Introduction
Bone mesenchymal stem cells (BMSCs) have been shown
to exhibit the ability to improve the neurological functional
outcome of central nervous system (CNS) disorders, including stroke
and traumatic brain injury (TBI) (1,2).
Moreover, BMSCs are capable of proliferating and differentiating
into neurons and glial cells in vivo and in
vitro(3,4). These studies affirm BMSCs as a
potential candidate for use in cellular therapy for CNS disorders.
However, this type of therapy presents difficulties in the
treatment of brain injury due to the limited plasticity of the
nervous system and the complicated pathological processes involved.
The viability of transplanted stem cells may be reduced in in
vivo conditions, including a low-oxygen environment or during
an immunoreaction. Moreover, neurotrophic factors are imperative
for tissue regeneration as an insufficient level of neurotrophins
weakens the proliferation and differentiation of transplanted stem
cells (5). Thus, increasing the
viability of grafts through the utilization of neurotrophic factors
is an important strategy in promoting the efficacy of cell therapy
for the treatment of CNS diseases. Among a large number of
neurotrophic factors, the basic fibroblast growth factor (bFGF) has
been shown as a potent mitogen for neural stem and progenitor cells
in vitro and in vivo. Evidently, bFGF fulfills a
mediative function in inducing neurogenesis (6) and enhancing neural survival and
outgrowth (7). Furthermore, bFGF
alone has been demonstrated to induce BMSC neuronal differentiation
effectively in vitro(8).
However, endogenous bFGF expression levels diminish with aging but
increase during brain development (9,10).
Thus, BMSC-derived neural functional recovery may be promoted
through the administration of exogenous bFGF following various
brain injuries in the adult mammal. To date, few studies have been
conducted on exogenous bFGF promotion of BMSC
transplantation-mediated functional recovery in adult rats
following TBI. The current study was performed to investigate the
therapeutic potential of exogenous bFGF to promote BMSC
transplantation for adult brain repair, as well as to examine the
effects of bFGF on BMSC transplantation in adult rats following
TBI.
Materials and methods
Animals
A total of 48 male Sprague-Dawley rats (age, 3
months; weight, ~300 g) were obtained from the Experimental Animal
Center, Fourth Military Medical University (Xi’an, China) to be
used in the present study. This study was conducted in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals of the National Institutes of Health
(1996). The animal use instructions were reviewed and approved by
the Institutional Animal Care and Use Committee of the Fourth
Military Medical University. Rats were divided into four groups: A,
(n=6) rats without TBI; B, (n=6), rats with TBI that underwent no
treatment; C, (n=18) TBI group treated with BMSC transplantation
and D, (n=18) TBI group treated with BMSC transplantation and
bFGF.
Cultivation of BMSCs
BMSCs were isolated and cultured according to a
typical method (11) with
modifications. Briefly, bone marrow was harvested from the femurs
and tibias of one month-old male Sprague-Dawley rats through
suction using a 20 ml sterile syringe. For anticoagulation, 5 ml
heparin (100 IU/ml) was used. The recovery of the nucleated cells
was accomplished by centrifugation at 900 × g. BMSCs
(<4×105) were loaded into 3 ml Percoll (Sigma, St.
Louis, MO, USA) with a density of 1.089 g/ml, in a 15 ml conical
tube. The cells were separated following centrifugation at 1,100 ×
g for 20 min at 20°C. The primary BMSCs were cultured in flasks at
2×105 cells/ml with Dulbecco’s modified Eagle’s medium
(DMEM) F12 (Gibco-BRL, Carlsbad, CA, USA) containing 15% fetal
bovine serum (Hyclone, Logan, UT, USA) in a carbon dioxide
incubator (37°C, 5% CO2). The medium was replaced every
three days. When adherent cells reached ~80% confluency, the cells
were propagated to the next passage. Following three passages of
culture and identification by flow cytometry (Biomedika Instruments
Inc., Dollard-des-Ormeaux, QC, Canada), BMSCs were ready for
analysis and transplantation by trypsinization using 0.25% trypsin
and 0.02% EDTA.
Labeling of BMSCs with bromodeoxyuridine
(BrdU)
When the cells had reached 60% confluency, rat BMSCs
were labeled with BrdU (Sigma) at 3 μg/ml concentration by the
addition of BrdU to the culture and the mixture was incubated for
two days. Following washing three times with sterile
phosphate-buffered saline (PBS), the BMSCs were trypsinized and
used in subsequent in vivo experiments.
TBI model
The TBI model was developed as described in a
previous study (12) with
modifications. Following administration of chloral hydrate (Kangjie
Technology and Development Co., Ltd., Wuhan, China) anesthesia [300
mg/kg, intraperitoneally (i.p.)], the scalps were incised and a
bone window was developed over the right forelimb motor cortex.
Specifically, the rats were placed into a stereotactic frame
(Wandong Instruments, Ltd., Wuhan, China). The center of the bone
window was 2.5 mm lateral to the midline and 1.5 mm posterior to
the bregma. The diameter of the bone window was 5.0 mm. The bone
was thinned over the right forelimb motor cortex using a high-speed
microdrill (Ruijing Medical Equipment Co., Ltd., Wuhu, China) and
the last layer of the bone was removed with forceps. A 40 g weight
was inserted into the top of the tube (25 cm) and was allowed to
slide through the tube to deliver a blow onto the motor cortex to
induce TBI. The bone flap was placed in situ following TBI,
without fixing.
Surgical procedures
One day following TBI modeling, the rats were
anesthetized using chloral hydrate (300 mg/kg, i.p.) and were
placed into a stereotactic frame (Wandong Instruments, Ltd.) for
the microinjection procedure. Prior to transplantation, BMSCs were
digested with trypsin, washed twice with DMEM and centrifuged. The
BMSCs were diluted in PBS at a density of 1×105
cells/μl. A microinjector was slowly inserted 4 mm vertically
through a hole drilled on the skull into the right lateral
ventricle of each animal. The microinjections were conducted at a
rate of 1 μl/min. BMSCs (1×106 cells/μl) were
unilaterally implanted into the left lateral ventricle of the rats.
Groups C and D received BMSC infusions, whereas groups A and B
received a normal saline infusion. The needle was left in place for
an additional 10 min prior to being slowly drawn back. Following
TBI, this intralateroventricular injection of bFGF solution
(PeproTech, Rocky Hill, NJ, USA) was performed for seven
consecutive days (400 ng/day). Group D received a bFGF infusion,
whereas groups A, B and C received a normal saline infusion. The
needle was kept in the targets for a further 5 min prior to being
slowly withdrawn. Six rats from groups C and D at days 10 and 20
post-TBI, respectively and six from groups A and B, in addition to
the remainder of groups C and D at day 30 post-TBI, were euthanized
by intraperitoneal injection of pentobarbital natrium and perfused
with 4% paraformaldehyde.
Immunohistochemical staining
Immunohistochemical staining was performed by BrdU
labeling to identify the transplanted BMSCs. Formalin-fixed and
paraffin-embedded brain tissue blocks were sliced into 4-μm thick
sections. The sections were deparaffinized in xylene, followed by
rehydration in a decreasing concentration gradient of ethanol
solution. The sections were subjected to microwave heat-induced
epitope retrieval in boiled EDTA for 2 min. Following washing with
0.1 M PBS, these sections were pretreated with 0.3%
H2O2 in methanol for 30 min at room
temperature to prevent endogenous peroxidase activity. The sections
were then blocked with PBS, 3% skimmed milk and 3% normal donkey
serum for 2 h at room temperature and anti-BrdU (sheep, 1:200;
Abcam, Cambridge, MA, USA) was applied onto the sections, which
were subsequently incubated for 24 h at 4°C in a humidified
chamber. Following rinsing with PBS with Tween-20, the samples were
reacted with biotinylated secondary antibody (Donkey anti-sheep
IgG, 1:500; Abcam Inc., Cambridge, MA, USA) diluted in 0.1 M PBS
(1:500) for 2 h at room temperature. Subsequently, these sections
were incubated with an avidin-biotin peroxidase complex (Sigma)
diluted in 0.1 M PBS (1:500) for 2 h at room temperature. The
samples were developed in a staining solution (DAB). All steps were
performed according to the manufacturer’s instructions. The
sections were examined under a light microscope, (Carl Zeiss,
Oberkochen, Germany). To confirm whether the staining
satisfactorily developed, cells were counterstained with
hematoxylin to facilitate the visualization of the immunostained
product. In addition, non-immune serum was used in the controls for
the primary antibodies or omission of the primary antibodies.
Immunofluorescent double-labeling
Double-immunostaining with immunofluorescence was
performed. The parallel sections were processed for
immunofluorescent double labeling with antibodies against BrdU and
markers for mature neurons (NeuN) and astrocytes (glial fibrillary
acidic protein, GFAP). The staining procedure used was similar to
the BrdU staining procedure described in the previous section with
modifications. The primary antibodies used were mouse anti-NeuN
(1:500, Millipore, Billerica, MA, USA), mouse anti-GFAP (1:500,
Millipore) and sheep anti-BrdU (1:500, Abcam). Secondary antibodies
used were Alexa Fluor 488 anti-mouse IgG (1:500, Millipore) or
Cy3-conjugated anti-goat IgG (1:500, Chemicon, Temecula, CA, USA).
Sections in EDTA buffer were boiled in a microwave for 2 min to
retrieve the antigen. Following DNA denaturation, endogenous
peroxidase and serum blocking, sections were incubated with primary
antibodies for 48 h at 4°C with constant shaking. Following washing
three times, sections were incubated with secondary antibodies for
4 h at room temperature. Vectashield cover slips (Kangjie
Technology and Development Co., Ltd.) were placed on the slides,
which were examined using an Olympus BX51 fluorescent microscope
(Olympus, Tokyo, Japan).
Quantification of cells
Sections were examined using an Olympus Image System
CAST program (Olympus BX-51) to quantify the number of
BrdU-positive cells in the dentate gyrus (DG) and cortex. Results
are expressed as the average number of cells per specimen in the
target areas that consist of the DG and cortex. Eight sections per
sample at the level of DG spanning between −2.56 and −5 mm of
bregma and eight sections per sample at the level of TBI cortex
spanning between −1 and −2 mm of bregma were assessed by a blinded
pathologist. All BrdU-positive cells in the DG and TBI cortex were
counted regardless of size or shape under magnification ×200. These
immunofluorescent double-labeling slides were examined using an
Olympus fluorescent microscope to quantify the number of
BrdU-positive cells that had differentiated to the varying cell
types. In counting the double-stained sections, all BrdU-labeled
cells, which were located in the DG and cortex, were examined for
colocalization with NeuN or GFAP in sections. Only those cells for
which the BrdU-positive cells were unambiguously associated with a
specific cell type-specific marker were considered to be
double-labeled. Immunofluorescent double-labeled images were
imported into Photoshop under magnification ×200 (Olympus BX-51)
for merging and the number of BrdU-positive cells (red fluorescence
image) which colocalized with NeuN or GFAP (green fluorescence
image) was counted on a computer monitor.
Neurological functional evaluation
All tests began at 5:00 pm, following the nocturnal
habits of rats. Beginning on the day following TBI modeling,
continuing every 10th day thereafter for one month and prior to
euthanasia, animals were examined using two standard tests to
assess the sensorimotor function in the limbs, as well as
vestibulomotor function (13).
Specifically, the forelimb placing test measures sensorimotor
function in each forelimb as the animal places the limb on a
tabletop in response to visual, tactile and proprioceptive stimuli
(total score=0–10; 10=maximally impaired) (13). The modified beam balance test
examines vestibulomotor activity as the animal balances on a narrow
beam (30×1.3 cm) for 60 sec (score range=1–7; 7=maximally impaired)
(13).
Statistical analyses
All statistical analyses were performed using
SPSS® version 16.0 software (SPSS Inc., Chicago, IL,
USA). One-way analysis of variance and the Bonferroni test were
used to compare the differences between three or four groups and
groups in pairs, respectively. The t-test was used to compare the
differences between those in the contralateral hemisphere and those
in the ipsilateral hemisphere. P<0.05 was considered to indicate
a statistically significant difference.
Results
The majority of BrdU-labeled cells were identified
in the injured hemisphere concentrated around the contusion cortex
and the DG, whereas the minority were located in the contralateral
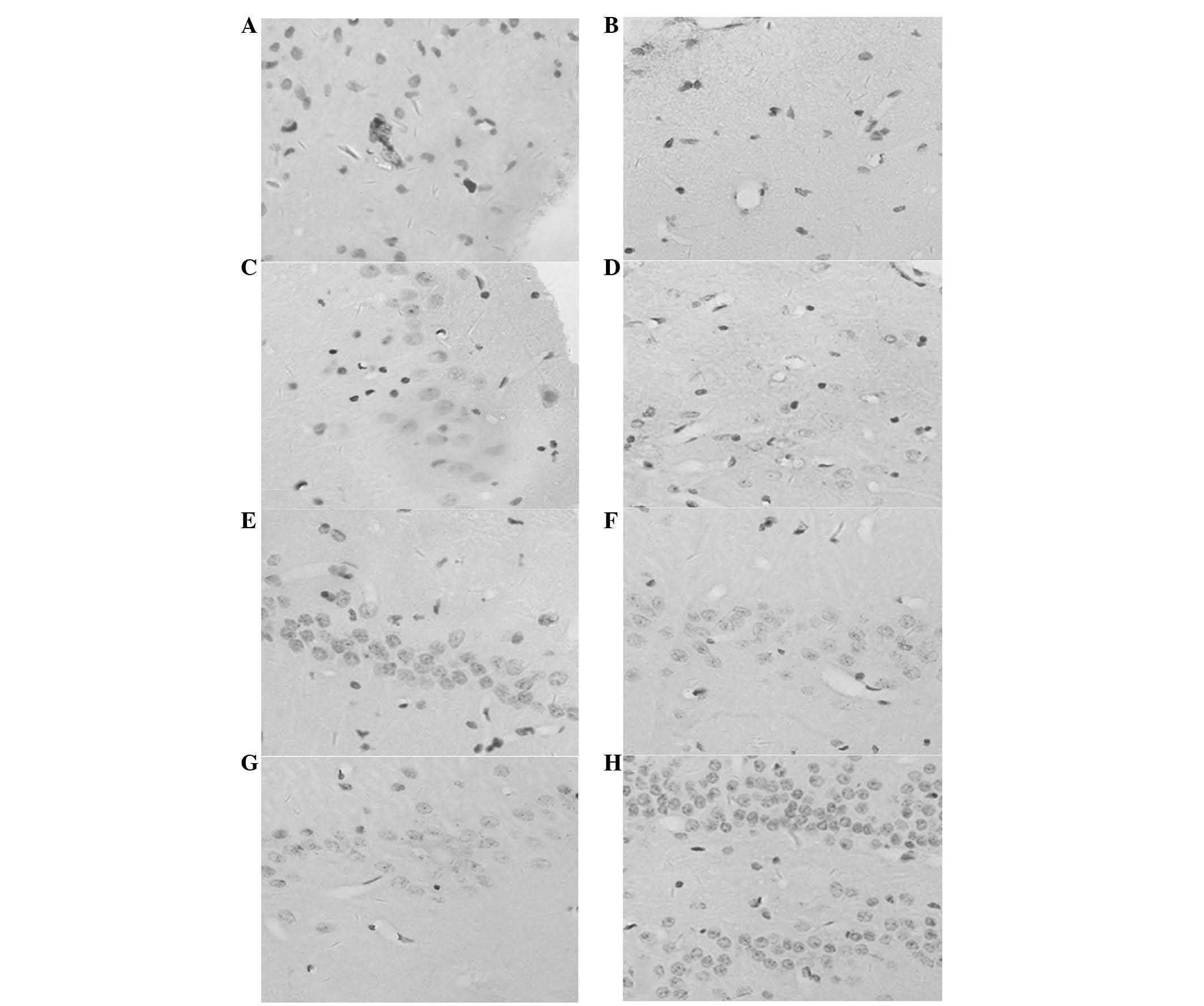
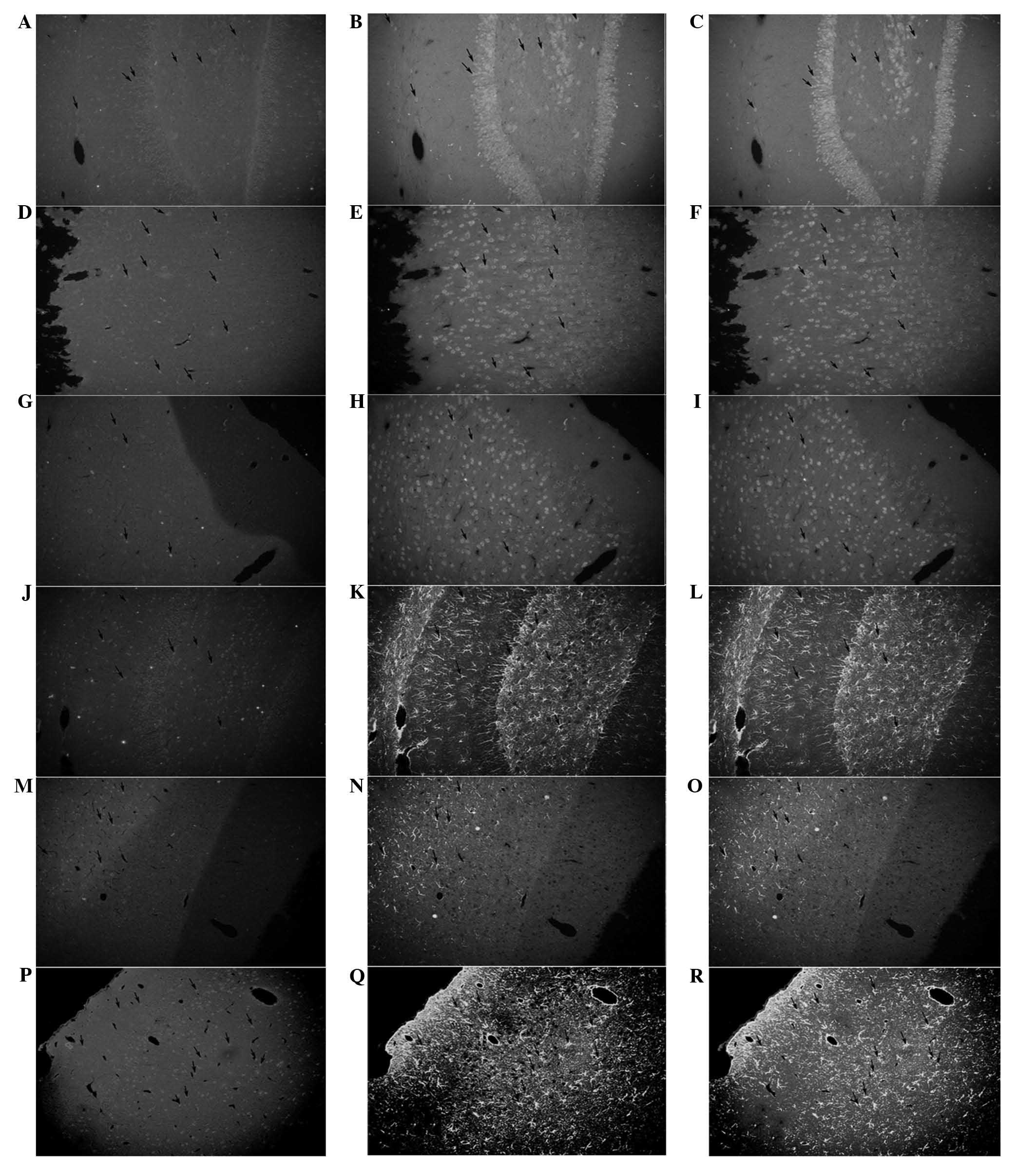
hemisphere (Fig. 1). In group D,
immunofluorescent double-labeling showed the highest expression of
astrocytes and neuron markers, with a small differentiation
proportion (P<0.05; Fig. 2A–I;
Fig. 3E–H). The majority of BMSCs
in the TBI cortex and DG showed differentiation along the glial
line, which was possibly enhanced by bFGF (Fig. 2J–R; Fig. 3G–H). Statistical significance was
observed in the number of BrdU-positive cells in the ipsilateral DG
and cortex between groups C and D at 10, 20 and 30 days post-injury
(P<0.05; Fig. 3C–D). The trend
in the ipsilateral DG in groups C and D was towards the significant
increase in the number of mitotically BrdU-positive cells
immediately following injury, which decreased marginally 20 days
post-injury (FC, 26.182; FD, 26.524;
P<0.05; Fig. 3C). The same
results were observed for groups C and D in the ipsilateral cortex
(FC, 22.962; FD, 12.180; P<0.05; Fig. 3D).
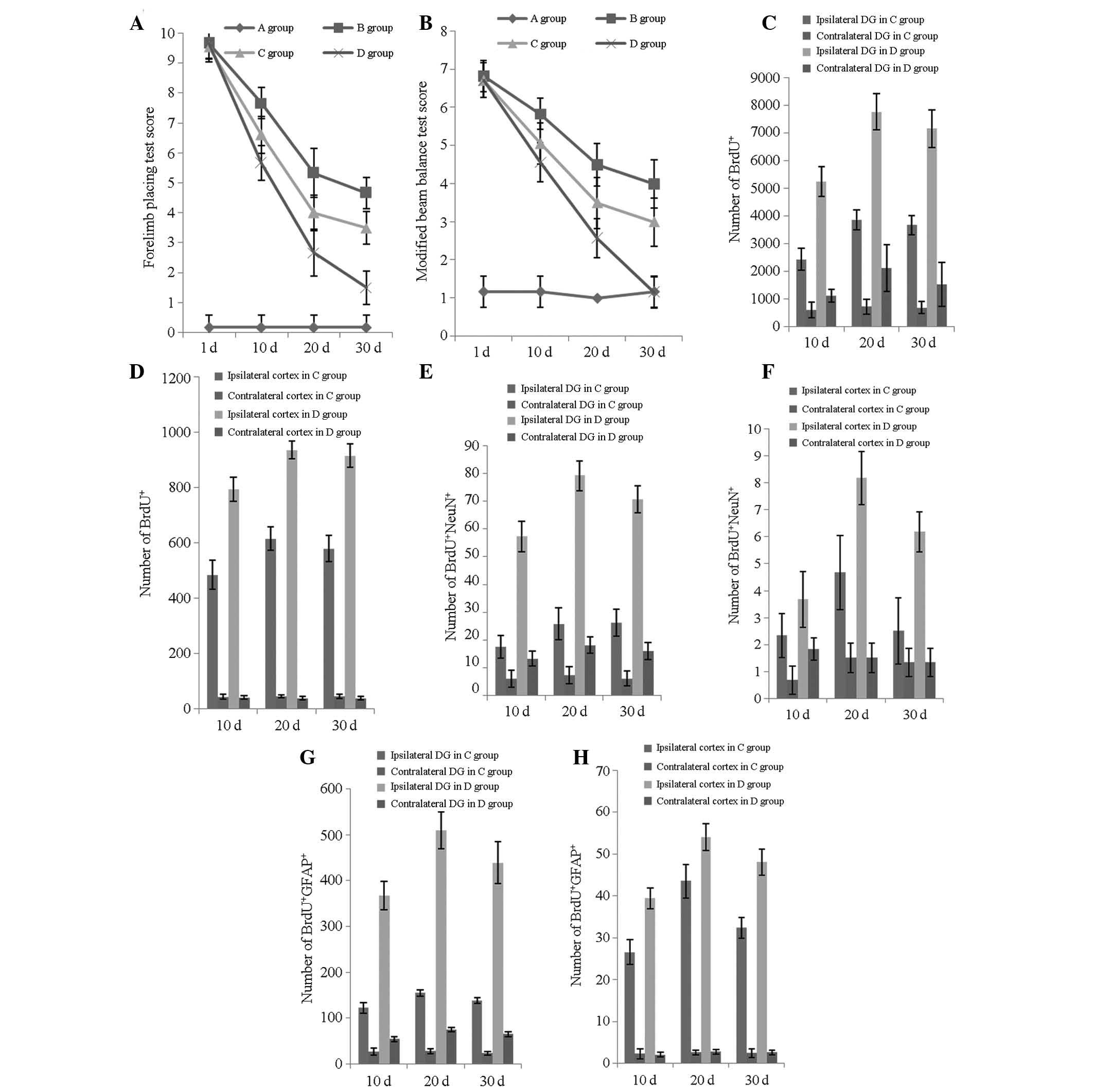 | Figure 3Neurological functional evaluation and
number of BrdU+/NeuN+/GFAP+ cells
at various time points. (A) Forelimb placing test scores of the
four groups at 1, 10, 20 and 30 days post-TBI; (B) modified beam
balance test scores of the four groups at 1, 10, 20 and 30 days
post-TBI; (C) number of BrdU+ cells in the ipsilateral
and contralateral DG in groups C and D at 10, 20 and 30 days
post-TBI; (D) number of BrdU+ cells in the ipsilateral
and contralateral cortices in groups C and D at 10, 20 and 30 days
post-TBI; (E) number of BrdU+/NeuN+ cells in
the ipsilateral and contralateral DG in groups C and D at 10, 20
and 30 days post-TBI; (F) number of
BrdU+/NeuN+ cells in the ipsilateral and
contralateral cortices in groups C and D at 10, 20 and 30 days
post-TBI; (G) number of BrdU+/GFAP+ cells in
the ipsilateral and contralateral DG in groups C and D at 10, 20
and 30 days post-TBI and (H) number of
BrdU+/GFAP+ cells in the ipsilateral and
contralateral cortices in groups C and D at 10, 20 and 30 days
post-TBI. Groups: A, rats without TBI; B, rats with TBI that
underwent no treatment; C, TBI treated with transplantation of
BMSCs; and D, TBI treated with transplantation of BMSCs and basic
fibroblast growth factor. DG, dentate gyrus; TBI, traumatic brain
injury; BrdU, bromodeoxyuridineBMSCs, bone mesenchymal stem
cells. |
The peak increase in the neuronal differentiation
rate in the DG occurred 20 days post-injury in groups C and D
(FC, 5.91; FD, 26.882; P<0.05; Fig. 3E). In the ipsilateral cortex, the
same results were observed for groups C and D (FC,
7.562; FD, 35.192; P<0.05; Fig. 3F).
A statistically significant improvement was observed
in the forelimb placing test scores (FC, 303.881;
FD, 456.813; P<0.001; Fig. 3A), as well as in the modified beam
balance test scores (FC, 111.825; FD,
281.817; P<0.001; Fig. 3B). The
recovery tempos of groups B and C were more rapid in the first 20
days (P<0.001), gradually decreasing for the remainder of the
study period, as evidenced in the forelimb placing (PB,
0.426; PC, 0.512; Fig.
3A) and modified beam balance test scores (PB,
0.623; PC, 0.474; Fig.
3B). At a different time point, group D constantly showed the
most significant improvement, which is inconsistent with the
neuronal differentiation rate, as demonstrated in the recovery of
the forelimb placing (F10, 224.626; F20,
67.216; F30, 93.925; P<0.001; Fig. 3A) and modified beam balance tests
(F10, 110.009; F20, 48.110; F30,
41.961; P<0.001; Fig. 3B).
Discussion
Currently available engrafted stem cells are
primarily classified into two types: Neural and non-neural stem
cells. As non-neural stem cells, BMSCs have an advantage over
neural and embryonic stem cells, which are more difficult to
prepare and culture and are limited in availability. Notably, the
utilization of BMSCs does not create ethical issues. In the current
study, the lower survival rate of the grafted cells is attributed
to the shortage of neurotrophic support due to the occurrence of
numerous newly engrafted cells and/or to the less conducive
environment for cell survival due to injury. However, the current
results indicate that following transplantation, cells survive
in vivo for at a minimum of one month even without bFGF
treatment.
bFGF is a mitogen for neuronal and non-neuronal stem
cells, showing multifunctional and pleiotropic activities.
Throughout the developmental stages and even in mature animals,
bFGF provides important extracellular signals for regulating
neurogenesis (14,15). Furthermore, the beneficial effect
of bFGF on functional recovery may be due to the increased
connectivity (16). These results
demonstrate that the survival, proliferation and differentiation of
cells within the CNS are crucially dependent on signals provided by
bFGF. In the present study, one of the essential contributions of
bFGF in BMSCs transplantation therapy for TBI is the enhancement of
the viability of transplanted cells. An increased viability of
transplanted stem cells is indispensable for tissue regeneration.
The therapeutic effect of BMSCs on the brain was evident in the
current experiments. Moreover, the therapeutic effect may be
further augmented with exogenous bFGF following TBI. Specifically,
an intraventricular injection of bFGF immediately following TBI
significantly increased the permeation of implanted cells into
damaged areas, that is, the subventricular zone and the DG.
Moreover, by determining the cell fate of these newly engrafted
cells, the growth factor treatment generated more neurons and
astrocytes than that identified in the group with injury alone or
that which was induced with a vehicle infusion.
Based on previous studies, an extremely low
percentage of differentiated neurons from transplanted cells were
observed following transplanting stem cells into TBI models
(17–19). Analogous results have been
demonstrated in the present study. The proportion of differentiated
neurons or astrocytes in this study was not significantly larger
than those of previous studies. The question of whether a majority
of BMSCs differentiate into neurons and astrocytes over time was
also addressed. The possibility that, as time elapsed, an increased
number of donor cells may present phenotypic markers of neurons and
astrocytes, exists. The present data indicated that the number of
donor cells expressing neuronal, astrocytic phenotypes was lesser
at later time points compared with earlier time points. Rats under
the effect of bFGF following TBI recovered most quickly. However,
the neuronal differentiation rate was not consistent with the
neurological functional recovery rate over time. The functional
improvement in the present experiments may have resulted from the
paracrine effect of the transplanted BMSCs or of bFGF itself,
rather than from the neuronal differentiation of the transplanted
stem cells. When the brain is damaged, reactions of neurotrophic
factors may lead to changes in the surrounding brain tissue
(20,21). Specific gene expression becomes
altered and a large number of potentially damaging and restorative
neurochemical factors are released and upregulated. Such factors
lead to delayed cellular dysfunction and death or tissue
regeneration following brain injuries, including TBI (22). These neurotrophic factors may be
the cause of the beneficial effect and may protect host neurons and
facilitate the host regeneration observed in other stem cells,
including neural stem cells (23,24).
Therefore, the beneficial effect of bFGF treatment on neural
function may not be due to its protective function on the
cyto-architecture, but rather due to other mechanisms. In the
current experiments, the exogenous injection that maintains bFGF
for an appropriate period enhanced bFGF efficacy for nerve
regeneration, as the endogenous bFGF content is low in normal
physical conditions, thus, following TBI, imbalance occurs.
Although the present results provide certain
reference values for the clinical utilization of BMSCs, further
studies on this preliminary research are required. Following TBI,
performing an immediate clinical administration is extremely
difficult due to the short window of opportunity. One-day
postinjury treatment, although clinically possible, may be
difficult due to a significant portion of patients with severe head
injury being transferred from smaller hospitals. For the majority
of patients, moving from the smaller hospitals to the central
hospitals would take more than a week. Hence, confirming the
efficacy of BMSCs when administered at more than one time point
following TBI, for example for one week or more, is essential.
Furthermore, the treatment dosage (BMSCs and bFGF) at various time
points following TBI also has a key function in affecting the
outcome of BMSC transplantation. Previous results showed that a
larger dose, specifically, 4×106 BMSCs, was required to
be efficacious compared with the 2×106 BMSC dose, which
was effective when administered one day following injury (17–19).
These observations are in agreement with those of other studies
concerning neural injury. Methylprednisolone treatment administered
within 3 h of spinal cord injury requires a shorter treatment
(smaller total dose) to be efficacious than when initiated 3 h
post-injury (25,26). An intervention early in the injury
phase requires less tissue repair than at later stages as damage
from a neural injury does not simply occur at the time of injury,
but is progressive and continues over time (27). To date, few studies regarding the
correlation between the dosage, time windows, intervention and
therapeutic effect of BMSCs have been conducted.
In conclusion, exogenous bFGF has become a booster
for BMSC transplantation-mediated functional recovery following
TBI, which has generated great interest in the neuroscience
community. The results of the present study add to the
understanding of the biological characteristics of cells with
neurotrophic factors, and augments their potential for clinical
utilization.
Acknowledgements
The authors would like to thank Ling Sun and Xiaoyan
Chen for their assistance in this study. This study was funded by
grants from the Foundation of Health Department Scientific Research
Project in Sichuan Province (grant nos. 2010-100301 and
2011-110547), Mianyang City Health Bureau (grant no. 201102) and
the Third Hospital of Mianyang (grant no. 2010–2012).
References
|
1
|
Li Y, Chen J, Chen XG, et al: Human marrow
stromal cell therapy for stroke in rat: neurotrophins and
functional recovery. Neurology. 59:514–523. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lu D, Li Y, Mahmood A, Wang L, Rafiq T and
Chopp M: Neural and marrow derived stromal cell sphere
transplantation in a rat model of traumatic brain injury. J
Neurosurg. 97:935–940. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu D, Li Y, Wang L, Chen J, Mahmood A and
Chopp M: Intraarterial administration of marrow stromal cells in a
rat model of traumatic brain injury. J Neurotrauma. 18:813–819.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanchez-Ramos J, Song S, Cardozo-Pelaez F,
et al: Adult bone marrow stromal cells differentiate into neural
cells in vitro. Exp Neurol. 164:247–256. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brundin P, Karlsson J, Emgård M, et al:
Improving the survival of grafted dopaminergic neurons: a review
over current approaches. Cell Transplant. 9:179–195.
2000.PubMed/NCBI
|
|
6
|
Wagner JP, Black IB and DiCicco-Bloom E:
Stimulation of neonatal and adult brain neurogenesis by
subcutaneous injection of basic fibroblast growth factor. J
Neurosci. 19:6006–6016. 1999.PubMed/NCBI
|
|
7
|
Ramirez JJ, Finklestein SP, Keller J,
Abrams W, George MN and Parakh T: Basic fibroblast growth factor
enhances axonal sprouting after cortical injury in rats.
Neuroreport. 10:1201–1204. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang H, Xia Y, Lu SQ, Soonq TW and Feng
ZW: Basic fibroblast growth factor-induced neuronal differentiation
of mouse bone marrow stromal cells requires FGFR-1, MAPK/ERK, and
transcription factor AP-1. J Biol Chem. 283:5287–5295. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caday CG, Klagsbrun M, Fanning PJ,
Mirzabegian A and Finklestein SP: Fibroblast growth factor (FGF)
levels in the developing rat brain. Brain Res Dev Brain Res.
52:241–246. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shetty AK, Hattiangady B and Shetty GA:
Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF
exhibit early decline during the course of aging in the
hippocampus: role of astrocytes. Glia. 51:173–186. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feeney DM, Boyeson MG, Linn RT, Murray HM
and Dail WG: Responses to cortical injury: I. Methodology and local
effects of contusions in the rat. Brain Res. 211:67–77. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawamata T, Dietrich WD, Schallert T, et
al: Intracisternal basic fibroblast growth factor enhances
functional recovery and up-regulates the expression of a molecular
marker of neuronal sprouting following focal cerebral infarction.
Proc Natl Acad Sci USA. 94:8179–8184. 1997. View Article : Google Scholar
|
|
14
|
Jin K, Sun Y, Xie L, et al: Neurogenesis
and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and
subventricular zone of aged mice. Aging Cell. 2:175–183. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Raballo R, Rhee J, Lyn-Cook R, Leckman JF,
Schwartz ML and Vaccarino FM: Basic fibroblast growth factor (Fgf2)
is necessary for cell proliferation and neurogenesis in the
developing cerebral cortex. J Neurosci. 20:5012–5023.
2000.PubMed/NCBI
|
|
16
|
Monfils MH, Driscoll I, Vavrek R, Kolb B
and Fouad K: FGF-2-induced functional improvement from neonatal
motor cortex injury via corticospinal projections. Exp Brain Res.
185:453–460. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu D, Mahmood A, Wang L, Li Y, Lu M and
Chopp M: Adult bone marrow stromal cells administered intravenously
to rats after traumatic brain injury migrate into brain and improve
neurological outcome. Neuroreport. 12:559–563. 2001. View Article : Google Scholar
|
|
18
|
Mahmood A, Lu D, Lu M and Chopp M:
Treatment of traumatic brain injury in adult rats with intravenous
administration of human bone marrow stromal cells. Neurosurgery.
53:697–703. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahmood A, Lu D, Wang L, Li Y, Lu M and
Chopp M: Treatment of traumatic brain injury in female rats with
intravenous administration of bone marrow stromal cells.
Neurosurgery. 49:1196–1204. 2001.PubMed/NCBI
|
|
20
|
Bondanelli M, Ambrosio MR, Margutti A, et
al: Evidence for integrity of the growth hormone/insulin-like
growth factor-1 axis in patients with severe head trauma during
rehabilitation. Metabolism. 51:1363–1369. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Winter CD, Pringle AK, Clough GF and
Church MK: Raised parenchymal interleukin-6 levels correlate with
improved outcome after traumatic brain injury. Brain. 127:315–320.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McIntosh TK, Saatman KE, Raghupathi R, et
al: The Dorothy Russel Memorial Lecture. The molecular and cellular
sequelae of experimental traumatic brain injury: pathogenetic
mechanisms. Neuropathol Appl Neurobiol. 24:251–267. 1998.
View Article : Google Scholar
|
|
23
|
McKay R: Stem cells in the central nervous
system. Science. 276:66–71. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ourednik J and Ourednik V: Graft-induced
plasticity in the mammalian host CNS. Cell Transplant. 13:307–318.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bracken MB, Shepard MJ, Holford TR, et al:
Administration of methylprednisolone for 24 to 48 hours or
tirilazad mesylate for 48 hours in the treatment of acute spinal
cord injury. Results of the third national acute spinal cord injury
randomized controlled trial. National Acute Spinal Cord Injury
Study. JAMA. 277:1597–1604. 1997.
|
|
26
|
Bracken MB, Shepard MJ, Holford TR, et al:
Methylprednisolone or tirilazad mesylate administration after acute
spinal cord injury: 1-year follow-up. Results of the third National
Acute Spinal Cord Injury randomized controlled trial. J Neurosurg.
89:699–706. 1998.
|
|
27
|
Liau LM, Bergsherder M and Becker DP:
Pathology and pathophysiology of head injury. Neurological Surgery.
Youmans JR: 4th edition. WB Saunders; Philadelphia: pp. 1549–1594.
1996
|