Introduction
Acrylonitrile (ACN), an industrial nitrile, is a
widely used intermediate in the manufacturing of acrylic fibers,
plastics, synthetic rubbers, adhesives and pesticides. Potential
human exposure to ACN may occur during its production and through
the use of ACN-containing products. In clinical applications, ACN
is used in the synthesis of biomaterials, including high permeable
dialysis tubing (1) and artificial
membranes to encapsulate Langerhans islet implants (2). Furthermore, ACN has been detected in
drinking water, cigarette smoke, food and occupational environments
(3–5). An increase in cancers, mainly of the
lung, gastrointestinal and hemopoietic-lymphatic system, in workers
occupationally exposed to ACN has been reported (6). The International Agency for Research
on Cancer (IARC) classified ACN as ‘possibly carcinogenic to
humans’ (7).
ACN is acutely toxic to humans. While the America
National Institute for Occupational Safety and Health recommended
permissible exposure level to ACN is relatively low (1 ppm),
high-level exposure may be reached via skin contact in the case of
accidental exposure (8). Studies
performed on animals indicated that ACN exhibits mutagenic,
embryotoxic (9), carcinogenic,
immunotoxic (10) and
hematotoxicity (11) effects. An
earlier study demonstrated that blood retains high levels of ACN
(12) and indicated a metabolic
incorporation and macromolecular interaction of ACN in the liver,
spleen, bone marrow, lung and adipose tissues of rats (13). A previous study also indicated that
ACN may interfere with their proliferative activity and with the
complex regulation pathways, by modulation of gene and protein
expression in hemopoietic cells (14).
N-acetyl-L-cysteine (NAC) is the acetylated
precursor of the amino acid L-cysteine and glutathione (GSH). The
biological activity of NAC is attributed to its protection against
oxidative and metabolic processes (15). NAC is a powerful nucleophile
capable of scavenging free radicals, stimulating GSH synthesis and
enhancing glutathione-S-transferase activity. It has been observed
that NAC may prevent ACN-induced damage in glial cells (16).
Stem cells have been observed to be significant in
predicting toxicity by working with in vitro systems
(17) and mesenchymal stem cells
(MSCs) have attracted more attention due to its multipotency to
differentiate into a variety of cell types of mesodermal lineage
(18,19). In addition, in clinical practice,
human umbilical cord MSCs (hUC-MSCs) may be harvested in a safer
and more non-invasive manner than bone marrow (BM) and have emerged
as a possible alternative cell source to BM-MSCs with less ethical
controversy than embryonic stem cells.
There is a lack of information with regard to the
potential toxicity of ACN in MSCs. Therefore, the objective of the
present study was to investigate the potential cytotoxic effects,
as well as the underlying mechanisms of toxicity, induced by ACN in
hUC-MSCs.
Materials and methods
Isolation of human umbilical cord
cells
Fresh human umbilical cords were obtained from the
Fourth Hospital of Zhenjiang and maintained in sterile conditions
at 4°C. The surface of each cord was rinsed with phosphate-buffered
saline (PBS) to remove as much blood as possible and the cord was
sliced into 3–5 cm-long sections using sharp, sterile scissors.
Blood vessels were removed from each piece of cord then the rest of
the tissue was sliced into small fragments ~1 mm3. The
fragments were seeded onto the surface of a culture dish (with a
diameter of 3 cm) with low glucose Dulbecco’s modified Eagle’s
medium (L-DMEM; Gibco-BRL, Carlsbad, CA, USA) supplemented with 10%
(v/v) fetal bovine serum (FBS), penicillin (100 U/l) and
streptomycin (100 μg/ml) at 37°C in 5% CO2-95% air
atmosphere for two weeks. Following two weeks incubation, the
explants were removed leaving the cells that had adhered to the
plate. When cells grew to 70% confluency, they were harvested and
plated onto a 25-cm2 culture flask. The experimental
procedure was approved by Jiangsu University Ethics Committee
(Zhenjiang, China) and written informed consent was obtained from
the patients.
Cell proliferation and survival
assay
The viability and proliferation of cells were
determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay. Briefly, the cells were plated in 96-well
plates and once cells reached 70–80% confluency, the medium was
removed and incubated with various concentrations of ACN-dissolved
serum-free culture medium for 12 and 24 h, respectively. MTT (20
μl) was added to each well in the final 4 h. The supernatant was
discarded and 150 μl dimethyl sulfoxide (DMSO) was added to each
well. Following uniform oscillation for 10 min to fully dissolve
the crystals, the absorption values were examined using a
microplate reader (Biotek, Winooski, VT, USA) with a wavelength of
570 nm.
The hUC-MSCs were plated in 96-well plates and once
70–80% cell confluency was reached, the medium was removed with
specific concentrations of NAC serum-free culture medium. After 30
min, 0.1 μg/ml ACN was added to each well. Following 24 h
incubation, the viability of the cultured hUM-MSCs was determined
by MTT.
Osteogenic differentiation
The multipotent differentiation of the hUC-MSCs was
analyzed for osteogenic ability. The cells were inoculated into
24-well plate at 3,000 cells/well in DMEM supplemented with 10% FBS
and incubated in a modified version of differentiation medium
(containing 4 mg/l basic fibroblast growth factor). The cells were
treated for two weeks with osteogenic induction medium (0.1 μM
dexamethasone, 10 mM-glycerophosphate, 4 mg/l basic fibroblast
growth factor and 50 μg/ml ascorbic acid).
Quantitative polymerase chain reaction
(qPCR) assay
Cell lysis and total RNA extraction from control and
treated cells treated with ACN using TRIzol Reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA) was performed according to
the reverse transcription kit instructions (Fermentas, Waltham, MA,
USA). The cDNA samples were subjected to PCR using specific
primers. The primers were designed and synthesized by Invitrogen
Life Technologies according to the serial number from GenBank
(Table I). The reaction was
started at 94°C for 5 min, denaturation at 94°C for 30 sec,
annealing at 55–70°C for 30 sec and extension for 30 sec at 72°C
followed by 30 cycles and a final polymerization at 72°C for 10
min. β-actin mRNA was used as an internal control. The products
were checked by electrophoresis on a 1.5% agarose gel, with
ethidium bromide staining and analyzed using the Gel Image Analysis
System (Syngene, Cambridge, UK).
 | Table ISpecific primers for control and
target genes. |
Table I
Specific primers for control and
target genes.
| Gene | Primers sequence, 5′
to 3′ | Size (bp) | Annealing,°C |
|---|
| Flt3-ligand-F |
CTGGAGCCCAACAACCTATC | 353 | 60.0 |
| Flt3-ligand-R |
TCTGGACGAAGCGAAGACA | | |
| SCF-F |
TGGATAAGCGAGATGGTA | 189 | 54.0 |
| SCF-R |
TTCTGGGCTCTTGAATGA | | |
| VEGF-F |
CCTTGCTCTACCTCCAC | 280 | 61.0 |
| VEGF-R |
ATCTGCATCCTGTTGGA | | |
| ALP-F |
AGCTTCAAACCGAGATACAA | 220 | 56.5 |
| ALP-R |
ATTCTGCCTCCTTCCACC | | |
| β-actin-F |
CACGAAACTACCTTCAACTC | 256 | 56.0 |
| β-actin-R |
CATACTCCTGCTTGCTGATC | | |
Cytochemical staining
Following hUC-MSC differentiation, osteogenic
characteristics were confirmed through alkaline phosphatase (ALP)
expression by cytochemical staining. Cells were fixed with 4%
paraformaldehyde. The ALP staining kit (Sun Biotech Co., Ltd.,
Shanghai, China) was used according to the manufacturer’s
instructions.
Cell cycle assay
hUC-MSCs were treated with ACN and NAC for 24 h.
Cells were harvested and washed twice with PBS and stained with
propidium iodide (PI; Sigma-Aldrich, St. Louis, MO, USA) for 30 min
in dark conditions. The stained cells were analyzed by flow
cytometry (FACSCalibur; BD Biosciences, San Diego, CA, USA).
Apoptosis assay
hUC-MSCs were treated with ACN and NAC for 24 h.
Following treatment, the cells were trypsinized with 0.25%
trypsin-EDTA, washed twice with PBS and stained according to the
recommendation of the manufacturer with propidium iodide (PI) and
Annexin V-fluorescein isothiocyanate (FITC). The stained cells were
analyzed by flow cytometry (FACSCalibur).
Results
Cell morphology and immunological
phenotype
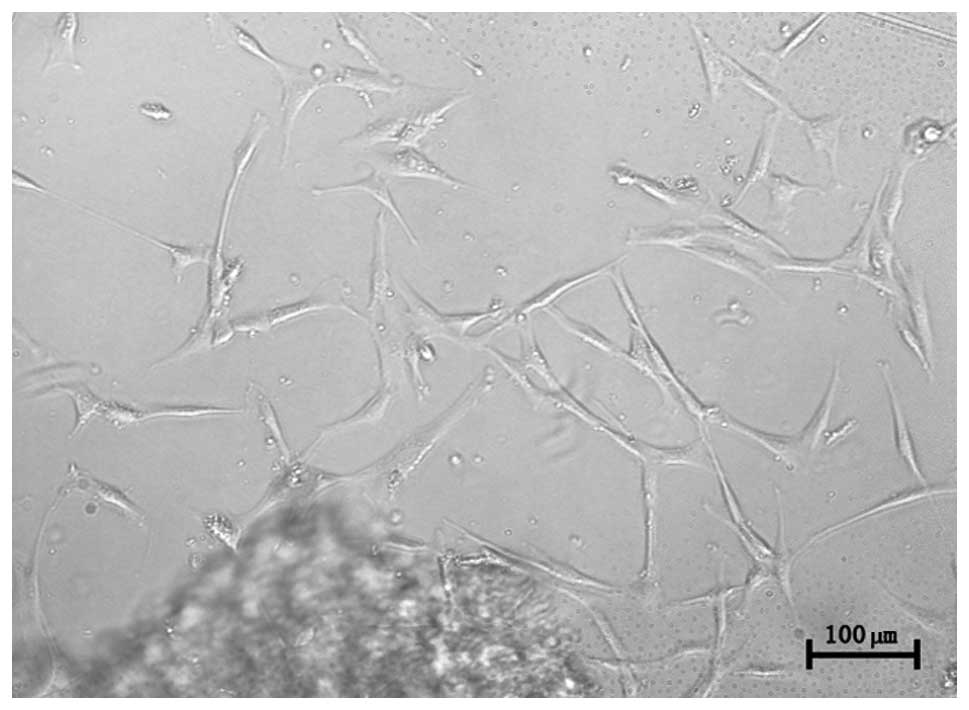
hUC-MSCs were observed following an initial three
days of primary culture. The cells adhered to plastic surfaces and
presented as a small population of single cells with spindle shape.
After 7–10 days, the cells appeared as long spindle-shaped
fibroblastic cells (Fig. 1).
Following re-plating, the fibroblast-like cells appeared polygonal
or spindly, with a long process and were considered normal, on the
basis of typical morphology.
Effect of ACN on cell proliferation
To investigate the cytotoxicity of ACN on hUC-MSC
proliferation, cells were exposed to specific concentrations of ACN
(0, 0.05, 0.1, 0.2,1, 10, 20 and 50 μg/ml) for 12 and 24 h. The MTT
assay was used to determine ACN-induced toxicity in hUC-MSC.
Following ACN treatment, the activation of hUC-MSC was markedly
decreased in a dose- and time-dependent manner (Fig. 2), indicating that ACN is cytotoxic
in hUC-MSC.
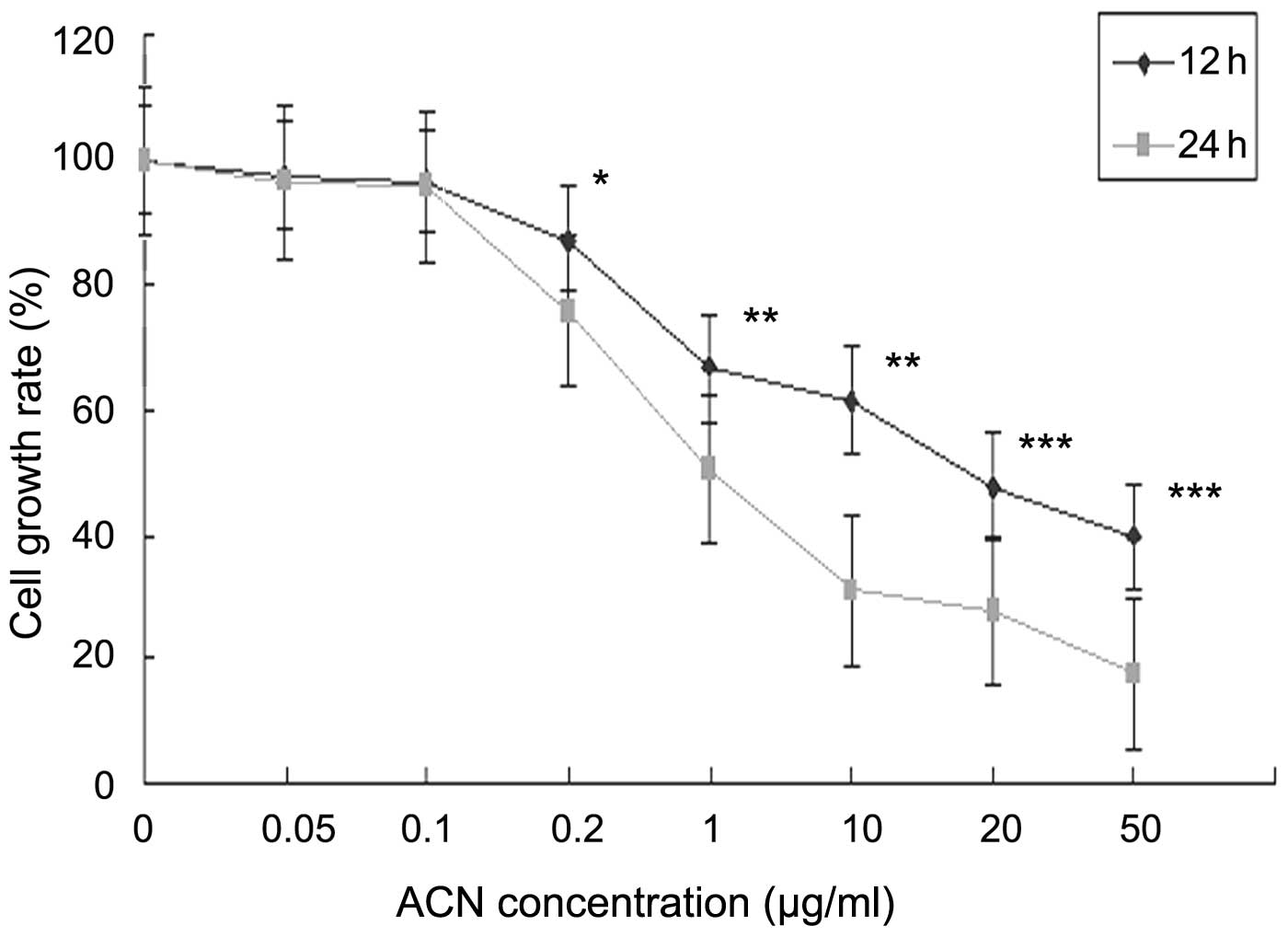 | Figure 2Effects of cell viability of ACN on
hUC-MSCs. Cells were treated with 0.05, 0.1, 0.2, 1, 10, 20 and 50
μg/ml ACN for 12 or 24 h and cell viability was determined by an
MTT assay. *P<0.05, **P<0.01 and
***P<0.001, vs. the control. hUC-MSCs, human
umbilical cord mesenchymal stem cells; ACN, acrylonitrile; MTT,
3-(4,5-dimethylthiazol-2-yl-)-2,5-diphenyl tetrazolium bromide. |
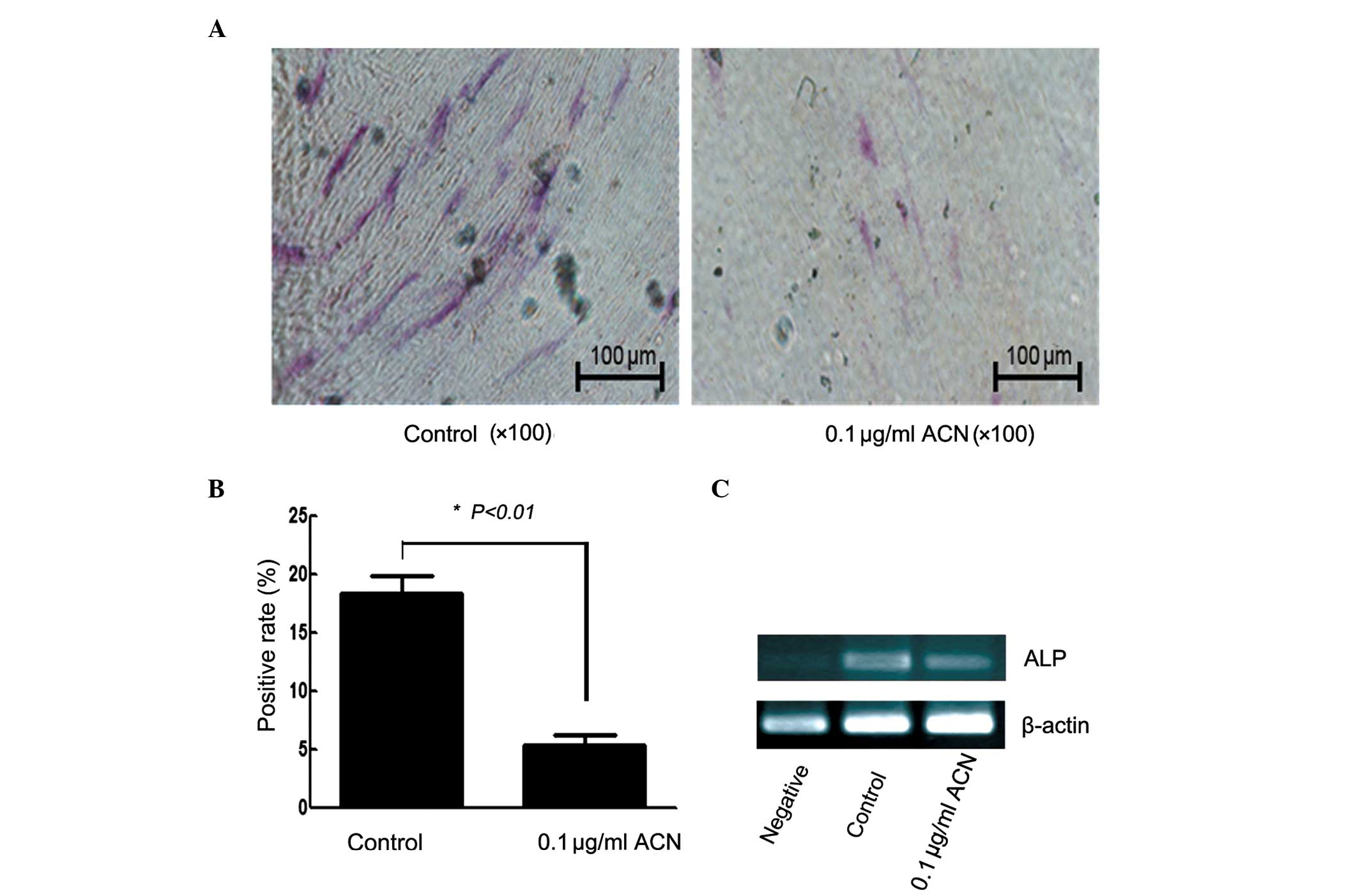
Effect of ACN on osteogenic
differentiation
To examine the potential effect of ACN on hUC-MSC to
differentiate into osteocytes, the cells were cultured in
osteogenic medium. Following 14 days, ALP stain and qPCR was
applied for characterization of the osteogenic differentiation.
Compared with the control group, the positive rate of ALP was
reduced in the ACN group (Fig. 3A and
B; P<0.01). ACN induced a statistically significant
difference in the expression of the ALP gene (Fig. 3C), which is considered to be a
marker for osteocytes. These results indicated that ACN inhibits
the osteogenic differentiation of MSCs.
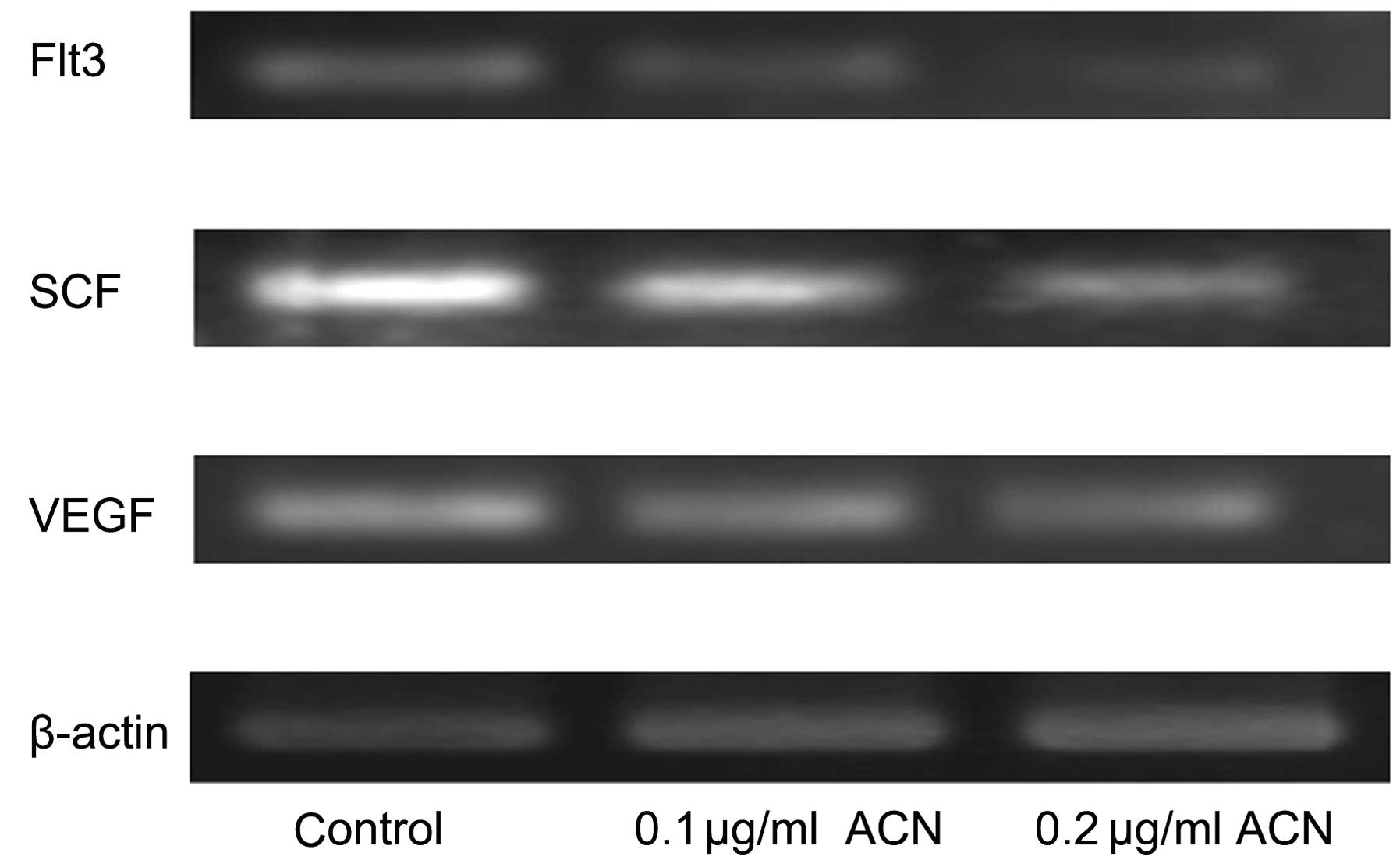
Effect of ACN on specific genes of
hematogenesis
qPCR was applied for characterization of expression
of cytokines in hUC-MSCs, which are important components of the
hematopoietic microenvironment, in addition to MSCs. There were
dose-dependent decreases in the expression of cytokine vascular
endothelial growth factor (VEGF), stem cell factor (SCF) and
Fms-like tyrosine kinase 3 (Flt3) showed with treatment of ACN
(Fig. 4). These results indicated
that ACN may injure the hematopoietic system by inhibiting the
hematopoiesis-supportive function of MSCs.
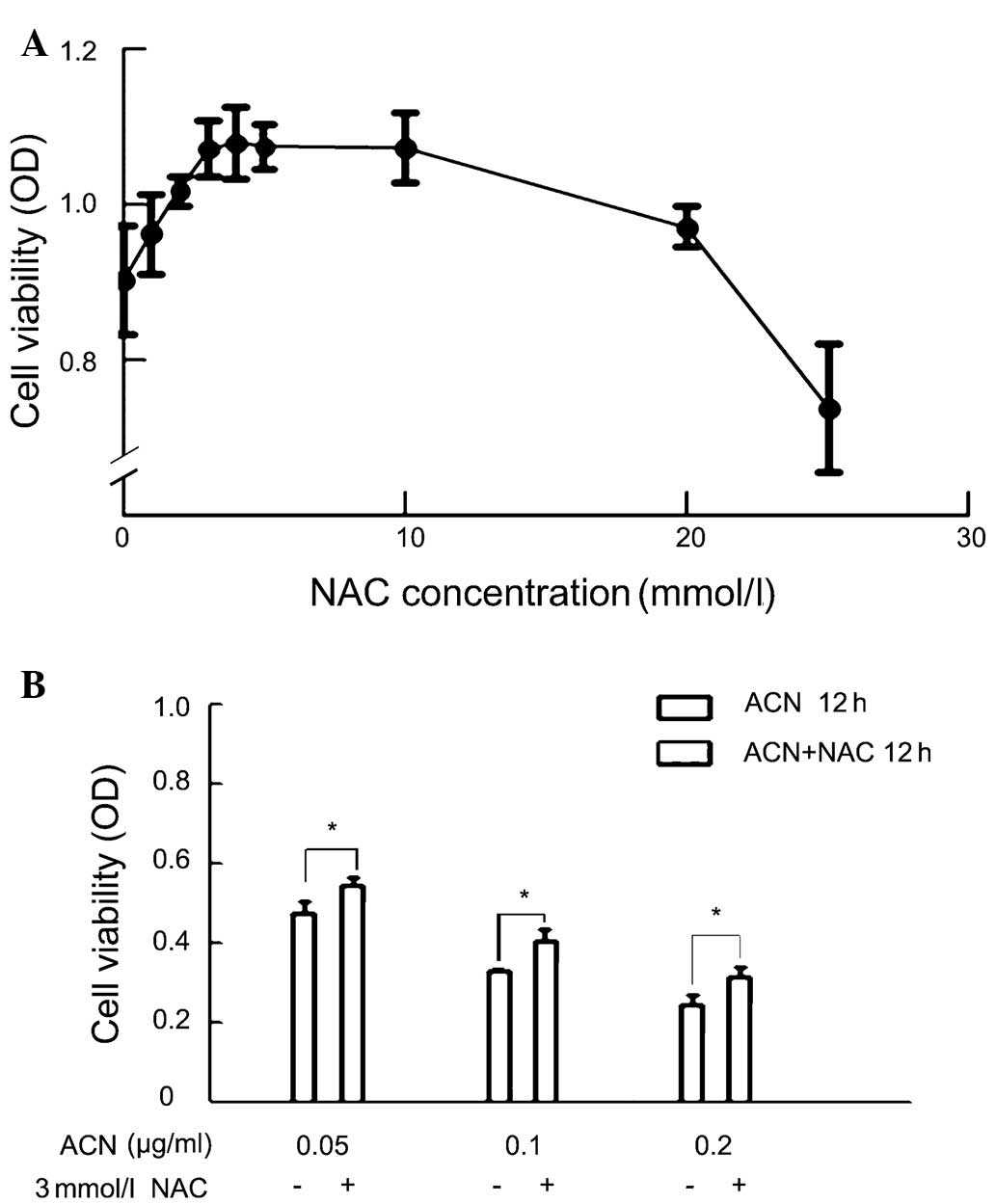
NAC attenuates ACN-induced
cytotoxicity
Since ACN is capable of inducing oxidative stress,
it was investigated whether ACN was capable of inducing oxidative
stress in MSCs. Cells were pretreated with an antioxidant, NAC, at
different concentrations followed by ACN treatment and cytoactivity
was determined. The MTT assay showed that treatment with NAC
resulted in a dose-dependent increase in cytoactivity at specific
concentrations. The optimal effect was observed at 3 mM, at which
point a further increase in NAC did not show any additional benefit
(Fig. 5A). Thus, 3 mM was selected
as the concentration of NAC for further studies.
The protection of NAC on ACN-treated cells was
further investigated. They all showed significant differences in
ACN-treated only groups of concentration 0.05, 0.1, and 0.2 μg/ml,
as compared with the corresponding ACN+NAC groups (Fig 5).
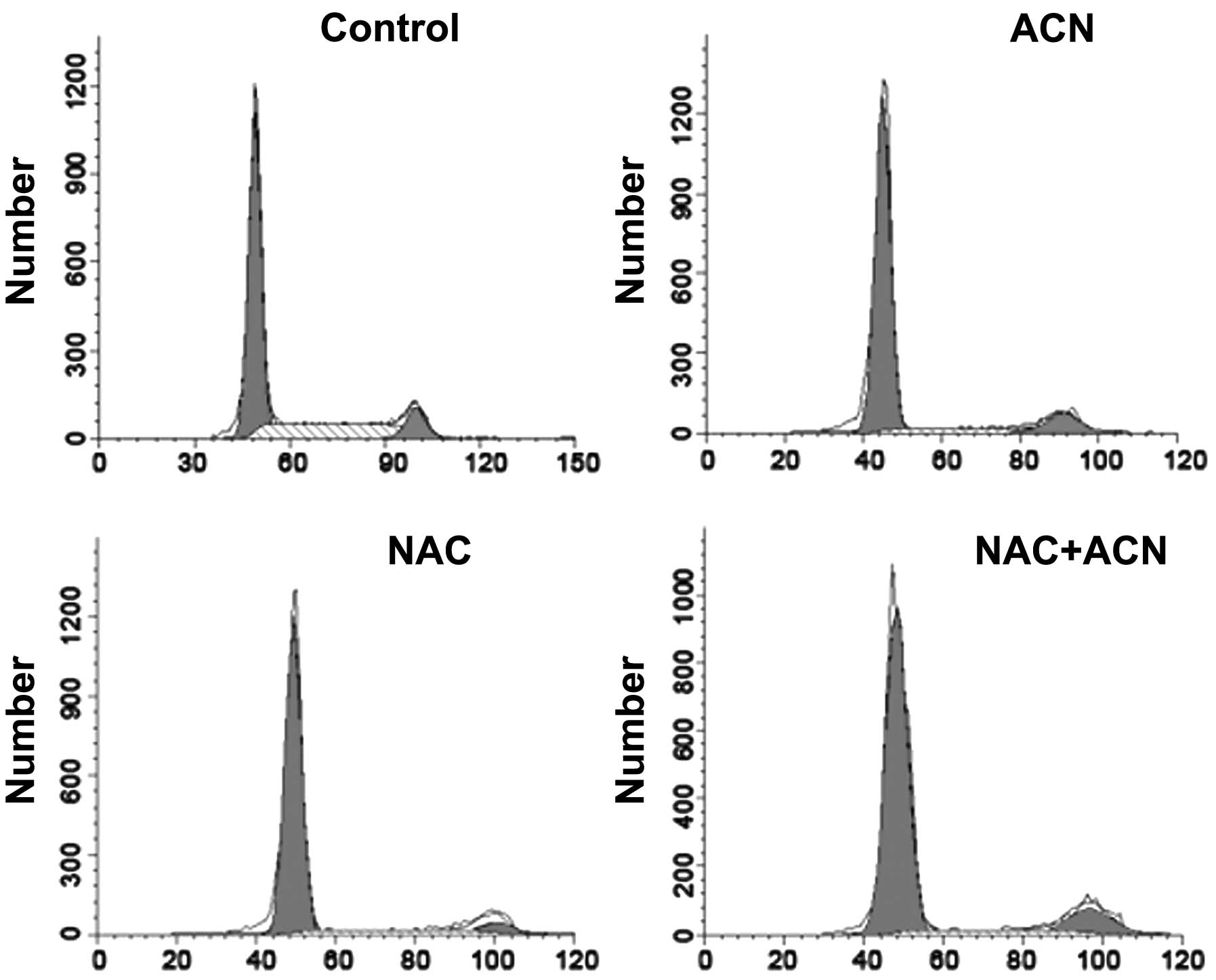
Effect of ACN on cell cycle
Flow cytometry was used to determine whether the
inhibitory effect of ACN on MSC proliferation was mediated, at
least in part, by affecting cell cycle progression. The results
demonstrated that pretreatment with NAC attenuated ACN-induced cell
cycle arrest at the G2/M phase and suggested that ACN suppresses
cell proliferation by controlling the G2/M checkpoint and inducing
a specific block in cell cycle progression (Fig. 6).
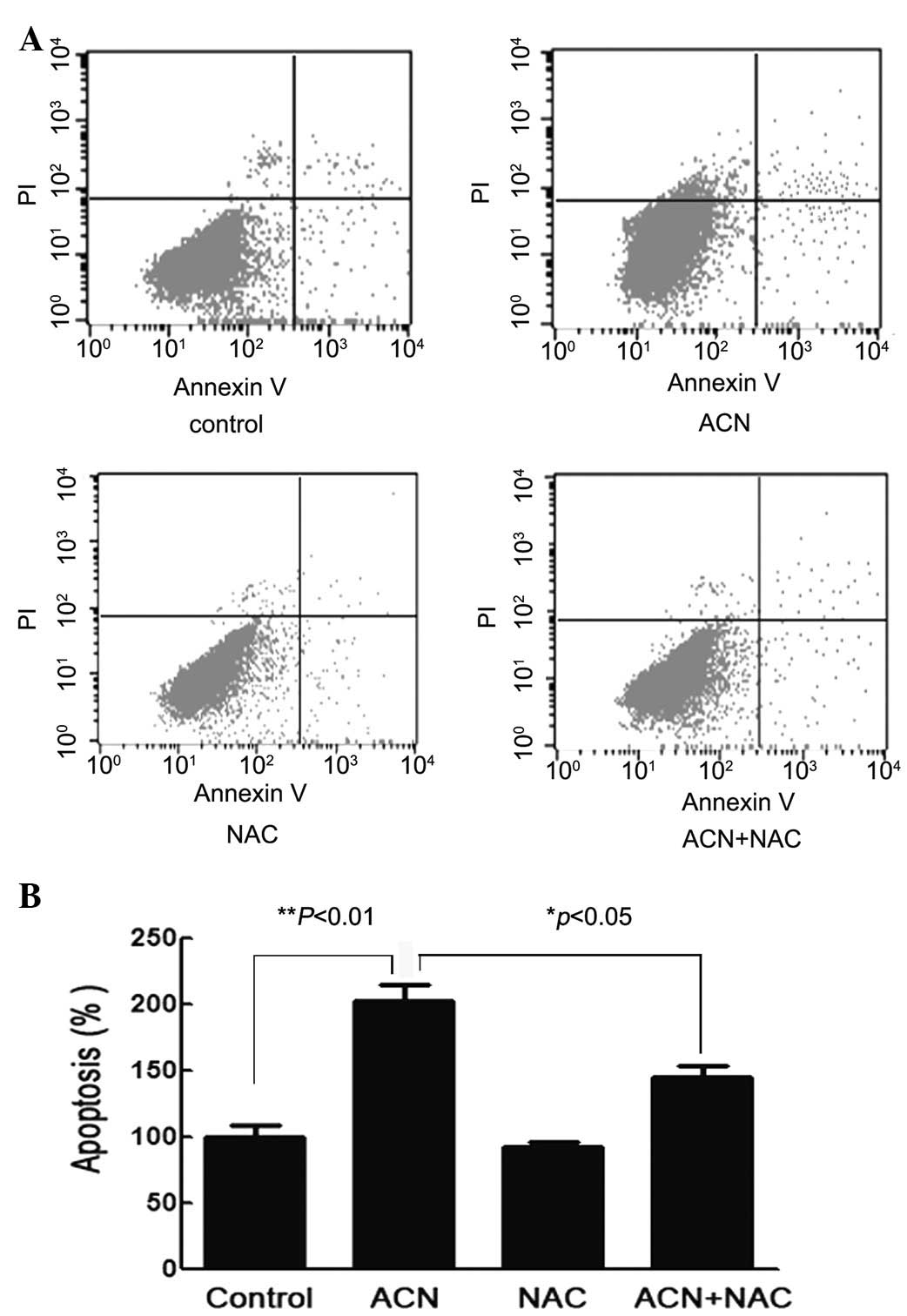
ACN induces cellular apoptosis
To further study the effect of ACN on hUC-MSC
apoptosis, cells were stained with Annexin V/FITC and PI and
subsequently analyzed by flow cytometry. Flow cytometric analysis
showed that the percentage of hUC-MSCs undergoing apoptosis
following ACN-treatment were significantly higher compared with
that of the control cells (P<0.01) and the NAC pretreatment
group (P<0.05), thus implying that ACN may induce the apoptosis
of hUC-MSCs and may be counteracted with the use of NAC (Fig. 7).
Discussion
There is evidence that the metabolism of ACN to
epoxide intermediate 2-cyanoethylene oxide, to form adducts with
DNA, may contribute to the toxicity and carcinogenicity of ACN.
BM is the site for hematopoiesis to occur, as well
as in the cord blood, where there are MSCs that are capable of
differentiating into multiple cell types, including adipocytes,
chondrocytes, osteocytes and cardiomyocytes (20). The present study provides evidence
that ACN suppresses cytoactivity, differentiation and causes
apoptosis in hUM-MSCs. Doses for the current study were selected on
the basis of the results of a 24 h in vitro study. ACN (0.1
μg/ml) was observed to affect the proliferation and morphology of
MSCs.
The present study demonstrated that ACN disturbed
the balance of cell proliferation and induced cell apoptosis, as
well as the potential for differentiation. Following osteogenic
induction, qPCR analysis of the gene expression of an early marker
of cells oriented towards osteogenic production demonstrated
downregulation of ALP (21)
following ACN treatment, which is an intracellular enzyme required
for mineralization. Histological staining supported the qPCR data
by demonstrating the presence of ALP positive cells. Therefore, in
the present study, ACN exhibited down-regulation of the osteogenic
capacity of MSCs.
MSCs produce a number of cytokines and extracellular
matrix proteins and express cell adhesion molecules, which are
critical for hematopoiesis (22),
thus, the ACN effect on the hematopoiesis by MSCs was investigated.
In the present study, qPCR experiments determined mRNA marker
expression for a number of the hematopoietic cytokines. VEGF, Flt3
and SCF were downregulated following exposure to ACN. These results
indicate that ACN may destroy the hematopoietic microenvironment.
It has been reported that ACN was extremely reactive with rat
tissue proteins in vivo(23). Blood was the most reactive tissue
studied and hemoglobin was the most reactive protein in blood. MSCs
exhibit multilineage differentiation potential and are capable of
generating progenitors with restricted developmental potential
(24).
In addition, ACN inhibited cell proliferation with a
smaller increase in apoptosis. A previous study demonstrated that
ACN induces apoptosis (25) and
further identified that the mechanism of ACN-induced cell death in
hUC-MSCs is through induction of apoptosis involved in the
generation of oxidative stress. ACN was observed to cause apoptosis
in hUC-MSCs, as was observed by flow cytometry. A number of studies
have been published stating that the antioxidant, NAC, protects the
rat neurocyte from ACN toxicity, suggesting the involvement of
oxidative stress in ACN toxicity. Using NAC as a potential
protective agent may induce the apoptosis of hUC-MSCs. Oxidative
stress is important in the initiation of apoptosis.
Despite a considerable amount of research, the
mechanism directly responsible for ACN toxicity remains unclear.
Three distinct pathways have been proposed: i) Reactive oxygen
species (ROS) generated as by-products of ACN metabolism via
cytochrome P450 2E1 oxidation (26). ii) Liberation of cyanide from ACN
metabolism. Cyanide is a potent generator of ROS production as well
as an inhibitor of the activities of several antioxidant enzymes
(27,28). iii) The process of ACN conjugation
with GSH, results in a rapid depletion of GSH and an overall
decrease in cellular antioxidant contents. A number of studies have
indicated the role of stress in the toxicity of ACN. Esmat et
al(29) reported that NAC
protects glial cells from ACN toxicity, suggesting the attribution
of oxidative stress in ACN toxicity.
In conclusion, ACN may inhibit the proliferation,
differentiation and cytokine gene expression of hUC-MSCs and the
present study supports the hypothesis that ACN causes apoptosis in
hUC-MSCs through a mechanism involving the generation of oxidative
stress. We, therefore, require further investigation of the
antagonism of antioxidant in these ACN effects.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 30840053) and the
Foundation of the Jiangsu University for Senior talented Scholars
(grant no. 11JDG0089).
References
|
1
|
Ward RA, Schaefer RM, Falkenhagen D, et
al: Biocompatibility of a new high-permeability modified cellulose
membrane for haemodialysis. Nephrol Dial Transplant. 8:47–53.
1993.PubMed/NCBI
|
|
2
|
Kessler L, Pinget M, Aprahamian M,
Dejardin P and Damgé C: In vitro and in vivo studies of the
properties of an artificial membrane for pancreatic islet
encapsulation. Horm Metab Res. 23:312–317. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Léonard A, Gerber GB, Stecca C, et al:
Mutagenicity, carcinogenicity, and teratogenicity of acrylonitrile.
Mutat Res. 436:263–283. 1999.
|
|
4
|
Miller SL, Branoff S and Nazaroff WW:
Exposure to toxic air contaminants in environmental tobacco smoke:
an assessment for California based on personal monitoring data. J
Expo Anal Environ Epidemiol. 8:287–311. 1998.PubMed/NCBI
|
|
5
|
Rubio PA: Use of adhesive tape for primary
closure of surgical skin wounds. Int Surg. 75:189–190.
1990.PubMed/NCBI
|
|
6
|
Ward CE and Starr TB: Comparison of cancer
risks projected from animal bioassays to epidemiologic studies of
acrylonitrile-exposed workers. Regul Toxicol Pharmacol. 18:214–232.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fechter LD, Klis SF, Shirwany NA, et al:
Acrylonitrile produces transient cochlear function loss and
potentiates permanent noise-induced hearing loss. Toxicol Sci.
75:117–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pouyatos B, Gearhart CA, Nelson-Miller A,
et al: vulnerability of the cochlear Basal turn to acrylonitrile
and noise. J Toxicol. 2009:9085962009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parent RA and Casto BC: Effect of
acrylonitrile on primary Syrian golden hamster embryo cells in
culture: transformation and DNA fragmentation. J Natl Cancer Inst.
62:1025–1029. 1979.PubMed/NCBI
|
|
10
|
Hamdy NM, Al-Abbasi FA, Alghamdi HA, Tolba
MF, Esmat A and Abdel-Naim AB: Role of neutrophils in
acrylonitrile-induced gastric mucosal damage. Toxicol Lett.
208:108–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghanayem BI, Elwell MR and Eldridge SR:
Effects of the carcinogen, acrylonitrile, on forestomach cell
proliferation and apoptosis in the rat: comparison with
methacrylonitrile. Carcinogenesis. 18:675–680. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahmed AE, Farooqui MY, Upreti RK and
El-Shabrawy O: Comparative toxicokinetics of 2,3-14C- and
1-14C-acrylonitrile in the rat. J Appl Toxicol. 3:39–47. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jacob S and Ahmed AE: Effect of route of
administration on the disposition of acrylonitrile: quantitative
whole-body autoradiographic study in rats. Pharmacol Res.
48:479–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Diodovich C, Malerba I, Ferrario D, et al:
Gene and protein expressions in human cord blood cells after
exposure to acrylonitrile. J Biochem Mol Toxicol. 19:204–212. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sjödin K, Nilsson E, Hallberg A and Tunek
A: Metabolism of N-acetyl-L-cysteine. Some structural requirements
for the deacetylation and consequences for the oral
bioavailability. Biochem Pharmacol. 38:3981–3985. 1989.PubMed/NCBI
|
|
16
|
Carrera MP, Antolín I, Martin V, et al:
Antioxidants do not prevent acrylonitrile-induced toxicity. Toxicol
Lett. 169:236–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chapin RE and Stedman DB: Endless
possibilities: stem cells and the vision for toxicology testing in
the 21st century. Toxicol Sci. 112:17–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagaya N, Fujii T, Iwase T, et al:
Intravenous administration of mesenchymal stem cells improves
cardiac function in rats with acute myocardial infarction through
angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol.
287:H2670–H2676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prockop DJ: Marrow stromal cells as stem
cells for nonhematopoietic tissues. Science. 276:71–74. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orbay H, Tobita M and Mizuno H:
Mesenchymal stem cells isolated from adipose and other tissues:
basic biological properties and clinical applications. Stem Cells
Int. 2012:4617182012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugawara Y, Suzuki K, Koshikawa M, Ando M
and Iida J: Necessity of enzymatic activity of alkaline phosphatase
for mineralization of osteoblastic cells. Jpn J Pharmacol.
88:262–269. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weissman IL: Stem cells: units of
development, units of regeneration, and units in evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Campian EC and Benz FW: The acute
lethality of acrylonitrile is not due to brain metabolic arrest.
Toxicology. 253:104–109. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fibbe WE: Mesenchymal stem cells. A
potential source for skeletal repair. Ann Rheum Dis. 61(Suppl 2):
ii29–ii31. 2002.PubMed/NCBI
|
|
25
|
Watcharasit P, Suntararuks S,
Visitnonthachai D, Thiantanawat A and Satayavivad J: Acrylonitrile
induced apoptosis via oxidative stress in neuroblastoma SH-SY5Y
cell. J Appl Toxicol. 30:649–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Chanas B and Ghanayem BI:
Cytochrome P450 2E1 (CYP2E1) is essential for acrylonitrile
metabolism to cyanide: comparative studies using CYP2E1-null and
wild-type mice. Drug Metab Dispos. 30:911–917. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gunasekar PG, Sun PW, Kanthasamy AG,
Borowitz JL and Isom GE: Cyanide-induced neurotoxicity involves
nitric oxide and reactive oxygen species generation after
N-methyl-D-aspartate receptor activation. J Pharmacol Exp Ther.
277:150–155. 1996.
|
|
28
|
Li L, Prabhakaran K, Shou Y, Borowitz JL
and Isom GE: Oxidative stress and cyclooxygenase-2 induction
mediate cyanide-induced apoptosis of cortical cells. Toxicol Appl
Pharmacol. 185:55–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esmat A, El-Demerdash E, El-Mesallamy H
and Abdel-Naim AB: Toxicity and oxidative stress of acrylonitrile
in rat primary glial cells: preventive effects of N-acetylcysteine.
Toxicol Lett. 171:111–118. 2007. View Article : Google Scholar : PubMed/NCBI
|