Introduction
The prevalence of diabetes is reaching epidemic
proportions at an alarming rate worldwide. Diabetes mellitus (DM)
is a group of metabolic diseases induced by insulin secretion
deficiency and/or insulin resistance, which result in chronic
hyperglycemia. Diabetic nephropathy (DN) and diabetic retinopathy
(DR), as major microvascular complications, are the leading cause
of end-stage renal disease and loss of vision, respectively
(1). It is well known that
prolonged hyperglycemia is an important risk factor (2,3). An
important conceptual consideration is that the diseases manifest in
individuals with genetic predisposition coupled with environmental
triggers (4,5). Previous studies (6–8) have
focused on the genetic basis of diabetes and its complications by
highlighting methods for improving and understanding the mechanisms
involved in the disease.
Lipoprotein oxidation was previously found to be
involved in the development of cerebrovascular and coronary artery
diseases, as well as microvascular complications of diabetes
(9–11). Paraoxonase (PON) is a
high-density lipoprotein (HDL)-associated enzyme, protecting
lipoproteins from oxidation (12).
The PON gene clusters (PON1 and PON2) mapped
on human chromosome 7q21.3 with several polymorphisms, particularly
specific functional variants with possible biological effects on
enzyme activity, have been extensively evaluated as genetic
candidates for diabetic microvascular complications. These include
the following: PON1 rs662 (c.575A>G or p.Gln192Arg or
p.Q192R), PON1 rs854560 (c.163T>A or p.Leu55Met or
p.L55M), PON2 rs7493 (c.932C>G or p.Ser311Cys or p.S311C)
and PON2 rs12026 (c.443C>G or p.Ala148Gly or p.A148G)
(6–8,13–19).
These emerging observations in genetic predisposition to DN and DR
have drawn particular attention and therefore, have garnered
research interests. The correlation between PON
polymorphisms and disorders, as aforementioned, remain unclear
since the reproducibility of a number of initial associations have
not been forthcoming and specific results from small sample sizes
are often controversial. Therefore, a meta-analysis was conducted
in the present study to mitigate these shortcomings and evaluate
the genetic effects of PON1 and PON2 genes on the
risk of DN and DR.
Materials and methods
Search strategy and inclusion
criteria
Online databases, MEDLINE (Medical Literature
Analysis and Retrieval System Online) and EMBASE (via Ovid) were
used for the literature search between the starting dates of the
databases and 6 January, 2013. The keywords were used as free words
and also as MeSH terms: ‘paraxonase’, ‘PON1’, ‘PON2’,
‘diabet(es/ic)’, ‘nephropathy(ies)’, ‘retinopathy(ies)’,
‘microvascular complication (s)’, ‘polymorphism(s)’, ‘variant(s)’
and ‘mutation(s)’. Reference lists of the retrieved articles and
reviews were also screened for additional articles not obtained by
the electronic search.
The inclusion criteria were defined as follows: i)
original case-control studies evaluating the association between
DN/DR and PON polymorphisms; ii) numbers or frequencies in
case and control groups reported for each genotype or allele; iii)
study samples of unrelated individuals drawn from clearly defined
populations; and iv) studies using diabetic patients free from any
form of complications as the control group. Animal studies, case
reports, reviews, abstracts, conference proceedings, editorials,
reports with incomplete data and studies based on pedigree data
were excluded.
Literature review and data
extraction
All the articles retrieved were reviewed and data
extracted by two independent investigators with standardized
datasheets. Uncertainties were resolved by consensus with a third
reviewer. Information collected from each study included: first
author, year of publication, country of study, ethnicity,
diagnostic methods of DN and DR, sample size, polymorphisms studied
and allelic and genotypic frequencies.
If genotype or allele data were not available in the
publication, calculations were based on the tests for
Hardy-Weinberg equilibrium (HWE) in the original study. If the test
for HWE was not reported, it was tested by genotype data.
Statistical analysis
HWE was evaluated using the χ2 test.
Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were
estimated with the DerSimonian and Laird random-effects model.
Although random-effects analyses exhibited less power than analyses
based on fixed-effects models, they yielded a more conservative CI
(20). For the genotypic
comparison, dominant, homozygote, heterozygote and recessive models
were applied into the investigation of the disease association with
reference to the common variation (Q for p.Q192R; L for p.L55M; S
for p.S311C; and A for p.A148G). Cochran's Q statistic was used to
test heterogeneity across studies and the index
I2 statistic was used to quantify the
proportion of total variation attributable to between-study
heterogeneity. P<0.1 was considered to indicate a statistically
significant difference for Q-statistic and
I2>50% was considered to indicate large
heterogeneity. The sensitivity analysis was applied to assess the
stability of the results. Funnel plot asymmetry and modified
Egger's regression test were used to statistically assess the
potential bias. Data management and statistical analyses were
conducted with ‘metafor’ package v1.6-0 and ‘Hardy Weinberg’
package v1.3 in R language v2.15.0. α was set to 0.05.
Results
Study identification and
characteristics
Major bibliographic databases were screened,
searching for studies focusing on the associations of PON1
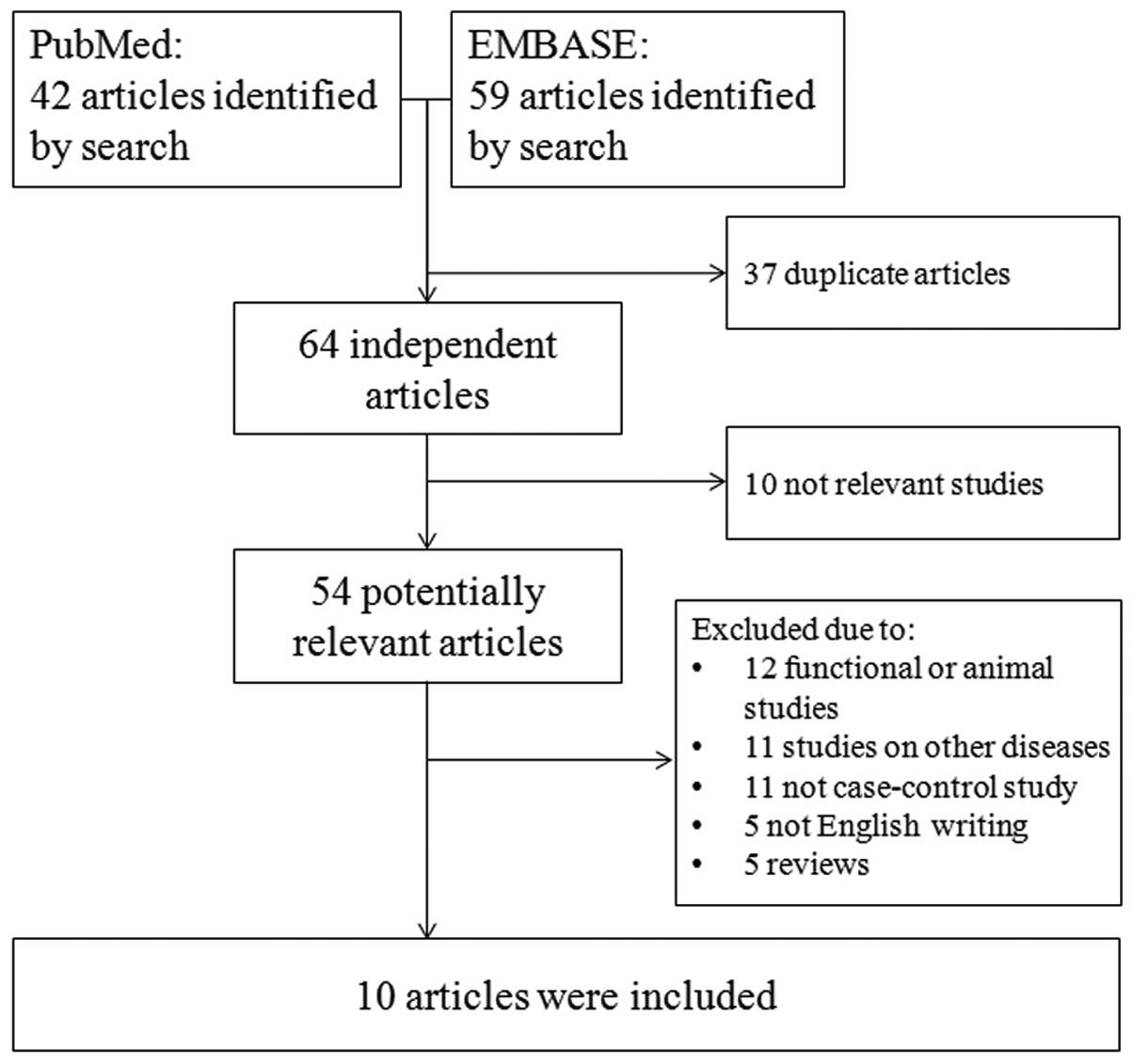
and PON2 polymorphisms with DR and DN. A schematic
representation of the selection process with specific reasons is
presented in Fig. 1. The initial
search strategy retrieved 101 potentially relevant studies.
Following screening, a total of 10 studies with 23 outcomes met the
inclusion criteria used for the meta-analysis. General
characteristics and genotypic frequencies of these reports are
presented in Table I. Overall
estimates of PON gene (PON1 and PON2)
polymorphisms for DN and DR in dominant model are shown in Fig. 2.
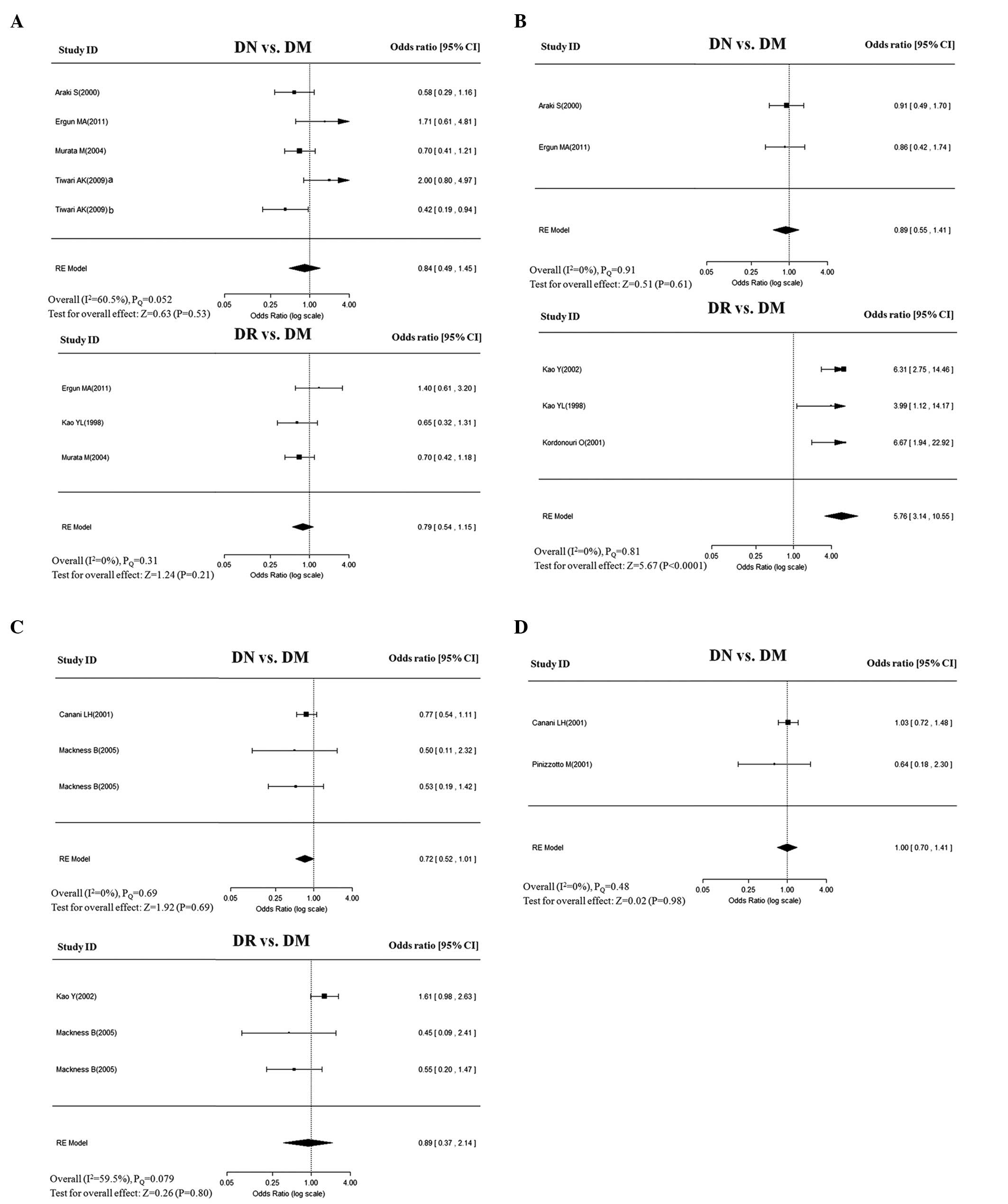 | Figure 2Overall estimates of PON gene
(PON1 and PON2) polymorphisms for DN and DR in
dominant model. The size of the box is proportional to the weight
of the study, horizontal lines indicate 95% CI and a diamond
indicates the summary OR with its corresponding 95% CI. (A)
PON1-Q192R, (B) PON1-L55M, (C) PON2-S311C, and
(D) PON2-A148G. PON, paraoxonase; DN, diabetic nephropathy;
DR, diabetic retinopathy; DM, diabetes mellitus; OR, odds ratio;
CI, confidence interval. |
 | Table IGeneral characteristics of the studies
included in the present meta-analysis. |
Table I
General characteristics of the studies
included in the present meta-analysis.
| | | | | | Age, mean ± SD,
years | M, % | Allele frequency,
R/A | Genotype frequency,
RR/RA/AA |
|---|
| | | | | |
|
|
|
|
|---|
| PON
(polymorphism) | First author (year)
(Refs.) | Country | Ethnicity | Case (n) | Diabetic control
(n) | Case | Control | Case | Control | Case | Control | Case | Control |
|---|
| PON1
(Q192R) | | | | | | | | | | | | | |
| Araki et
al(2000)(13) | USA | Caucasian | DN (188) | Type 1 (179) | 35.00±6.00 | 36.00±7.00 | 50.5 | 50.3 | 248/128 | 251/107 | 84/80/24 | 86/79/14 |
| Ergun et
al(2011)(14) | Turkey | Turkish | DN (41) | Type 2 (130) | NA | 47.00±6.53 | NA | NA | 60/22 | 172/88 | 24/12/5 | 67/38/25 |
| Murata et
al(2004)(18) | Japan | Japanese | DN (148) | Type 2 (92) | NA | 47.90±8.40 | 44.6 | 42.4 | 95/201 | 76/108 | 9/77/62 | 15/46/31 |
| Tiwari et
al(2009)(19) | India (South) | Indian | DN (104) | Type 2 (143) | 55.97±11.50 | 60.45±11.47 | 76.4 | 68.5 | 140/68 | 187/99 | 43/54/7 | 62/63/18 |
| Tiwari et
al(2009)(19) | India (North) | Indian | DN (82) | Type 2 (64) | 53.56±10.99 | 61.03±8.88 | 87.0 | 53.0 | 82/82 | 69/59 | 27/28/27 | 16/37/11 |
| Ergun et
al(2011)(14) | Turkey | Turkish | DR (68) | Type 2 (103) | NA | 47.00±6.53 | NA | NA | 98/38 | 134/72 | 40/18/10 | 51/32/20 |
| Kao et
al(1998)(15) | Australia | Caucasian | DR (80) | Type 1 (119) | 15.40 | 13.90 | 42.5 | 50.4 | 96/64 | 159/79 | 35/26/19 | 60/39/20 |
| Murata et
al(2004)(18) | Japan | Japanese | DR (188) | Type 2 (92) | 49.00±11.40 | 47.90±8.40 | 43.1 | 42.4 | 120/256 | 76/108 | 11/98/79 | 15/46/31 |
| PON1
(L55M) | | | | | | | | | | | | | |
| Araki et
al(2000)(13) | USA | Caucasian | DN (188) | Type 1 (179) | 35.00±6.00 | 36.00±7.00 | 50.5 | 50.3 | 244/132 | 226/132 | 80/84/24 | 68/90/21 |
| Ergun et
al(2011)(14) | Turkey | Turkish | DN (41) | Type 2 (130) | NA | 47.00±6.53 | NA | NA | 26/56 | 89/171 | 8/10/23 | 27/35/68 |
| Ergun et
al(2011)(14) | Turkey | Turkish | DR (68) | Type 2 (103) | NA | 47.00±6.53 | NA | NA | 51/85 | 64/142 | 17/17/34 | 18/28/57 |
| Kao et
al(2002)(7) | Australia | Caucasian | DR (171) | Type 1 (198) | 14.80 | 13.00 | 41.5 | 50.0 | 253/109 | 201/195 | 89/75/7 | 45/111/42 |
| Kao et
al(1998)(15) | Australia | Caucasian | DR (80) | Type 1 (119) | 15.40 | 13.90 | 42.5 | 50.4 | 117/43 | 135/103 | 40/37/3 | 32/71/16 |
| Kordonouri et
al(2001)(16) | Australia | Caucasian | DR (73) | Type 1 (117) | NA | NA | NA | NA | 115/31 | 122/112 | 45/25/3 | 31/60/26 |
| PON2
(S311C) | | | | | | | | | | | | | |
| Canani et
al(2001)(6) | USA | Caucasian | DN (267) | Type 1 (241) | 13±7 | 11.00±6.00 | 49.3 | 50.0 | NA | NA | 161/106b | 160/81b |
| Mackness et
al(2005a)(17) | UK | Caucasian | DN (62) | Type 1 (90) | 43.30±13.20a | 58.60a | | | 93/31 | 138/42 | 35/23/4 | 51/36/3 |
| Mackness et
al(2005b)(17) | UK | Caucasian | DN (79) | Type 2 (161) | 59.10±11.30a | 64.30a | | | 115/43 | 236/86 | 44/27/8 | 84/68/9 |
| Kao et
al(2002)(7) | Australia | Caucasian | DR (171) | Type 1 (198) | 14.80 | 13.00 | 41.5 | 50.0 | 192/150 | 191/205 | 54/84/33 | 48/95/55 |
| Mackness et
al(2005)(17) | UK | Caucasian | DR (82) | Type 1 (70) | 43.30±13.20a | 58.60a | | | 127/37 | 104/36 | 50/27/5 | 36/32/2 |
| Mackness et
al(2005)(17) | UK | Caucasian | DR (94) | Type 2 (146) | 59.10±11.30a | 64.30a | | | 134/54 | 217/75 | 49/36/9 | 79/59/8 |
| PON2
(A148G) | | | | | | | | | | | | | |
| Canani et
al(2001)(6) | USA | Caucasian | DN (267) | Type 1 (241) | 13.00±7.00 | 11.00±6.00 | 49.3 | 50.0 | NA | NA | 167/100b | 149/92b |
| Pinizzotto et
al(2001)(8) | Switzerland | Caucasian | DN (147) | Type 2 (152) | NA | NA | NA | NA | 235/59 | 255/49 | 94/47/6 | 107/41/4 |
| PON1
(T-107C) | | | | | | | | | | | | | |
| Araki et
al(2000)(13) | USA | Caucasian | DN (188) | Type 1 (179) | 35.00±6.00 | 36.00±7.00 | 50.5 | 50.3 | 198/178 | 176/182 | 51/96/41 | 45/86/48 |
Meta-analysis of PON1-Q192R
The association of Q192R with DN was assessed in
five studies. Of these, two studies were performed in Caucasian
populations and three in Asian populations. All the control groups
were in HWE, with the exception of the study by Ergun et
al(14). A random-effects
model that takes into account the intra- and inter-study
variability did not reveal any significant association of Q192R
with DN, under any of the following genetic models: allele (Q vs.,
R: OR=0.90; 95% CI, 0.73–1.11), dominant (QQ+QR vs., RR: OR=0.84;
95% CI, 0.49–1.15), homozygote (QQ vs., RR: OR=0.79; 95% CI,
0.41–1.53), heterozygote (QR vs., RR: OR=0.84; 95% CI, 0.44–1.60),
and recessive (QQ vs., QR+RR: OR=0.91; 95% CI, 0.64–1.31) (Table II). These ORs were moderately
heterogeneous across studies in overall comparisons. The frequency
of the Q allele in Indian populations was found to be the major
allele, similar to that of Caucasian populations, while the Q
allele was found to be the minor allele in the single Japanese
population. When stratifying by ethnicity, no evident associations
were found in the Caucasian or Asian populations. Publication bias
was assessed using funnel plots, which indicated symmetry of the
genetic effects for these ORs (data not shown).
 | Table IIPooled analyses on the correlation
between PON gene polymorphisms and DN and DR. |
Table II
Pooled analyses on the correlation
between PON gene polymorphisms and DN and DR.
| PON
(polymorphism) | Disease | Sample size,
cases/controls | Genetic model | OR (95% CI) | P-value |
I2, % |
PQ |
|---|
| PON1
(Q192R) | DN | 563/608 | Allele | 0.90
(0.73–1.11) | 0.330 | 28.70 | 0.200 |
| | | Dominant | 0.84
(0.49–1.15) | 0.530 | 60.50 | 0.052 |
| | | Homozygote | 0.79
(0.41–1.53) | 0.490 | 61.10 | 0.040 |
| | | Heterozygote | 0.84
(0.44–1.60) | 0.590 | 67.90 | 0.025 |
| | | Recessive | 0.91
(0.64–1.31) | 0.630 | 42.70 | 0.095 |
| DR | 336/314 | Allele | 0.87
(0.56–1.33) | 0.510 | 69.00 | 0.045 |
| | | Dominant | 0.79
(0.54–1.15) | 0.210 | 0.00 | 0.310 |
| | | Homozygote | 0.65
(0.26–1.67) | 0.370 | 73.80 | 0.026 |
| | | Heterozygote | 0.84
(0.56–1.27) | 0.410 | 0.00 | 0.760 |
| | | Recessive | 0.73
(0.32–1.68) | 0.470 | 78.70 | 0.015 |
| PON1
(L55M) | DN | 229/309 | Allele | 1.03
(0.79–1.34) | 0.820 | 0.00 | 0.540 |
| | | Dominant | 0.89
(0.55–1.41) | 0.610 | 0.00 | 0.910 |
| | | Homozygote | 0.97
(0.57–1.67) | 0.920 | 0.00 | 0.780 |
| | | Heterozygote | 0.83
(0.49–1.39) | 0.470 | 0.00 | 0.950 |
| | | Recessive | 1.15
(0.79–1.68) | 0.470 | 0.00 | 0.590 |
| DR | 392/537 | Allele | 2.42
(1.91–3.07) | <0.001 | 13.00 | 0.260 |
| | | Dominant | 5.76
(3.14–10.55) | <0.001 | 0.00 | 0.810 |
| | | Homozygote | 10.53
(5.59–19.86) | <0.001 | 0.00 | 0.740 |
| | | Heterozygote | 3.62
(1.94–6.74) | <0.001 | 0.00 | 0.890 |
| | | Recessive | 3.56
(2.61–4.86) | <0.001 | 0.00 | 0.520 |
| PON2
(S311C) | DN | 408/492 | Allele | 0.95
(0.68–1.33) | 0.760 | 0.00 | 0.980 |
| | | Dominant | 0.72
(0.52–1.01) | 0.055 | 0.00 | 0.690 |
| | | Homozygote | 0.73
(0.53–1.02) | 0.069 | 0.00 | 0.800 |
| | | Heterozygote | 0.47
(0.20–1.10) | 0.080 | 0.00 | 0.970 |
| | | Recessive | 0.89
(0.68–1.18) | 0.430 | 2.50 | 0.450 |
| DR | 347/414 | Allele | 1.14
(0.84–1.55) | 0.390 | 42.70 | 0.190 |
| | | Dominant | 0.89
(0.37–2.14) | 0.800 | 59.50 | 0.079 |
| | | Homozygote | 0.98
(0.39–2.47) | 0.960 | 60.10 | 0.073 |
| | | Heterozygote | 0.81
(0.33–1.98) | 0.640 | 57.80 | 0.091 |
| | | Recessive | 1.25
(0.91–1.69) | 0.160 | 2.97 | 0.380 |
| PON2
(A148G) | DN | 414/393 | Allele | 0.77
(0.51–1.16) | 0.210 | 0.00 | 0.920 |
| | | Dominant | 1.00
(0.7–1.41) | 0.980 | 0.00 | 0.480 |
| | | Homozygote | 0.99
(0.70–1.40) | 0.960 | 0.00 | 0.410 |
| | | Heterozygote | 0.78
(0.22–2.75) | 0.700 | 0.00 | 0.930 |
| | | Recessive | 0.92
(0.67–1.24) | 0.570 | 9.70 | 0.290 |
To assess the association of Q192R with DR, three
studies were conducted. No significant associations were found in
the genetic models when the studies were pooled into the
meta-analysis (Table II). In
addition, no evidence of publication bias was observed. Subgroup
analysis by ethnicity was not performed due to a small number of
studies.
Meta-analysis of PON1-L55M
The association of L55M with risk of DN in Caucasian
populations was assessed in two studies. The pooled analysis showed
that no significant association was found in any of the genetic
models. The ORs for genetic effect were homogenous across the
studies (PQ>0.1; I2=0%;
Table II).
Four studies were eligible for pooling of the
genetic effects of L55M on DR. The allele model (L vs., M) yielded
a pooled OR of 2.42 (95% CI, 1.91–3.07) with mild heterogeneity
(PQ=0.54; I2=13%), indicating
that the L allele was significantly higher in DR patients compared
with that of DM controls. Similar or even more significant
associations were also observed in the following genotype models:
dominant (LL+LM vs., MM: OR=5.76; 95% CI, 3.14–10.55); homozygote
(LL vs., MM: OR=10.53; 95% CI, 5.59–19.86); heterozygote (LM vs.,
MM: OR=3.62; 95% CI, 1.94–6.74); and recessive (LL vs., LM+MM:
OR=3.56; 95% CI, 2.61–4.86). The genotypic effects were homogenous,
with I2 values of 0% for the
above-mentioned inherited models (Table II). Sensitivity analyses by
excluding and including the study [Ergun et al(14)] that deviated from HWE yielded
similar results, but was accompanied with moderate heterogeneity.
No evidence of publication bias was identified.
Meta-analysis of PON2-S311C
Each of the three studies was performed to assess
the association of S311C with DN and DR in Caucasian populations.
The genetic effects were homogenous across DN studies
(I2=0%), but mildly to moderately
heterogeneous across studies in DR (PQ=0.073–0.38;
I2=3.0–60.1%). The pooled analysis showed
no significant associations of S311C with DN or DR in any of the
genetic models (Table II). No
evidence of asymmetry was identified in the shape of the funnel
plots (data not shown).
Meta-analysis of PON2-A148G
With regard to A148G, two studies exclusively
assessed the association of A148G with DN only. The analysis showed
no significant association between A148G and DN in any of the
following genetic models: allele (A vs., G: OR=0.77; 95% CI,
0.51–1.16), dominant model (AA+AG vs., GG: OR=1.00; 95% CI,
0.70–1.41), homozygote model (AA vs., GG: OR=0.99; 95% CI,
0.70–1.40), heterozygote (AG vs., GG: OR=0.78; 95% CI, 0.22–2.75),
and recessive model (AA vs., AG+GG: OR=0.92; 95% CI, 0.67–1.24).
The ORs for all genetic effects were homogenous across studies
(I2=0%), with the exception of mildly
heterogeneous in the recessive model (PQ=0.29;
I2=9.7%; Table II).
Discussion
In the present study, a systematic review and
meta-analysis was performed to examine the associations of four
well-evaluated polymorphisms in PON with DN and DR. The
results indicated that the PON1-L55M polymorphism was
significantly associated with DR, which remained following
sensitivity analyses. The observations were consistent with a
majority of the previous studies investigated. The conflicting
results obtained on a Turkish population by Ergun et
al(14) may be due to
differences in diabetes control selection and statistical power, as
well as ethnical background (14).
Genetic effect of L allele yielded a higher risk of having DR
(between 3.56- and 10.53-fold in various genetic models),
indicating that it is worthy of in-depth analysis, particularly its
biological functions. However, such an association was not detected
in DN, which may be due to the limited studies, various phenotypes
and heterogeneity in the genetic susceptibility between DN and DR.
Further examination in larger cohorts are therefore required.
Nevertheless, the present study highlighted results for the genetic
association of functional variant L55M and DR, which is definitely
likely to lead to increased research interest, particularly for its
biological effect.
As aforementioned, low-density lipoprotein (LDL)
oxidation is key for the development of microvascular diseases
(21). PON activity affects the
efficiency of HDL on the inhibition of LDL oxidation (22). Moreover, lower PON activity has
been examined in type 2 diabetes patients, which has been
implicated in the development of diabetic microvascular
complications (23). The
PON1-L55M polymorphism has been found to modify the serum
concentration and enzyme activity of PON (24,25).
Thus, these circumstantial and laboratory results suggest a
critical role for L55M in the development of DR, although, the
exact molecular mechanisms remain elusive.
In the current study, no evident associations were
found in the remaining three variants (PON1-Q192R,
PON2-S311C and PON2-A148G) with DN or DR under any of
the genetic models. Therefore, the results suggest that these
polymorphisms may not be associated with diabetic microvascular
complications, particularly for DN and DR.
The present study had a number of strengths.
Firstly, to the best of our knowledge, this is the first
meta-analysis to investigate the associations of PON gene
polymorphisms with DN and DR. Secondly, the methods of the
meta-analysis were carefully designed; explicit search strategy
based on computer-assisted and manual search methods allowed almost
all relevant studies to be included and the conclusions are based
on conservative estimations. However, specific limitations also
existed; the number of available studies is not sufficient enough
for every variant in the meta-analysis, particularly for specific
subgroups. Thus, certain analyses based on <2 studies may not be
powered to detect modest association and must be assessed
cautiously. Additional studies of larger sample sizes and
containing more detailed information are required. An additional
potential drawback is that the majority of studies were
clinic-based resources, which may produce overestimated genetic
effects. However, this is unlikely to be significant in the present
study, which considered the significantly statistical power,
together with its biological relevance.
In conclusion, the current meta-analysis highlighted
conclusive results for the robust association between
PON1-L55M polymorphisms with DR. The results also
demonstrated that the remaining three variants (PON1-Q192R,
PON2-S311C and PON2-A148G) may not be associated with
DN or DR. Larger association studies and functional analyses of
PON1 are required to elucidate the pathological mechanisms
of the diabetic microvascular complications.
Acknowledgements
The authors would like to thank Dr Liu Guodong from
the Department of Endocrinology (Harbin Medical University, Harbin,
China) and anonymous reviewers for their useful comments and
language editing, which have greatly improved the manuscript.
References
|
1
|
Resnikoff S, Pascolini D, Etya'ale D, et
al: Global data on visual impairment in the year 2002. Bull World
Health Organ. 82:844–851. 2004.PubMed/NCBI
|
|
2
|
Sheetz MJ and King GL: Molecular
understanding of hyperglycemia's adverse effects for diabetic
complications. JAMA. 288:2579–2588. 2002. View Article : Google Scholar
|
|
3
|
Stratton IM, Kohner EM, Aldington SJ, et
al: UKPDS 50: risk factors for incidence and progression of
retinopathy in Type II diabetes over 6 years from diagnosis.
Diabetologia. 44:156–163. 2001.PubMed/NCBI
|
|
4
|
Pettitt DJ, Saad MF, Bennett PH, Nelson RG
and Knowler WC: Familial predisposition to renal disease in two
generations of Pima Indians with type 2 (non-insulin-dependent)
diabetes mellitus. Diabetologia. 33:438–443. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
The Diabetes Control and Complications
Trial Research Group. Clustering of long-term complications in
families with diabetes in the diabetes control and complications
trial. Diabetes. 46:1829–1839. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Canani LH, Araki S, Warram JH and
Krolewski AS: Comment - to: Pinizzotto M, Castillo E, Fiaux M,
Temler E, Gaillard RC, Ruiz J (2001) Paraoxonase 2 polymorphisms
are associated with diabetic nephropathy in Type II diabetes.
Diabetologia 44: 104–107. Diabetologia. 44:1062–1064.
2001.PubMed/NCBI
|
|
7
|
Kao Y, Donaghue KC, Chan A, Bennetts BH,
Knight J and Silink M: Paraoxonase gene cluster is a genetic marker
for early microvascular complications in type 1 diabetes. Diabet
Med. 19:212–215. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pinizzotto M, Castillo E, Fiaux M, Temler
E, Gaillard RC and Ruiz J: Paraoxonase2 polymorphisms are
associated with nephropathy in Type II diabetes. Diabetologia.
44:104–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lyons TJ, Li W, Wells-Knecht MC and Jokl
R: Toxicity of mildly modified low-density lipoproteins to cultured
retinal capillary endothelial cells and pericytes. Diabetes.
43:1090–1095. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morrison AC, Bare LA, Luke MM, et al:
Single nucleotide polymorphisms associated with coronary heart
disease predict incident ischemic stroke in the atherosclerosis
risk in communities study. Cerebrovasc Dis. 26:420–424. 2008.
View Article : Google Scholar
|
|
11
|
Luke MM, Lalouschek W, Rowland CM, et al:
Polymorphisms associated with both noncardioembolic stroke and
coronary heart disease: vienna stroke registry. Cerebrovasc Dis.
28:499–504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Durrington PN, Mackness B and Mackness MI:
Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol.
21:473–480. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Araki S, Makita Y, Canani L, Ng D, Warram
JH and Krolewski AS: Polymorphisms of human paraoxonase 1 gene
(PON1) and susceptibility to diabetic nephropathy in type I
diabetes mellitus. Diabetologia. 43:1540–1543. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ergun MA, Yurtcu E, Demirci H, et al: PON1
55 and 192 gene polymorphisms in type 2 diabetes mellitus patients
in a Turkish population. Biochem Genet. 49:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kao YL, Donaghue K, Chan A, Knight J and
Silink M: A variant of paraoxonase (PON1) gene is associated with
diabetic retinopathy in IDDM. J Clin Endocrinol Metab.
83:2589–2592. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kordonouri O, James RW, Bennetts B, et al:
Modulation by blood glucose levels of activity and concentration of
paraoxonase in young patients with type 1 diabetes mellitus.
Metabolism. 50:657–660. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mackness B, McElduff P and Mackness MI:
The paraoxonase-2–310 polymorphism is associated with the presence
of microvascular complications in diabetes mellitus. J Intern Med.
258:363–368. 2005.
|
|
18
|
Murata M, Maruyama T, Suzuki Y, Saruta T
and Ikeda Y: Paraoxonase 1 Gln/Arg polymorphism is associated with
the risk of microangiopathy in Type 2 diabetes mellitus. Diabet
Med. 21:837–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiwari AK, Prasad P, BKT, et al: Oxidative
stress pathway genes and chronic renal insufficiency in Asian
Indians with Type 2 diabetes. J Diabetes Complications. 23:102–111.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleiss JL: The statistical basis of
meta-analysis. Stat Methods Med Res. 2:121–145. 1993. View Article : Google Scholar
|
|
21
|
Nivoit P, Wiernsperger N, Moulin P,
Lagarde M and Renaudin C: Effect of glycated LDL on microvascular
tone in mice: a comparative study with LDL modified in vitro or
isolated from diabetic patients. Diabetologia. 46:1550–1558. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mackness B, Durrington PN, Boulton AJ,
Hine D and Mackness MI: Serum paraoxonase activity in patients with
type 1 diabetes compared to healthy controls. Eur J Clin Invest.
32:259–264. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuzura S, Ikeda Y, Suehiro T, et al:
Correlation of plasma oxidized low-density lipoprotein levels to
vascular complications and human serum paraoxonase in patients with
type 2 diabetes. Metabolism. 53:297–302. 2004. View Article : Google Scholar
|
|
24
|
Humbert R, Adler DA, Disteche CM, Hassett
C, Omiecinski CJ and Furlong CE: The molecular basis of the human
serum paraoxonase activity polymorphism. Nat Genet. 3:73–76. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Garin MC, James RW, Dussoix P, et al:
Paraoxonase polymorphism Met-Leu54 is associated with modified
serum concentrations of the enzyme. A possible link between the
paraoxonase gene and increased risk of cardiovascular disease in
diabetes. J Clin Invest. 99:62–66. 1997. View Article : Google Scholar
|