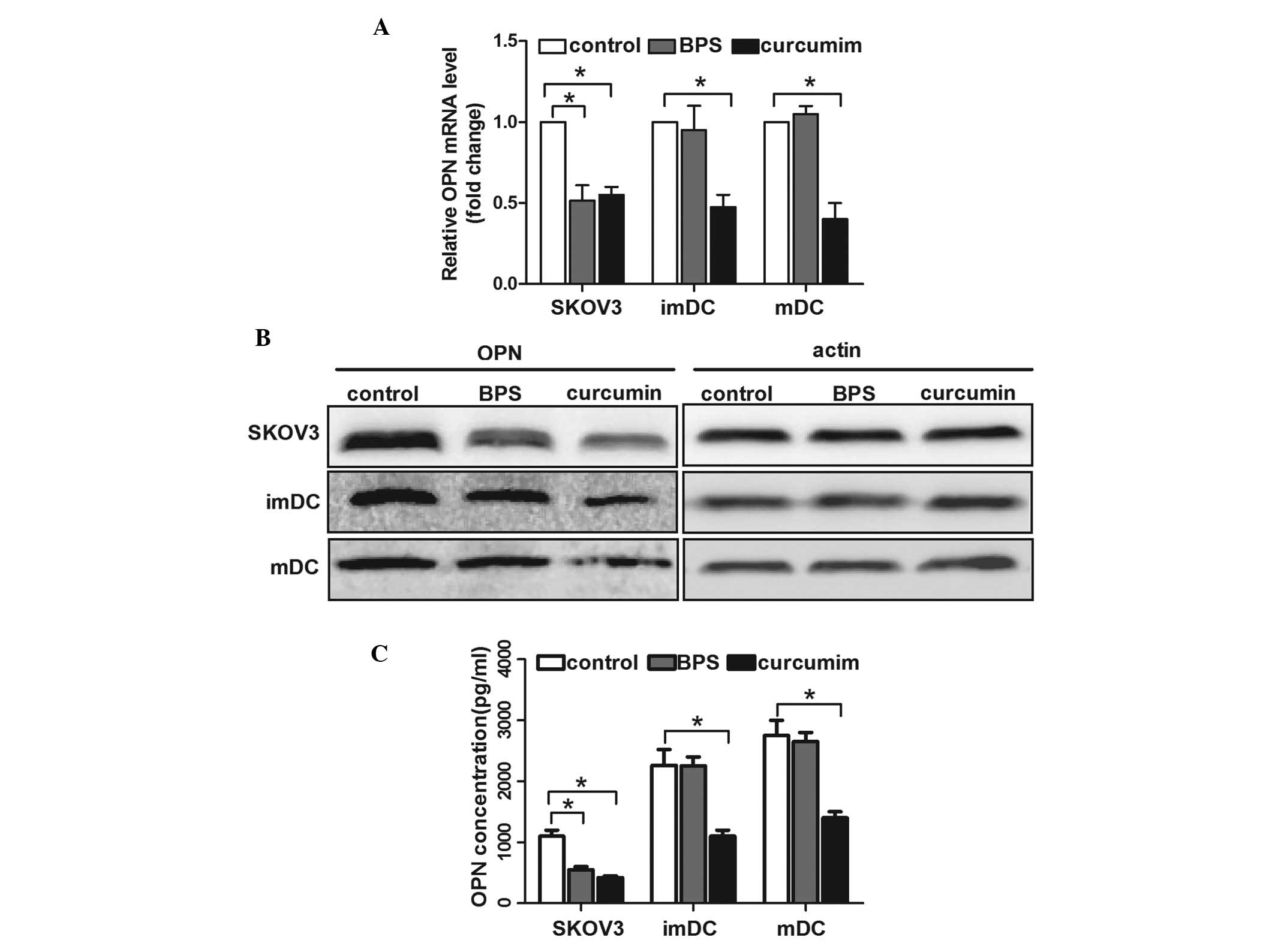 | Figure 2OPN expression of SKOV3 cells and DCs
following curcumin or basil polysaccharide (BPS) treatment. SKOV3
cells, imDCs and mDCs were treated with RPMI medium only (control),
RPMI with curcumin (50 μM) or RPMI with BPS (100 μg/ml) for certain
time periods. (A) Expression of OPN mRNA was measured by qPCR
analysis. The data are presented as the mean ± SD (n=3,
*P<0.05). (B) Western blot analysis was used to
evaluate OPN protein expression in cells. The image shown is
representative of 3 independent experiments. (C) Enzyme-linked
immunosorbent assay was used to analyze OPN levels in the cell
supernatant. The data are presented as the mean ± SD (n=3,
*P<0.05). OPN, Osteopontin; DCs, dendritic cells;
BPS, basil polysaccharide; imDC, immature DCs; mDCS, mature DCs;
qPCR, quantitative polymerase chain reaction. |
CD44 is downregulated in curcumin- but
not BPS-treated SKOV3 cells and DCs
The CD44 surface expression on SKOV3 and DCs was
analyzed following curcumin or BPS treatment by flow cytometry, as
CD44 is an important receptor of OPN and it facilitates the
invasion of cancer cells (8).
Results of the current study showed that CD44 surface expression
was significantly decreased in curcumin-treated SKOV3 cells and
DCs, but its expression was not affected by BPS (Fig. 3). As well as decreasing OPN
expression, curcumin was hypothesized to dampen its signaling
activity by downregulating the functional receptor, CD44, in
ovarian cancer cells and DCs. However, the effect of BPS on CD44
expression was not observed to be significant compared with that of
curcumin.
MMP-9 is involved in curcumin- and
BPS-modulated invasion of SKOV3 cells and DCs
OPN has been demonstrated to upregulate MMP-9
expression in the metastasis of a number of types of cancer
(9–11). In addition, MMP-9 overexpression is
markedly correlated with a higher risk of metastasis in ovarian
cancer patients (27). Therefore,
the effect of curcumin and BPS on MMP-9 expression of SKOV3 cells
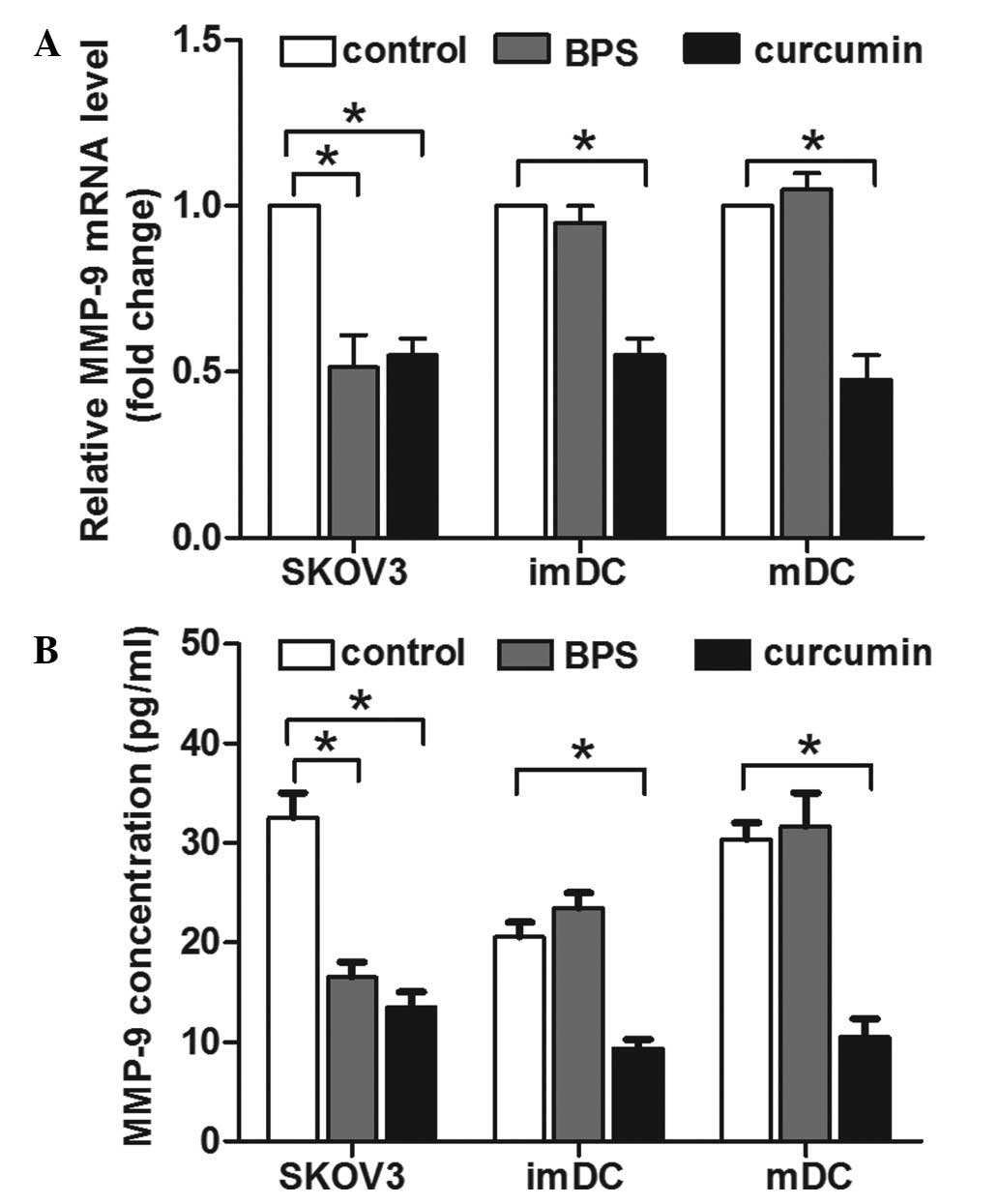
and DCs was investigated. qPCR results indicated that curcumin
decreased MMP-9 mRNA expression in SKOV3 cells and DCs (Fig. 4A). By contrast, BPS decreased MMP-9
expression in SKOV3 cells, but not in immature or mature DCs
(Fig. 4A). Consistent with its
mRNA level, ELISA results indicated that the concentration of MMP-9
in the supernatant of curcumin-treated SKOV3 cells and DCs was
significantly lower, compared with the control group. BPS decreased
MMP-9 concentration in the supernatant of SKOV3 cells, but the
MMP-9 level in the supernatant of DCs was not affected (Fig. 4B). This correlation between MMP-9
expression and invasion activity suggested that the alteration of
MMP-9 expression was involved in curcumin and BPS modulated
invasion of SKOV3 cells and DCs.
Discussion
In the current study, the regulation of two natural
products, curcumin and BPS, on the invasion of SKOV3 ovarian cancer
cells and DCs, and the underlying mechanisms were investigated. The
observations indicated that curcumin impeded the invasion of SKOV3
cells and immature or mature DCs. Compared with curcumin, BPS
showed similar inhibitory efficiency on SKOV3 cell invasion, but
its effect on DCs was minimal. Further investigation showed that
curcumin decreased the levels of OPN, CD44 and MMP-9 expression on
the surface of ovarian cancer cells and DCs, while BPS decreased
OPN and MMP-9 expression on ovarian cancer cells and showed no
inhibitory effect on CD44 expression. Therefore, these results
indicated that distinct regulation of OPN, CD44 and MMP-9
expression was at least partly responsible for the motility change
of two types of cells mediated by curcumin or BPS.
The therapeutic effect of curcumin on ovarian cancer
has been well established by in vitro and in vivo
studies (16,28–30).
Consistent with previous studies, which have demonstrated that
curcumin decreased ovarian cancer cell migration (23,24),
the current study confirmed that it significantly inhibited the
invasion of SKOV3 cells, which provided more evidence for its
anticancer mechanisms. However, the effect of curcumin on the
immune system appears to be controversial, primarily dependent on
different types of immunocytes. Although curcumin was shown to
directly enhance T cell-mediated antitumor immunity, it inhibited
the antigen-presenting properties of DCs by blocking maturation
marker expression and inducing differentiation towards a
tolerogenic phenotypes (25,31,32).
In the current study, curcumin was observed to inhibit the invasion
of immature and mature DCs. Compared with curcumin, BPS appears
more beneficial as a complementary therapy in ovarian cancer
treatment, as the results showed that it inhibited SKOV3 cell
invasion, almost as efficiently as curcumin, whereas no inhibition
was observed on the invasion of immature or mature DCs. These
results indicated that BPS may achieve more robust antitumor
immunity in ovarian cancer patients than curcumin as the invasion
of activated DCs to secondary lymph organs is vital for the
subsequent excitation of T cells. However, in addition to motility,
the immune-initiating potency of DCs is also dependent upon its
phagocytotic activity, surface molecule expression and cytokine
secretion. Therefore, this hypothesis may not be confirmed until
further studies regarding the modulation of BPS on DCs biological
properties are conducted.
OPN enhances cell invasion by interacting with
integrins and CD44 and initiating subsequent reactions (12,14,26),
for example, enhancing MMP secretion (9,11).
In the present study, curcumin was observed to inhibit OPN
expression in SKOV3 cells and DCs, while BPS decreased OPN
expression in SKOV3 cells, but not in DCs. The correlation between
the levels of OPN and cell motility indicated that curcumin and BPS
modulated SKOV3 cells and DC invasion by altering the expression of
OPN. The results indicated that the protein level and secretion of
OPN was significantly affected following treatment wotj curcumin or
BPS and a significant decrease of OPN mRNA may be responsible for
this change in protein levels. However, more modifications may have
occurred at the translational or post-translational level after the
mRNA was formed, such as methylation, phosphorylation and
acetylization. All these modulations would affect the protein
synthesis of OPN, but their effects were not investigated in the
present study. In addition, the distinct effect of BPS on OPN
expression in cancer cells and DCs is noteworthy. This may be
explained by various activation pathways of OPN expression in
cancer cells and DCs; however, further studies are required to
investigate the underlying mechanisms.
Cellular adhesion molecules (CAMs) mediate contact
among or between cells and the ECM, and dysregulation of their
expression is involved in tumor progression. CD44 has been shown to
be involved in cancer metastasis (33). In addition, the close correlation
between CD44 and OPN-mediated cell invasion has been well described
as OPN was observed to upregulate CD44 surface expression (34) and CD44 is known to promote cell
invasion by binding with hyaluronan in ECM. However, OPN directly
collaborates with CD44 to activate downstream signaling pathways in
an autocrine manner and subsequently promotes cell invasion and
chemotaxis (35,36). The current study demonstrated that
curcumin significantly downregulated the expression of CD44 on the
surface of SKOV3 cells and DCs, while BPS had no marked effect on
CD44 expression. These results indicated that curcumin not only
decreased OPN expression, but also dampened its activity by
inhibiting the expression of its functional receptor.
OPN induced MMP expression, was involved in the
metastasis of cancer cells (9,11)
and curcumin was shown to inhibit the expression of MMP-2 by
inactivating the OPN-mediated nuclear factor-κB (NF-κB) pathway
(37). Curcumin and BPS were
observed to modulate MMP-9 expression in a manner consistent with
the OPN level. This was achieved by decreasing the OPN level or
directly interfering with OPN-MMP-9 pathway activity or a
combination of the two. Moreover, activation of CD44 promotes the
binding of MMP-9 to the cell surface and its maturation (38), and CD44-MMP-9 aggregates, enhanced
cancer metastasis in a murine mammary carcinoma model (39). Although curcumin and BPS decreased
MMP-9 secretion in SKOV3 cells to a similar efficacy, the
maturation of MMP-9 may differ, given that curcumin significantly
downregulated CD44 expression when compared with BPS. The current
results showed that curcumin and BPS modulated MMP-9 secretion in a
manner that was consistent with cell invasion and OPN expression,
thereby it may be a potential mechanism for the modulation of cell
motility.
In conclusion, to the best of our knowledge, this
was the first study to demonstrate that BPS inhibited the invasion
of SKOV3 cells while curcumin affected SKOV3 cells and DCs. This
diversity was achieved, in part, by the distinct expression of OPN,
CD44 and MMP-9 in the two types of cells. The present study
indicated that curcumin and BPS may serve as efficient
complementary therapies for ovarian cancer. Notably however, BPS
may be a superior candidate for maintaining anticancer immunity
activity in ovarian cancer patients, considering its low inhibitory
effect on DC invasion. However, further investigation is required
to investigate whether these compounds are equally effective in
vivo.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant nos. 30472261,
31270971 and 81072406).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
Amati L, Pepe M, Passeri ME, Mastronardi
ML, Jirillo E and Covelli V: Toll-like receptor signaling
mechanisms involved in dendritic cell activation: potential
therapeutic control of T cell polarization. Curr Pharm Des.
12:4247–4254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu CS, Boyer J, Schullery DS, et al:
Phase I/II randomized trial of dendritic cell vaccination with or
without cyclophosphamide for consolidation therapy of advanced
ovarian cancer in first or second remission. Cancer Immunol
Immunother. 61:629–641. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Triozzi PL, Khurram R, Aldrich WA, Walker
MJ, Kim JA and Jaynes S: Intratumoral injection of dendritic cells
derived in vitro in patients with metastatic cancer. Cancer.
89:2646–2654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chakraborty G, Jain S, Behera R, et al:
The multifaceted roles of osteopontin in cell signaling, tumor
progression and angiogenesis. Curr Mol Med. 6:819–830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bulfone-Paus S and Paus R: Osteopontin as
a new player in mast cell biology. Eur J Immunol. 38:338–341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pagano PJ and Haurani MJ: Vascular cell
locomotion: osteopontin, NADPH oxidase, and matrix
metalloproteinase-9. Circ Res. 98:1453–1455. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khan SA, Cook AC, Kappil M, et al:
Enhanced cell surface CD44 variant (v6, v9) expression by
osteopontin in breast cancer epithelial cells facilitates tumor
cell migration: novel post-transcriptional, post-translational
regulation. Clin Exp Metastasis. 22:663–673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen YJ, Wei YY, Chen HT, et al:
Osteopontin increases migration and MMP-9 up-regulation via
alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in
human chondrosarcoma cells. J Cell Physiol. 221:98–108. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Desai B, Ma T, Zhu J and Chellaiah MA:
Characterization of the expression of variant and standard CD44 in
prostate cancer cells: identification of the possible molecular
mechanism of CD44/MMP9 complex formation on the cell surface. J
Cell Biochem. 108:272–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang G, Zhang Y, Wu J, et al: Osteopontin
regulates growth and migration of human nasopharyngeal cancer
cells. Mol Med Rep. 4:1169–1173. 2011.PubMed/NCBI
|
|
12
|
Schulz G, Renkl AC, Seier A, Liaw L and
Weiss JM: Regulated osteopontin expression by dendritic cells
decisively affects their migratory capacity. J Invest Dermatol.
128:2541–2544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Visintin I, Feng Z, Longton G, et al:
Diagnostic markers for early detection of ovarian cancer. Clin
Cancer Res. 14:1065–1072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bao LH, Sakaguchi H, Fujimoto J and Tamaya
T: Osteopontin in metastatic lesions as a prognostic marker in
ovarian cancers. J Biomed Sci. 14:373–381. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Zhang HQ, Zhu GH, et al: A novel
mono-carbonyl analogue of curcumin induces apoptosis in ovarian
carcinoma cells via endoplasmic reticulum stress and reactive
oxygen species production. Mol Med Rep. 5:739–744. 2012.
|
|
16
|
Selvendiran K, Ahmed S, Dayton A, et al:
HO-3867, a curcumin analog, sensitizes cisplatin-resistant ovarian
carcinoma, leading to therapeutic synergy through STAT3 inhibition.
Cancer Biol Ther. 12:837–845. 2011. View Article : Google Scholar
|
|
17
|
Sadzuka Y, Nagamine M, Toyooka T, Ibuki Y
and Sonobe T: Beneficial effects of curcumin on antitumor activity
and adverse reactions of doxorubicin. Int J Pharm. 432:42–49. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo F, Song X, Zhang Y and Chu Y: Low-dose
curcumin leads to the inhibition of tumor growth via enhancing
CTL-mediated antitumor immunity. Int Immunopharmacol. 11:1234–1240.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhattacharyya S, Md Sakib Hossain D,
Mohanty S, et al: Curcumin reverses T cell-mediated adaptive immune
dysfunctions in tumor-bearing hosts. Cell Mol Immunol. 7:306–315.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Badisa RB, Tzakou O, Couladis M and
Pilarinou E: Cytotoxic activities of some Greek Labiatae herbs.
Phytother Res. 17:472–476. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xia L, Zheng G, Zhou H, et al: The
application of BPS in the preparing of anti-metastasis therapies.
P.R. China Patent CN1498625. Filed, November 8, 2002; Issued, May
26, 2004 (In Chinese).
|
|
22
|
Renkl AC, Wussler J, Ahrens T, et al:
Osteopontin functionally activates dendritic cells and induces
their differentiation toward a Th1-polarizing phenotype. Blood.
106:946–955. 2005. View Article : Google Scholar
|
|
23
|
Ji C, Cao C, Lu S, et al: Curcumin
attenuates EGF-induced AQP3 up-regulation and cell migration in
human ovarian cancer cells. Cancer Chemother Pharmacol. 62:857–865.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seo JH, Jeong KJ, Oh WJ, et al:
Lysophosphatidic acid induces STAT3 phosphorylation and ovarian
cancer cell motility: their inhibition by curcumin. Cancer lett.
288:50–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shirley SA, Montpetit AJ, Lockey RF and
Mohapatra SS: Curcumin prevents human dendritic cell response to
immune stimulants. Biochem Biophys Res Commun. 374:431–436. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tilli TM, Franco VF, Robbs BK, et al:
Osteopontin-c splicing isoform contributes to ovarian cancer
progression. Mol Cancer Res. 9:280–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang W, Yang HC, Wang Q, et al: Clinical
value of combined detection of serum matrix metalloproteinase-9,
heparanase, and cathepsin for determining ovarian cancer invasion
and metastasis. Anticancer Res. 31:3423–3428. 2011.
|
|
28
|
Lin YG, Kunnumakkara AB, Nair A, et al:
Curcumin inhibits tumor growth and angiogenesis in ovarian
carcinoma by targeting the nuclear factor-kappaB pathway. Clin
Cancer Res. 13:3423–3430. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Watson JL, Greenshields A, Hill R, et al:
Curcumin-induced apoptosis in ovarian carcinoma cells is
p53-independent and involves p38 mitogen-activated protein kinase
activation and downregulation of Bcl-2 and survivin expression and
Akt signaling. Mol Carcinog. 49:13–24. 2010.
|
|
30
|
Ganta S, Devalapally H and Amiji M:
Curcumin enhances oral bioavailability and anti-tumor therapeutic
efficacy of paclitaxel upon administration in nanoemulsion
formulation. J Pharm Sci. 99:4630–4641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rogers NM, Kireta S and Coates PT:
Curcumin induces maturation-arrested dendritic cells that expand
regulatory T cells in vitro and in vivo. Clin Exp Immunol.
162:460–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cong Y, Wang L, Konrad A, Schoeb T and
Elson CO: Curcumin induces the tolerogenic dendritic cell that
promotes differentiation of intestine-protective regulatory T
cells. Eur J Immunol. 39:3134–3146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Samanna V, Wei H, Ego-Osuala D and
Chellaiah MA: Alpha-V-dependent outside-in signaling is required
for the regulation of CD44 surface expression, MMP-2 secretion, and
cell migration by osteopontin in human melanoma cells. Exp Cell
Res. 312:2214–2230. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jijiwa M, Demir H, Gupta S, et al: CD44v6
regulates growth of brain tumor stem cells partially through the
AKT-mediated pathway. PloS One. 6:e242172011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chagan-Yasutan H, Tsukasaki K, Takahashi
Y, et al: Involvement of osteopontin and its signaling molecule
CD44 in clinicopathological features of adult T cell leukemia. Leuk
Res. 35:1484–1490. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Philip S and Kundu GC: Osteopontin induces
nuclear factor kappa B-mediated promatrix metalloproteinase-2
activation through I kappa B alpha /IKK signaling pathways, and
curcumin (diferulolylmethane) down-regulates these pathways. J Biol
Chem. 278:14487–14497. 2003. View Article : Google Scholar
|
|
38
|
Bagnoli F, Oliveira VM, Silva MA, Taromaru
GC, Rinaldi JF and Aoki T: The interaction between aromatase,
metalloproteinase 2,9 and CD44 in breast cancer. Rev Assoc Med
Bras. 56:472–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu Q and Stamenkovic I: Cell
surface-localized matrix metalloproteinase-9 proteolytically
activates TGF-beta and promotes tumor invasion and angiogenesis.
Genes Dev. 14:163–176. 2000.PubMed/NCBI
|