Introduction
Over the past decade, increasing evidence has
indicated that adverse reactions are associated with volatile
anesthetics (1,2). Xie et al observed that
neurodegenerative disorders, including Alzheimer’s and Parkinson’s
diseases, may be accelerated by volatile anesthesia following long
surgery (3–5). More recently, they showed that
anesthesia with sevoflurane for 6 h induced caspase activation and
apoptosis, altered amyloid precursor protein (APP) processing and
increased amyloid-β levels in the brain tissue of transgenic mice
(6). Inhalation anesthetics also
led to pronounced immunosuppression by reducing the activity of the
natural killer cells (7,8), peripheral leukocytes and T
lymphocytes (9). Matsuoka et
al(10) observed that
sevoflurane and isoflurane induced apoptosis in human peripheral
lymphocytes in a dose- and time-dependent manner and that apoptosis
was strictly associated with overproduction of reactive oxygen
species (ROS) mediated by mitochondria (10,11).
Over the past few years, the majority of studies
have been focused on the exact mechanism of action of volatile
anesthetics using physicochemical models; however, precise
information of the effects on cell culture model systems in
vitro remains unclear, particularly the potent negative effect
on lung alveolar cells. During inhalation of anesthesia, lung
epithelial cells are the first barrier directly exposed to volatile
anesthetics prior to reaching the target neuronal cells. Type II
pneumocytes are the primary producers of pulmonary surfactant, a
liquid film that consists of ~90% liquid and ~10% proteins
(12). An important function of
type II pneumocytes is that they are the alveolar progenitor cells
responsible for normal tissue turnover and restoration of alveoli
following lung injury (13,14).
The A549 human lung carcinoma cell line, which is sensitive to
harmful agents, is widely accepted as a model for in vitro
investigations of type II alveolar pneumocytes (15).
There are well-documented examples that indicate
that halothane reduces the production of phophatidylcholine, which
is the major surfactant component of A549 pulmonary cells,
following treatment with 1.5–3 mM concentrations in
vitro(16,17). Studies also observed that halothane
decreased lung cell viability, impaired DNA integrality and
provoked stress-induced apoptosis by reduction of Bcl-2 expression
of the mitochondrial apoptotic pathway (18). However, the effect of sevoflurane,
halothane’s parent molecule on the action the lung-type 2 alveolar
pneumocytes is obscure and not yet completely clear. Numerous
pathophysiological mechanisms have been implicated in the
development of cell damage; however, the exact cascade leading to
sevoflurane-mediated development is unclear.
Sevoflurane, the most popular anesthetic used daily,
as with halothane, was hypothesized to induce A549 lung alveolar
cell apoptosis in vitro. Apoptosis is the predominant
mechanism regulating immunological homeostasis and the termination
of surgical injury-induced inflammation on lung epithelial cells
(19,20). However, to avoid these side effects
and evaluate cellular injury, identification of the various
apoptotic changes in sevoflurane-treated lung cells is required.
Thus, the current study aimed to evaluate the apoptotic changes on
sevoflurane-treated A549 human lung alveolar cells and to assess
the effects of 1 mM sevoflurane on apoptotic-bodies, DNA damage and
caspase 3/7, the most important indicators of apoptosis.
Materials and methods
Cell culture
All experiments were performed with A549 cells, a
lung-derived human carcinoma cell line (no. CCL-185), obtained from
our laboratory, maintaining the morphological and biochemical
characteristics of type II pneumocytes (21). The cells were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; HyClone Laboratories
Inc., South Logan, UT, USA), supplemented with 10% fetal bovine
serum (HyClone Laboratories Inc.), 2 mM glutamine, 100 U penicillin
and 100 μg/ml streptomycin (all Gibco Invitrogen Canada Inc.,
Burlington, ON, Canada). The cultures were equilibrated with
humidified 5.0% (v/v) CO2 in air at 37ºC in a Series II
Water CO2 Jacketed Incubator (Thermo Fisher Scientific
Inc., Marietta, OH, USA).
Exposure to sevoflurane
A549 cells were exposed to 5% (v/v) sevoflurane
(Sevoflurane®; Maruishi Pharmaceutical Co., Ltd., Osaka,
Japan) by calibrated vaporizers (Datex-Ohmeda Inc., Madison, WI,
USA) with a 2 l/min flow of 95% air and 5% CO2 using an
anesthesia machine (Excel 210SE; Ohmeda Inc., Madison, WI, USA) for
2 h in a gas tight glass chamber, which was maintained at 37ºC by
electric-heated thermostatic water bath (ZHWY-110×30; Zhicheng
Inc., Shanghai, China). Humidification was achieved with water
evaporation inside the chamber. The sevoflurane concentration was a
constant 5.0% (v/v), which was detected with an infrared anesthetic
gas module (M1026B; Philips Inc., Berlin, Germany). Simultaneously,
a 60-mm culture dish containing 5 ml medium was used to measure the
fluid concentrations of sevoflurane dissolved in the cell culture
medium by gas chromatography (Agilent 4890D; Agilent Technologies
Inc., Palo Alto, CA, USA) in the chamber of every experiment. The
coefficient of sevoflurane in DMEM-gas was observed at 0.38±0.08 by
gas chromatography. The concentration of sevoflurane in DMEM was
1.0±0.1 mM, which was higher than those attained in the plasma of
patients during administration of sevoflurane for general
anesthesis. An untreated control group was obtained in a standard
95% air, 5% CO2 incubator without anesthetic exposure. A
positive control group was treated with 2 μM daunorubicin
hydrochloride (DRB; Sigma-Aldrich, St. Louis, MO, USA). Following
treatment under the respective conditions at 37ºC for 2 h, cells
were washed three times with phosphate-buffered saline and used in
a number of experiments 24 h later.
Cell viability by adenosine triphoshpate
(ATP) assay
The cells were seeded into a 96-microwell plate at a
density of 1×104 cells/well. The activity of ATP was
detected by Wallac Victor 2 1420 Multilabel Counter with
Enliten® rLuciferase/Luciferin reagent kit (cat. no.
FF2021; Promega Corporation, Madison, WI, USA). Wearing new,
disposable gloves when preparing samples and performing the ATP
assay was required. Untreated, DRB- and sevoflurane-treated cells
were added, assuming 0.1 ml reagent/well, mixed gently to avoid
generating aerosols that may contaminate assay reagents and
incubated at room temperature for 30 min. When ATP was the limiting
component in the luciferase reaction, the intensity of the emitted
light was proportional to the concentration of ATP and the number
of viable cells was assessed based on the quantity of ATP
available.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) staining
The histochemical TUNEL kit (cat. no. 1168409910;
Roche Diagnostics, Mannheim, Germany) was used to detect
apoptotic-bodies on A549 cells. The cells (1×105
cells/ml) were cultured on sterile coverslips on 35-mm dish
(Corning Inc., New York, NY, USA) overnight. Following treatment,
cells were fixed with 4% paraformaldehyde and TUNEL was performed
according to the manufacturer’s instructions. TUNEL-positive cells
were defined using a microscope (DP70; Olympus Corporation, Tokyo,
Japan), which was equipped with a digital camera (BX51; Olympus
Corporation). There were different numbers of dead cells that
detached from the cover between the untreated, DRB and sevoflurane
groups. In addition to cell counts, cell morphology was also
examined. Cells with nuclear abnormalities, including chromatin
compaction and clustering, fragmented nuclei, bi- and tri-nucleated
cells that represent an apoptosis-like or necrotic-like cell, were
counted. Data were received for the number of surviving cells due
to the effect of sevoflurane, DRB and untreated control with CAST
software (Revision 0.9.5; Olympus Danmark A/S, Ballerup, Denmark).
The ratios of positively stained cells to total cells were
calculated. Ten sites were selected at random for each slide for
image analysis (Image-Pro Plus version 4.5.0.19; Media Cybernetics,
Carlsbad, CA, USA). All examinations were performed by blinded
observers. Furthermore, the values were obtained from three
repeated experiments and ten images per experiment and the averages
are presented as mean ± standard deviation.
DNA isolation and electrophoresis
The cells were seeded into 60-mm2 corning
culture dishes at a density of 1×106 cells/dish.
Following treatment, cells were collected and DNA isolation was
performed using the cells/tissue genomic DNA extraction kit (cat.
no. GK0121; Generay Biotech Co., Ltd., Shanghai, China), according
to the manufacturer’s instructions. DNA integrality in cells was
analyzed by 0.8% agarose gel (Sigma-Aldrich) electrophoresis for 1
h at 80 V. Visualization of DNA was analyzed by ethidium bromide
fluorescence using UVP EC3 Imaging system (UVP Inc., Upland, CA,
USA) with VisionWorks®Ls Image Acquisition and Analysis
software (version 5.5.3; UVP Inc., Upland, CA, USA).
Analysis of caspases 3/7 activity
The cells were cultured in a 96-microwell plate at a
density of 1×104 cells/well overnight. The groups of
cells were then exposed to each pre-set condition and the activity
of caspase 3/7 was detected by Wallac Victor 2 1420 Multilabel
Counter (PerkinElmer Inc., Wellesley, MA, USA) with
caspase-Glo® 3/7 assay kit (cat. no. G8090; Promega
Corporation) according to the manufacturer’s instructions.
Following treatment, 100 μl caspase 3/7 substrate was added to the
cell suspension in each well. Following immediately mixing, the
plate was incubated at room temperature for 30 min. A ‘no-cell’
control, in addition to an untreated control to account for the
signal generated from serum alone, was performed. The ‘no-cell’
control signal was subtracted from signals produced by the treated
and untreated cells. The luminescent signal was recorded, which was
proportional to the amount of caspase 3/7 activity present.
Statistical analysis
Each experiment was performed three times. Data are
presented as the mean ± standard deviation and analyzed using a
one-way analysis of variance and Student-Newman-Keuls (SNK) test
(non-parametric) for multiple comparisons. P<0.05 was considered
to indicate a statistically significant difference. All statistical
analyses were performed by the software SPSS 15.0 for windows
(SPSS, Inc., Chicago, IL, USA).
Results
Sevoflurane decreases cell viability as
shown by an ATP assay
Apoptosis is a genetically determined active form of
cell death that is essential under physiological and pathological
conditions throughout the embryonic development and later life of
multicellular organisms (22).
Since ATP is an indicator of metabolically active cells, the number
of viable cells may be assessed based on the quantity of ATP
available. The ATP assay showed improved reproducibility and
sensitivity compared with the MTT assay and is particularly useful
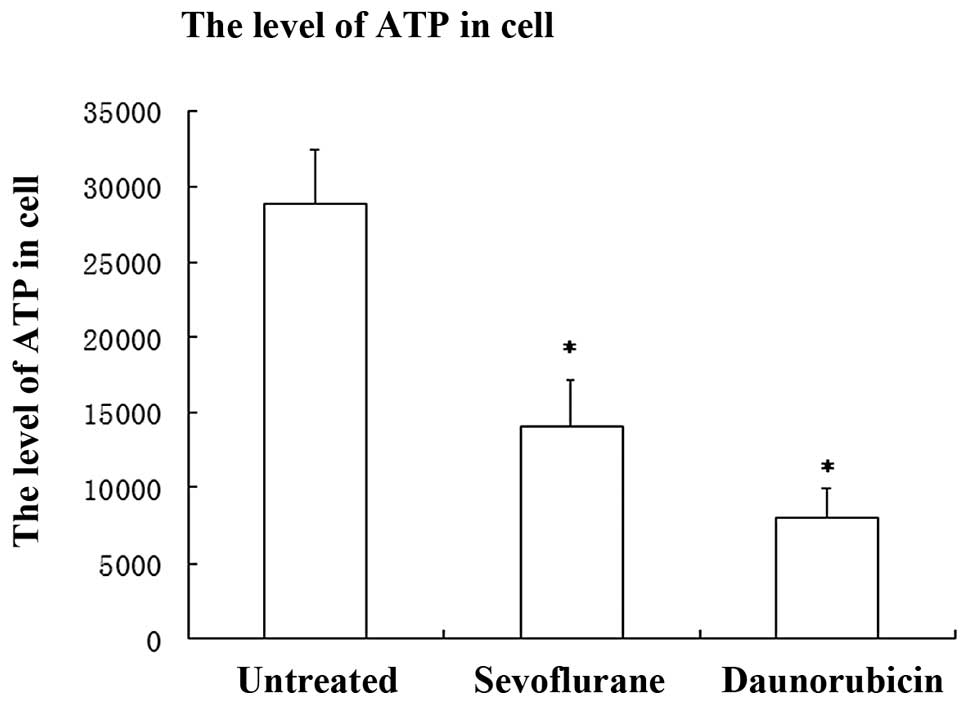
for the measurement of viability with low cell numbers (23). Cells treated with sevoflurane
showed a significant reduction in ATP (reduction, ~51.3%) compared
with untreated cells (P<0.001, SNK). The reduction by the DRB
group was more evident (reduction, ~72%) compared with the
untreated group (P<0.001, SNK). These results were consistent
with TUNEL staining of the remaining cell number which showed that
A549 cells exposed to sevoflurane underwent programmed cell death
(Fig. 1).
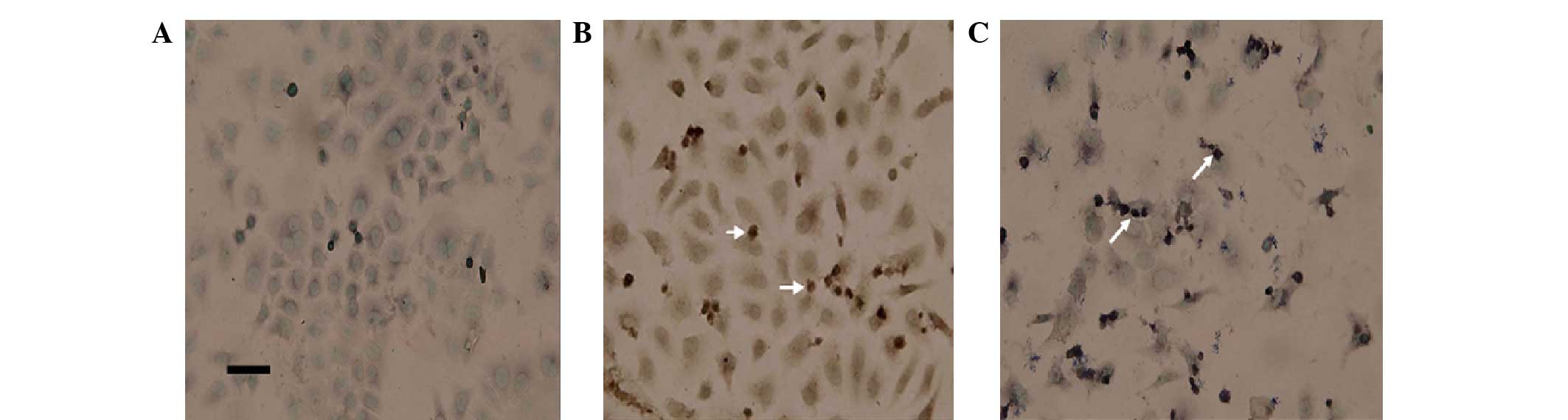
Sevoflurane inducing apoptotic body
formation shown by TUNEL staining
According to the images, obtained by light
microscopy with a digital camera, of the three groups, sevoflurane
induced morphological changes in A549 cells; the cells became more
rounded and detached from the dish. The number of detached cells in
the sevoflurane group was greater compared with the untreated
group, but less than the DRB group (data not shown).
The quantitative analysis of the effect of
sevoflurane on A549 cells was evaluated by TUNEL staining. In
apoptosis, cells became spherical, ballooned and dispersed into
smaller groups and apoptotic-bodies were observed. In the three
groups the proportion of cells with apoptotic-bodies were:
Untreated, 2.00±0.08%; sevoflurane, 18.0±0.1% and DRB, 43.0±0.2%.
The difference between the sevoflurane and untreated groups was
considered statistically significant (P=0.001, SNK) and the DRB
group to untreated group was also statistically significant
(P<0.001, SNK; Fig. 2).
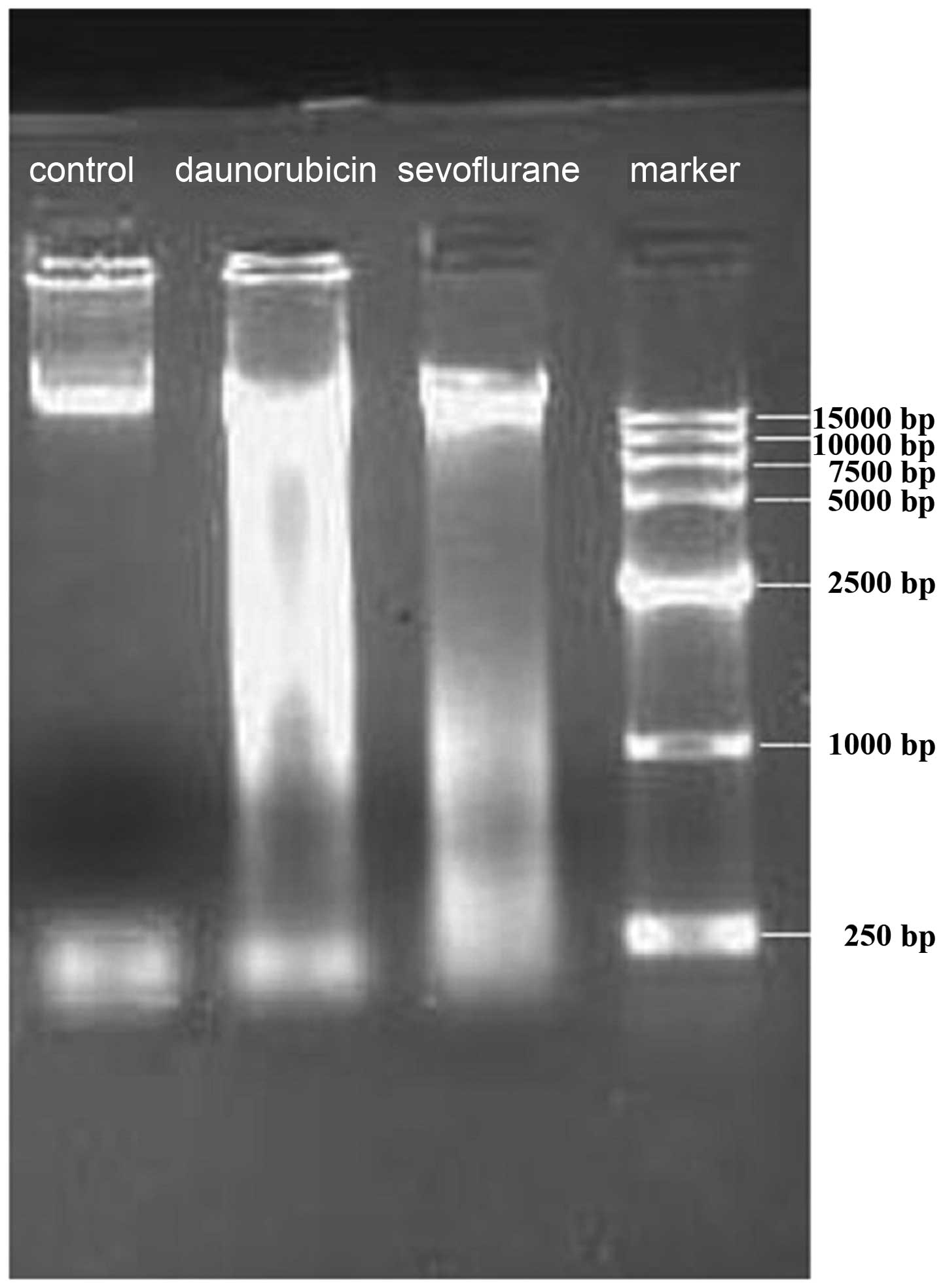
Sevoflurane increasing DNA damage
observed by electrophoresis of DNA
The DNA was digested by specific endonucleases into
fragments and ultimately packed into vesicles. DNA integrity in
cells exposed to sevoflurane and controls was analyzed by 0.8%
agarose gel electrophoresis. Electrophoresis of DNA isolated from
A549 cells following exposure to sevoflurane and control is shown
in Fig. 3. A DNA fragment was
observed with sevoflurane and DRB group, but not with untreated
group.
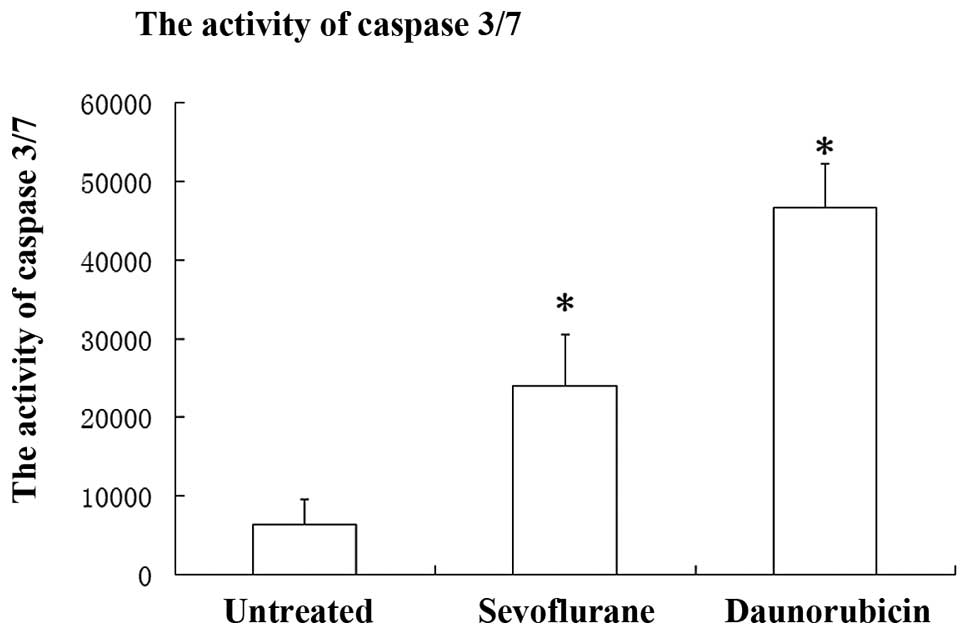
Sevoflurane provoking caspase 3/7
A specific class of cysteine proteases, termed
caspases, are activated in an amplifying proteolytic cascade,
including caspase 3/7, which exhibits an important role in cell
apoptosis (24). As shown in
Fig. 4, the activity of caspase
3/7 on A549 cells of three groups was as follows: Sevoflurane,
24055.4±6435.1; DRB, 46624.3±5663.5 and untreated, 6423.6±3194.7.
Cells treated with sevoflurane exhibited significantly increased
levels of caspases 3/7 (~2.74 times growth) as compared with the
untreated cells (P<0.001, SNK). The DRB-group exhibited a ~6.25
times increase compared with the untreated group (P<0.001, SNK).
These data indicate that the activation of caspases 3/7 is involved
in sevoflurane-induced cellular injury (Fig. 4).
Discussion
In this study, 5% sevoflurane was observed to induce
cellular damage and apoptosis of A549 lung alveolar epithelial
cells by decreasing cell viability, increasing apoptotic bodies,
impairing DNA integrity and provoking caspase 3/7. Sevoflurane,
with its low pungency, non-irritating odor and low blood/gas
partition coefficient, is a potent novel inhalation anesthetic
agent widely used for general anesthesia (25). Although increasing evidence of the
noxious effects of volatile anesthetics has attracted attention,
particularly for their potential toxic effects following long
surgery (25), the potential
significant lung epithelial cell damage resulting from sevoflurane
treatment has not been fully understood. This area of research may
contribute to elucidating the role of stress-induced apoptosis in
lung diseases following long surgery using volatile anesthetics,
including sevoflurane and thus, may be of value in clinical
practice.
Previous evidence with regard to apoptotic effects
of volatile anesthetics has been conflicting. This may result from
differences in study designs, as well as the inherent limitations
of in vitro models (9,10,26,27).
The difficulties of the various experimental approaches in the
literature are associated with the handling of the concentration of
volatile agents in vitro. However, after 20 years,
significant progress has been made by a number of researchers.
Muckter et al(28) reported
an apparatus for the exposure of cultured A549 cells to volatile
anesthetics in 1998. Matsuoka et al(10) controlled the concentration with the
container equipped with two sealing cocks in normal peripheral
lymphocytes in vitro in 2001.
In the present study the concentration was monitored
in two parallel methods of each test. It is principally feasible to
test the concentration of effluent gas with infrared spectroscopy
and fluid with gas chromatography. The methods ensure not only the
concentration of sevoflurane in the air of the tight chamber, but
also the liquids that the cells survive in. The data showed that
the DMEM medium-gas partition coefficient of sevoflurane determined
in this study was 0.38±0.08, which was similar with the reports
that the coefficient in cells cultured just like the DMEM was 0.36
(10). Therefore, the
concentrations of 1.0±0.1 mM were calculated to be equivalent to 5%
sevoflurane in the gas phase of the DMEM-gas system. Stephanova
et al(18) observed that
A549 cells treated with halothane at 1.5–3 mM in vitro
induced morphological alererations, as well as DNA damage. The
authors also reported that an irreversible impairment of the cell
genome is initiated at a concentration as low as 1.5 mM, defining
the threshold for cell survival (16–18).
In the current study, the concentration of 1.0±0.1 mM of
sevoflurane on A549 cells <1.5 mM of halothane induced
apoptosis-like changes by decreasing cell viability, increasing the
number of apoptotic bodies, impairing DNA integrity and provoking
caspase 3/7. Further study at a lower concentration is
required.
In the dead cells, the ATP level was markedly
decreased, thus, the ATP levels better reflected the cell
viability. The ATP assay is a popular assay and is used to evaluate
pharmacological toxicity sensitively and accurately (29). The data indicated that the level of
ATP in A549 cells exposed to sevoflurane had decreased compared
with the untreated group. This indicated that cell viability of
human A549 cells reduced to ~52% following 2-h exposure to 5%
sevoflurane. The DRB group that was used to treat lung cancer
reduced to ~72% of A549 cell death. Bakand et al(24) observed that cell viability was
reduced in a concentration dependent manner following exposure of
human A549 cells to NH3 with ATP assay. However, it
remains unkown whether sevoflurane also promotes A549 cell
apoptosis in a concentration-dependent manner and the mechanism of
apoptosis on A549 cells induced by sevoflurane is unknown.
The number of remaining cells treated with
sevoflurane was reduced compared with the untreated group but not
all cells swelled and detached from the subculture. Apoptotic
bodies may be observed by a TUNEL assay via a light microscope.
These results were consistent with the ATP assay results that
showed that A549 cells exposed to sevoflurane underwent programmed
cell death. Information of the noxious effects of volatile
anesthetics and the apoptosis of peripheral polymorphonuclear
neutrophils (26), T lymphocytes,
natural killer cells, colon carcinoma and larynx carcinoma (HEP-2)
have been previously reported (10,30,31).
Apoptotic events in type II pneumocytes are usually accompanied
with severe pulmonary complications. Clinical studies have provided
data that apoptotic cells are observed in humans during the
resolution phase following acute lung injury (32). Thus, clarifying the mechanism that
induced cell death may contribute to the understanding of chronic
or temporary disorders following inhalation anesthesia (14). However, investigations in
vivo are usually more complex and clinical experiments or
animal experiments of the adverse effects of volatile anesthetics
are required in the future.
Studies have highlighted the cyto- and genotoxic
effect of anesthetics in a series of model systems in
vitro(16,33–43).
As apoptotic morphology was observed, the fragmentation of genomic
DNA that usually represented a critical event in cell cycle and may
lead to cell death was investigated. The current data showed DNA
fragmentation in A549 cells treated with sevoflurane, even at a
concentration of 1.0±0.1 mM, without a ladder pattern of fragments.
These data were in agreement with the in vitro genotoxic
effect of inhalation anesthetics on human lymphocytes (43). Hacker et al(41) observed a phenomenon associated with
DNA cleavage and degradation of nuclear lamina by caspase 3 during
apoptosis. The results aided in the explanation that
sevoflurance-induced apoptosis in cells by DNA degradation may lead
to caspase-dependent signaling events; however, the potential
genotoxic mechanism remains unclear.
The caspase family of cysteine proteases is the
central mediator of the proteolytic cascade leading to cell death
and elimination of compromised cells (33,34).
Caspase 3 is key in apoptosis. A previous study demonstrated that
caspase 3/7 activation is an important step in glutathione
depletion-induced apoptosis in resting and inflammatory neutrophils
(10). The current data showed
that the percentage of caspase 3/7 activity of A549 increased
following exposure to sevoflurane. This result suggested that
caspase 3/7 may be involved in the apoptosis of A549 following
exposure to sevoflurane for 2 h, however, further investigations
are required to determine the precise pathway. Furthermore, a
previous study observed that sevoflurane may induce caspase 3/7
activation and increase amyloid-β levels in H4-APP cells and key
changes associated with Alzheimer’s disease neuropathogenesis
(36). Therefore,
sevoflurane-induced caspase 3 activation indicated that induced
alterations in apoptosis processing were, at least partially,
dependent upon caspase activation. Roesslein et al(27) observed that 8% sevoflurane (1,170
μmol/l) is a specific activator of the apoptotic signal p38 MAP
kinase cascade by incubating Jurkat T cells with 8% sevoflurane for
24-h treatment. That signal appears to act as a proapoptotic
intermediate and multifunctional stress-sensing kinase through
mitochondrial-dependent caspase activation and is required for
apoptosis induced by oxidative stress, tumor necrosis factor and
endoplasmic reticulum stress (44–46).
Thus, it is possible that sevoflurane may activate the p38 MAP
kinase pathway of apoptosis with caspase 3/7 activation in A549
lung cells in vitro. A number of the results may be a
consequence of the cancer cell character. However, it is crucial to
perform the same experiments in primary rats or mice cultures of
this cell type to verify the current findings. In the future,
studies of the mechanism of p38 MAPK caspase apoptosis in lung
alveolar cells lower concentrations of sevoflurane in vivo
are required.
No data are available concerning the mechanism of
apoptosis in sevoflurane lung cytotoxicity. A number of
experimental data have revealed sevoflurane’s parent molecule,
halothane, increased the cytosolic concentration of
Ca2+, where calcium acts as an essential signal by
releasing cytochrome c and other factors, provoking apoptosis of
cells (47,48). Significant disturbances in the
function and conformation of the Ca2+-ATPase were also
reported for volatile anesthetics applied at clinically relevant
concentrations (49). However, it
is difficult to assess in vivo whether sevoflurane or
surgical stress are the primary cause of the lung tissue damage and
cell apoptosis. Experimental support for this hypothesis is
required.
In conclusion, the results of the present study
suggest that 1 mM sevoflurane exerts an apoptotic effect on the
A549 lung alveolar epithelial cells in vitro by decreasing
cell viability, increasing apoptotic body formatiom, impairing DNA
integrity and activating caspase 3/7. The exact mechanism of the
effect of sevoflurane on alveolar cells is not well understood and
further studies are required to specify the signaling pathways
involved. The current findings may ultimately contribute to
recognizing the role of lung injury in diseases following a long
surgery under sevoflurane anesthesia, which is likely to facilitate
the design of safe anesthetics and improved anesthesia care for
patients. Taking into consideration that sevoflurane may modulate
respiratory function, its use in susceptible patients should be
avoided.
Acknowledgements
The authors would like to thank Yun-Xia Zuo and
Guo-Hua Li (Professor of Laboratory of Anesthesiology and Critical
Care Medicine, West China Hospital, Sichuan University, Chengdu,
China) for their scientific discussions for this study. This study
was supported by a grant from WJTU11BR071 (Southwest Jiaotong
University One Hundred Young Teachers project Fund, Chengdu,
China).
References
|
1
|
Tang J, Eckenhoff MF and Eckenhoff RG:
Anesthesia and the old brain. Anesth Analg. 110:421–426. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terrando N, Brzezinski M, Degos V,
Eriksson LI, Kramer JH, Leung JM, Miller BL, Seeley WW, Vacas S,
Weiner MW, et al: Perioperative cognitive decline in the aging
population. Mayo Clin Proc. 86:885–893. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu X, Lu Y, Dong Y, Zhang G, Zhang Y, Xu
Z, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE and Xie Z: The
inhalation anesthetic isoflurane increases levels of
proinflammatory TNF-α, IL-6, and IL-1β. Neurobiol Aging.
33:1364–1378. 2012.
|
|
4
|
Xie Z and Tanzi RE: Alzheimer’s disease
and post-operative cognitive dysfunction. Exp Gerontol. 41:346–359.
2006.
|
|
5
|
Xu Z, Dong Y, Wu X, Zhang J, McAuliffe S,
Pan C, Zhang Y, Ichinose F, Yue Y and Xie Z: The potential dual
effects of anesthetic isoflurane on Aβ-induced apoptosis. Curr
Alzheimer Res. 8:741–752. 2011.
|
|
6
|
Lu Y, Wu X, Dong Y, Xu Z, Zhang Y and Xie
Z: Anesthetic sevoflurane causes neurotoxicity differently in
neonatal naive and Alzheimer disease transgenic mice.
Anesthesiology. 112:1404–1416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pollock RE, Lotzová E and Stanford SD:
Surgical stress impairs natural killer cell programming of tumor
for lysis in patients with sarcomas and other solid tumors. Cancer.
70:2192–2202. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Salo M: Effects of anaesthesia and surgery
on the immune response. Acta Anaesthesiol Scand. 36:201–220. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Loop T, Dovi-Akue D, Frick M, Roesslein M,
Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, et al:
Volatile anesthetics induce caspase-dependent,
mitochondria-mediated apoptosis in human T lymphocytes in vitro.
Anesthesiology. 102:1147–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Matsuoka H, Kurosawa S, Horinouchi T, Kato
M and Hashimoto Y: Inhalation anesthetics induce apoptosis in
normal peripheral lymphocytes in vitro. Anesthesiology.
95:1467–1472. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delogu G, Moretti S, Famularo G, Antonucci
A, Signore L, Marcellini S, Lo Bosco L and De Simone C: Circulating
neutrophils exhibit enhanced apoptosis associated with
mitochondrial dysfunctions after surgery under general anaesthesia.
Acta Anaesthesiol Scand. 45:87–94. 2001. View Article : Google Scholar
|
|
12
|
Lalchev ZI: Surface properties of lipids
and proteins at bio-interfaces. Handbook of Surface and Colloid
Chemistry. Birdi KS: CRC Press; New York, NY: pp. 625–687. 1997
|
|
13
|
White MK, Baireddy V and Strayer DS:
Natural protection from apoptosis by surfactant protein A in type
II pneumocytes. Exp Cell Res. 263:183–192. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fehrenbach H: Alveolar epithelial type II
cell: defender of the alveolus revisited. Respir Res. 2:33–46.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe N, Dickinson DA, Krzywanski DM,
Iles KE, Zhang H, Venglarik CJ and Forman HJ: A549 subclones
demonstrate heterogeneity in toxicological sensitivity and
antioxidant profile. Am J Physiol Lung Cell Mol Physiol.
283:L726–L736. 2002.PubMed/NCBI
|
|
16
|
Topouzova-Hristova T, Daza P,
Garcia-Herdugo G and Stephanova E: Volatile anaesthetic halothane
causes DNA damage in A549 lung cells. Toxicol In Vitro. 20:585–593.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Topouzová-Hristova T, Hazarosova R,
Bandreva B and Stephanova E: Halothane does not directly interact
with genome DNA of A549 cells. Folia Biol (Praha). 53:176–182.
2007.PubMed/NCBI
|
|
18
|
Stephanova E, Topouzova-Hristova T and
Konakchieva R: Mitochondria are involved in stress response of A549
alveolar cells to halothane toxicity. Toxicol In Vitro. 22:688–694.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oka M, Hirazawa K, Yamamoto K, Iizuka N,
Hazama S, Suzuki T and Kobayashi N: Induction of Fas-mediated
apoptosis on circulating lymphocytes by surgical stress. Ann Surg.
223:434–440. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sasajima K, Inokuchi K, Onda M, Miyashita
M, Okawa KI, Matsutani T and Takubo K: Detection of T cell
apoptosis after major operations. Eur J Surg. 165:1020–1023. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Petty RD, Sutherland LA, Hunter EM and
Cree IA: Comparison of MTT and ATP-based assays for the measurement
of viable cell number. J Biolumin Chemilumin. 10:29–34. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bakand S, Winder C and Hayes A:
Comparative in vitro cytotoxicity assessment of selected gaseous
compounds in human alveolar epithelial cells. Toxicol In Vitro.
21:1341–1347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghatge S, Lee J and Smith I: Sevoflurane:
an ideal agent for adult day-case anesthesia? Acta Anaesthesiol
Scand. 47:917–931. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wong CH, Liu TZ, Chye SM, Lu FJ, Liu YC,
Lin ZC and Chen CH: Sevoflurane-induced oxidative stress and
cellular injury in human peripheral polymorphonuclear neutrophils.
Food Chem Toxicol. 44:1399–1407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roesslein M, Frick M, Auwaerter V, Humar
M, Goebel U, Schwer C, Geiger KK, Pahl HL, Pannen BH and Loop T:
Sevoflurane-mediated activation of p38-mitogen-activated
stresskinase is independent of apoptosis in Jurkat T-cells. Anesth
Analg. 106:1150–1160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mückter H, Zwing M, Bäder S, Marx T,
Doklea E, Liebl B, Fichtl B and Georgieff M: A novel apparatus for
the exposure of cultured cells to volatile agents. J Pharmacol
Toxicol Methods. 40:63–69. 1998.PubMed/NCBI
|
|
29
|
Lundin A and Thore A: Analytical
information obtainable by evaluation of the time course of firefly
bioluminescence in the assay of ATP. Anal Biochem. 66:47–63. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loop T, Scheiermann P, Doviakue D,
Musshoff F, Humar M, Roesslein M, Hoetzel A, Schmidt R, Madea B,
Geiger KK, et al: Sevoflurane inhibits
phorbol-myristate-acetate-induced activator protein-1 activation in
human T lymphocytes in vitro: potential role of the p38-stress
kinase pathway. Anesthesiology. 101:710–721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kvolik S, Glavas-Obrovac L, Bares V and
Karner I: Effects of inhalation anesthetics halothane, sevoflurane,
and isoflurane on human cell lines. Life Sci. 77:2369–2383. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bardales RH, Xie SS, Schaefer RF and Hsu
SM: Apoptosis is a major pathway responsible for the resolution of
type II pneumocytes in acute lung injury. Am J Pathol. 149:845–852.
1996.PubMed/NCBI
|
|
33
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O’Neill AJ, O’Neill S, Hegarty NJ, Coffey
RN, Gibbons N, Brady H, Fitzpatrick JM and Watson RW: Glutathione
depletion-induced neutrophil apoptosis is caspase 3 dependent.
Shock. 14:605–609. 2000.PubMed/NCBI
|
|
36
|
Dong Y, Zhang G, Zhang B, Moir RD, Xia W,
Marcantonio ER, Culley DJ, Crosby G, Tanzi RE and Xie Z: The common
inhalational anesthetic sevoflurane induces apoptosis and increases
beta-amyloid protein levels. Arch Neurol. 66:620–631. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Molliex S, Dureuil B, Aubier M,
Friedlander G, Desmonts JM and Clerici C: Halothane decreases
Na,K-ATPase, and Na channel activity in alveolar type II cells.
Anesthesiology. 88:1606–1613. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sardaş S, Aygün N, Gamli M, Unal Y, Unal
N, Berk N and Karakaya AE: Use of alkaline comet assay (single cell
gel electrophoresis technique) to detect DNA damages in lymphocytes
of operating room personnel occupationally exposed to anaesthetic
gases. Mutat Res. 418:93–100. 1998.
|
|
39
|
Jaloszyński P, Kujawski M, Wasowicz M,
Szulc R and Szyfter K: Genotoxicity of inhalation anesthetics
halothane and isoflurane in human lymphocytes studied in vitro
using the comet assay. Mutat Res. 439:199–206. 1999.PubMed/NCBI
|
|
40
|
Patel AB, Sokolowski J, Davidson BA,
Knight PR and Holm BA: Halothane potentiation of hydrogen
peroxide-induced inhibition of surfactant synthesis: the role of
type II cell energy status. Anesth Analg. 94:943–947. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Häcker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000.
|
|
42
|
Valtcheva R, Stephanova E, Jordanova A,
Pankov R, Altankov G and Lalchev Z: Effect of halothane on lung
carcinoma cells A 549. Chem Biol Interact. 146:191–200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Szyfter K, Szulc R, Mikstacki A, Stachecki
I, Rydzanicz M and Jaloszyński P: Genotoxicity of inhalation
anaesthetics: DNA lesions generated by sevoflurane in vitro and in
vivo. J Appl Genet. 45:369–374. 2004.PubMed/NCBI
|
|
44
|
Kanamoto T, Mota M, Takeda K, Rubin LL,
Miyazono K, Ichijo H and Bazenet CE: Role of apoptosis
signal-regulating kinase in regulation of the c-Jun N-terminal
kinase pathway and apoptosis in sympathetic neurons. Mol Cell Biol.
20:196–204. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hofmann TG, Möller A, Hehner SP, Welsch D,
Dröge W and Schmitz ML: CD95-induced JNK activation signals are
transmitted by the death-inducing signaling complex (DISC), but not
by Daxx. Int J Cancer. 93:185–191. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hatai T, Matsuzawa A, Inoshita S, Mochida
Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H and Takeda
K: Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced
apoptosis by the mitochondria-dependent caspase activation. J Biol
Chem. 275:26576–26581. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rithalia A, Qureshi MA, Howarth FC and
Harrison SM: Effects of halothane on contraction and intracellular
calcium in ventricular myocytes from streptozotocin-induced
diabetic rats. Br J Anaesth. 92:246–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu WF, Yang LQ, Zhou MT, Liu ZQ and Li Q:
Ca2+ cytochemical changes of hepatotoxicity caused by
halothane and sevoflurane in enzyme-induced hypoxic rats. World J
Gastroenterol. 11:5025–5028. 2005.
|
|
49
|
Lopez MM and Kosk-Kosicka D: How do
volatile anesthetics inhibit Ca(2+)-ATPases? J Biol Chem.
270:28239–28245. 1995.
|