Introduction
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide),
a vanilloid receptor agonist, is the principal pungent constituent
of the plant genus Capsicum that is present in chilli peppers
(1). The relationship of capsaicin
to genotoxicity, carcinogenicity, and prevention and treatment of
cancer has been widely explored (2). This alkaloid compound has been found
to inhibit cancer growth and progression in vitro and in
vivo and to induce apoptosis in various types of cancer cells
(3–8). However, several investigations of the
carcinogenic potential of capsaicin in animal models have also been
reported (2,8,9). The
molecular mechanisms underlying capsaicin-induced apoptosis are
cell-type dependent. Despite the cumulative evidence for the tumor
suppressive effects of capsaicin, few studies have been undertaken
concerning the effects of capsaicin on cell signaling and molecular
pathways leading to apoptosis in gastric cancer (10–12).
In the present study, we investigated the effects of
capsaicin on human gastric cancer cells and demonstrated that
capsaicin induced apoptosis and autophagy in human gastric cancer
cells.
Materials and methods
Cell cultures and culture condition
Human gastric carcinoma AGS cells were purchased
from the American Type Culture collection (Manassas, VA, USA). The
cells were cultured at 37°C in a 5% CO2 atmosphere with
Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
bovine serum and 1% penicillin-streptomycin. Capsaicin was
purchased from Sigma Chemical Co. (St. Louis, MO, USA) and prepared
as an ethanol stock solution, which was then diluted in
intracellular solution prior to use.
Measurement of cell proliferation
Cell viability was determined using the
3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide
assay. Briefly, 5×105 cells/well were seeded in 96-well
plates and allowed to adhere overnight. The concentrations of
capsaicin used in this experiment were 0, 10, 20, 30, 60, 80, 100,
200 and 300 μM. Each group contained three wells. After the samples
were treated with capsaicin for 12 h, 10 μl of MTT (0.5 mg/ml;
Sigma) was added to each well, and the cells were incubated at 37°C
for 4 h. The reaction was then stopped by lysing the cells with 200
μl of dimethyl sulfoxide (DMSO) for 15 min. Quantification
measurements (optical density) were obtained at a wavelength of 450
nm using spectrophotometric analysis.
Effect of capsaicin on apoptosis of AGS
cells as determined by flow cytometry
To evaluate the impact of capsaicin on apoptosis,
FACS analysis was performed. Cell apoptosis was assessed using the
Annexin V assay (Annexin V-FITC apoptosis detection kit; BD
Pharmingen, San Jose, CA, USA). AGS cells (1×105 cells)
were treated with various concentrations (0–300 μM) of capsaicin
for 12 h, respectively. Annexin V-FITC and propidium iodide (PI)
labeling was then performed according to the manufacturer’s
instructions. The cells were double-stained with Annexin
V-fluorescein isothiocyanate (FITC) and PI, followed by flow
cytometry. To quantify early apoptotic cells, flow cytometry was
used to assess binding of fluorescence-labeled Annexin V to
externalized phosphatidylserine. PI uptake was measured to assess
cells in the late stages of apoptosis or cells that sustained
direct plasma membrane damage.
Western blotting
Total protein was lysed with RIFA buffer (1 M
Tris-HCl, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA) with 1 mM
phenylmethanesulfonyl fluoride (PMSF) and Halt™ protease inhibitor
cocktail (Thermo Scientific, Rockford, IL, USA). Protein
concentrations of the cell lysate were quantified using BCA™
protein assay (Thermo Scientific) with bovine serum albumin (BSA)
as a standard. Total protein (10–20 μg) was subjected to
electrophoresis on 10% SDS-polyacrylamide gels and then transferred
to a polyvinylidene fluoride membrane (Millipore, Billerica, MA,
USA). The membrane was incubated for 1 h in blocking solution (5%
BSA in TBS-Tween 20 buffer) and sequentially blotted with primary
antibodies at 4°C overnight. Antibodies against c-Jun N-terminal
kinase (JNK), phospho-JNK, extracellular signal-regulated kinase
1/2 (ERK 1/2), phospho-ERK 1/2 (1:1,000 dilution; Cell Signaling
Technology, Inc., Danvers, MA, USA), p38 mitogen-activated protein
kinase (MAPK), phospho-p38 MAPK, caspase 3, cleaved caspase 3,
Bcl-2, and β-actin were purchased from Cell Signaling Technology
(Beverly, MA, USA). After rinsing in TST, the membrane was
incubated with horseradish peroxidase (HRP)-labeled anti-rabbit or
anti-mouse immunoglobulins as the secondary antibody (1:5,000
dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, USA) at room
temperature for 1 h. Enhanced chemiluminescence was used to
visualize the bands by LAS 3000 (Fuji Film, Tokyo, Japan).
Statistical analysis
The Student’s t-test was used to determine the
significance of differences between treatments and respective
controls. Values are expressed as means ± SE from at least three
independent experiments. P<0.05 was considered to indicate
statistical significance. Statistical analysis was assessed using
Statistical Package for the Social Sciences (SPSS/PC 20.0, Chicago,
IL, USA).
Results
Capsaicin suppresses proliferation in
human gastric cancer cells
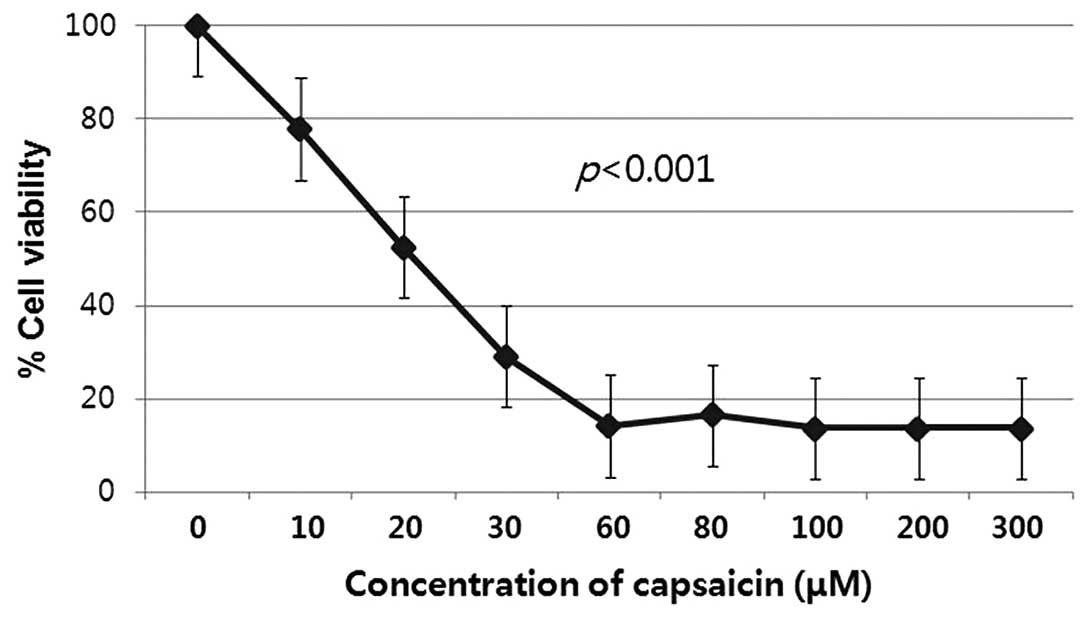
An MTT assay was used to assess the potential
effects of capsaicin on the proliferation of human gastric cancer
cells that had been exposed to capsaicin for 12 h at different
concentrations. After treatment with capsaicin at concentrations
ranging from 10 to 300 μM for 12 h, the cell proliferation was
significantly lower than that of the untreated corresponding
control groups (P<0.001). Capsaicin decreased the proliferation
of the gastric cancer cell line in vitro in a dose-dependent
manner (Fig. 1).
Capsaicin induces apoptosis in human
gastric cancer cells
Regulation of apoptosis is crucial for the
preservation of homeostasis and morphogenesis of human tissue
(3). Disturbance of these
processes by aberrantly extending cell viability or favoring
accumulation of transforming mutation is thought to contribute to
carcinogenesis (3,4). To evaluate the impact of capsaicin on
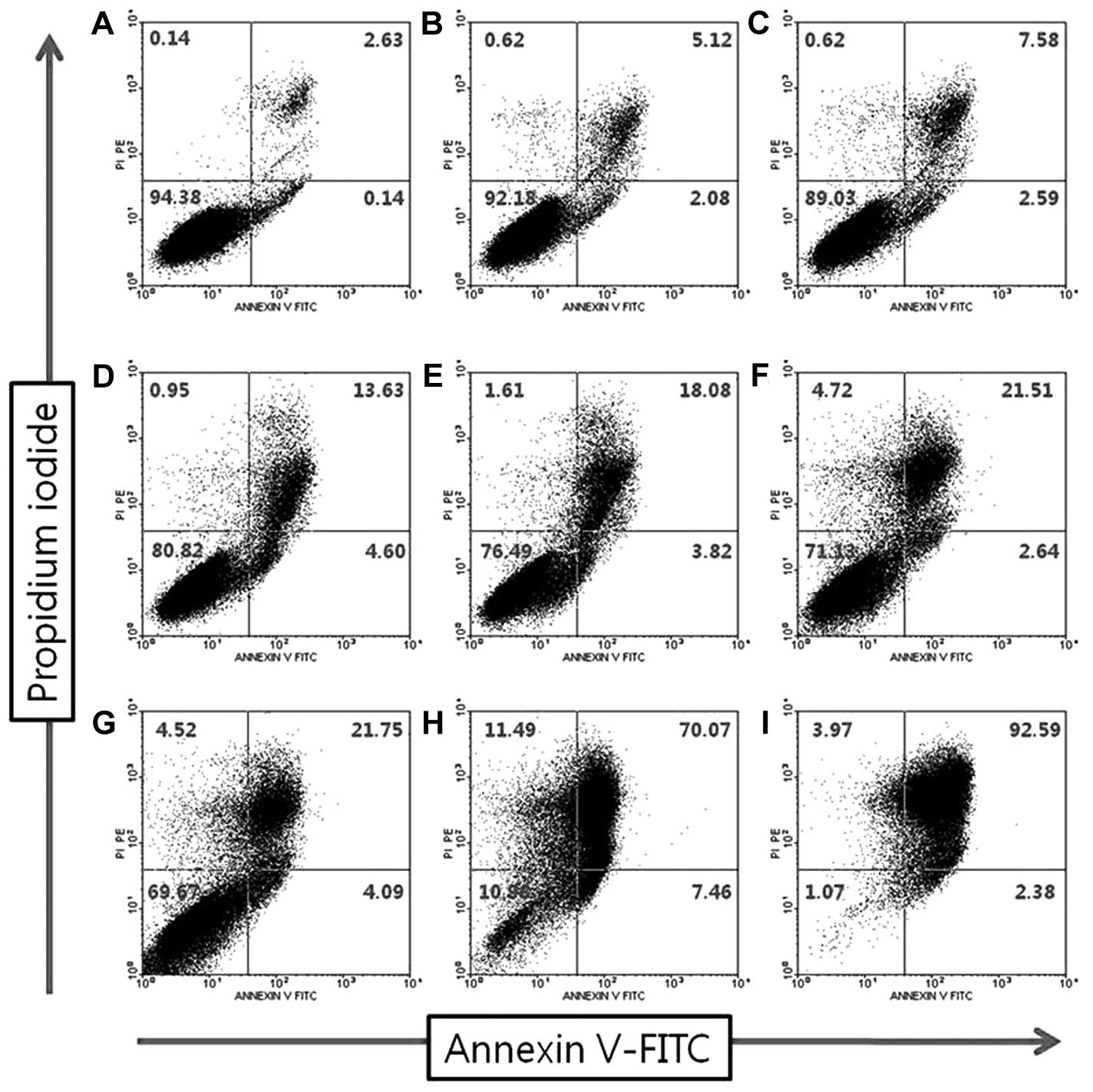
apoptosis, we performed FACS analysis. Fig. 2 shows flow cytometric plots
obtained with Annexin V-FITC/PI assay after a 12-h exposure to
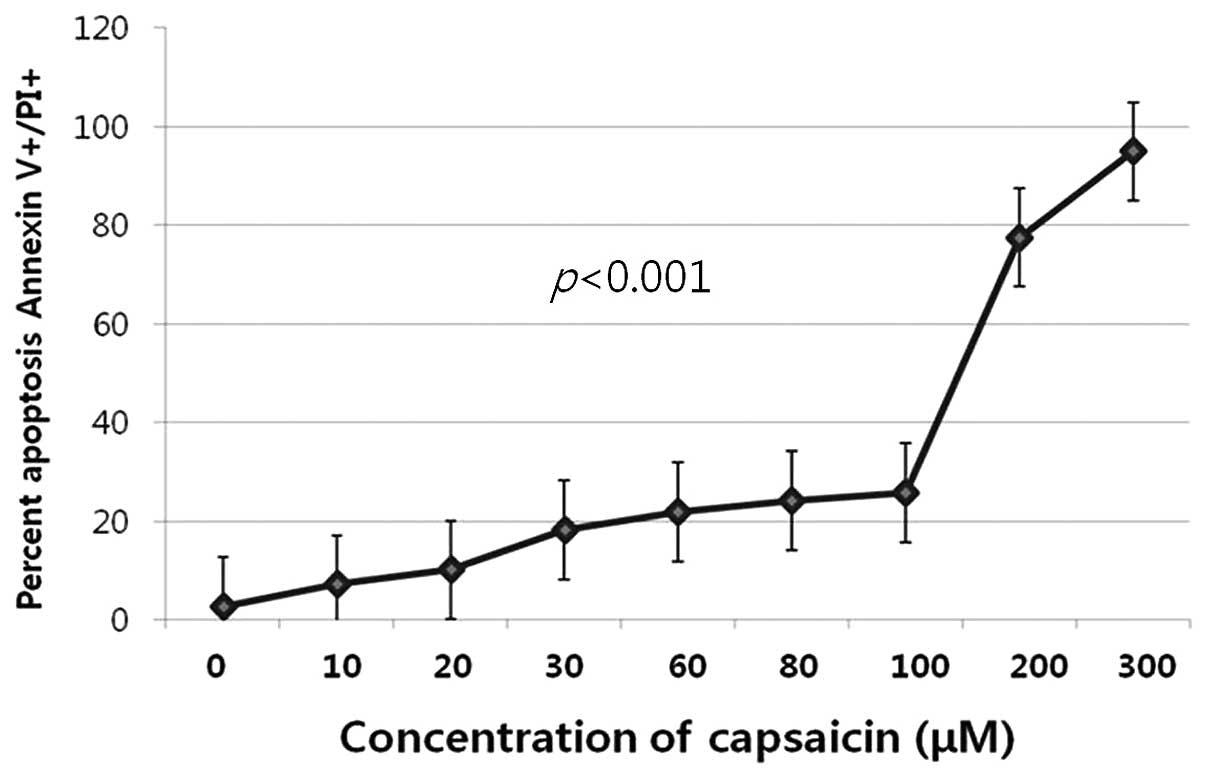
different concentrations (0–300 μM). The number of early and late
apoptotic cells or directly damaged cells increased subsequent to
exposure of the cells to capsaicin (P<0.001, Fig. 3).
Capsaicin-induced apoptosis is associated
with the modulation of apoptotic regulatory proteins in human
gastric cancer cells
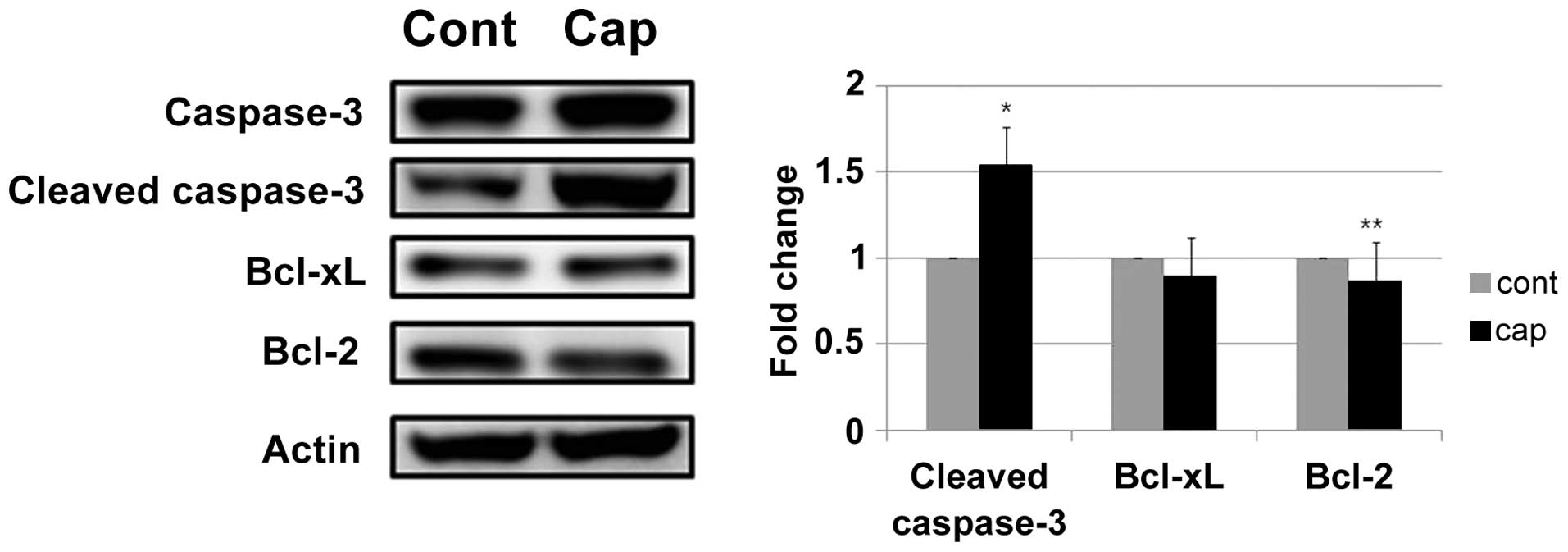
An increase in cleaved caspase-3 expression was
detected in the AGS cells following treatment with capsaicin
(Fig. 4). Apoptosis is regulated
by the balance between pro- and anti-apoptotic genes. As shown in
Fig. 4, the protein levels of the
anti-apoptotic gene Bcl-2 was reduced by treatment with capsaicin
in AGS cells, whereas the protein levels of Bcl-xL were not altered
in response to the treatment with capsaicin.
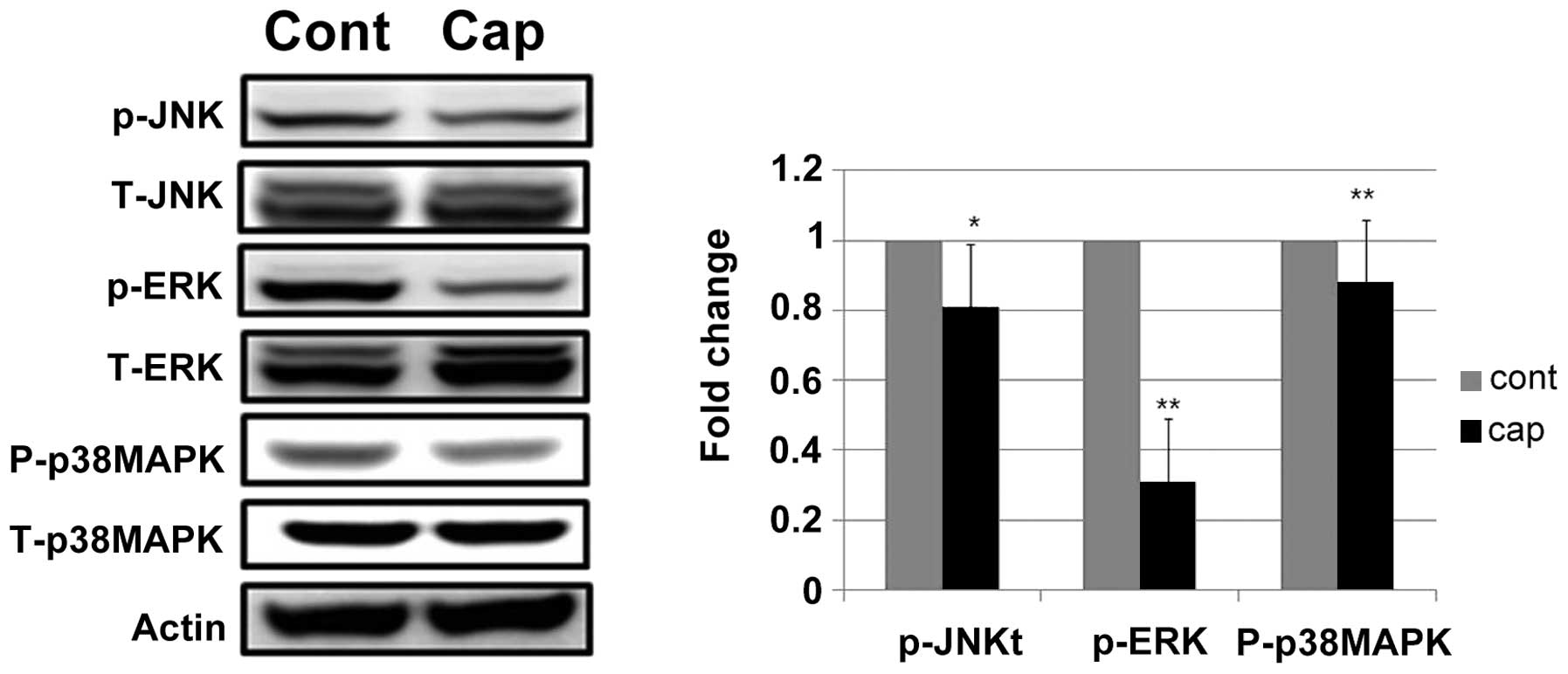
Capsaicin modulates the phosphorylation
of MAPK cascade signaling proteins in human gastric cancer
cells
The effect of capsaicin on the MAPK signal pathway,
which is essential for cell survival and growth during development
and carcinogenesis, was examined. Capsaicin treatment decreased the
expression of phosphorylated ERK 1/2 (Fig. 5). Since crosstalk between signal
transduction pathways is common, we examined whether capsaicin
prevented the phosphorylation of p38 or JNK in human gastric cancer
cells. Capsaicin treatment slightly decreased the phosphorylation
of p38 and JNK. The levels of total ERK 1/2, JNK and p38 MAPK were
not altered after capsaicin treatment (Fig. 5).
Discussion
Although previous studies (3–8) have
focused on the tumor suppressive effects of capsaicin in human
cancer cells, few studies have been undertaken to elucidate
capsaicin-induced apoptosis in human gastric cancer cells. Previous
results of an XTT assay, DNA fragmentation and flow cytometric
analysis revealed that capsaicin (3.5–10 μmol/l) induced apoptotic
cell death in an in vitro Korean stomach tumor cell (SNU1)
(13). This induction of cell
death may be due to a Bcl-2 sensitive apoptotic pathway in a human
gastric adenocarcinoma cell line (AGA cells) (12). In this study, cell proliferation
significantly decreased in a dose-dependent manner following
incubation of AGS cells with capsaicin for 12 h. The results have
also demonstrated that the proportion of early and late apoptotic
cells induced by capsaicin increased in a dose-dependent manner.
The anticancer effects of capsaicin are both concentration- and
time-dependent in vitro (7). Previous findings have shown the
effects of capsaicin to be in the low micromolar range and to
become maximal at 200–300 μM (2).
Our results have shown that a considerable antiproliferative or
apoptotic effect of capsaicin occurred at 60–300 μM.
The apoptotic response was evidenced by the
activation of caspase-3 generation and the expression of
anti-apoptotic markers. The activity of caspase-3 was found to
increase with the exposure to capsaicin. In addition, western
blotting revealed a reduction of the Bcl-2 gene. Recently, the
Bcl-2 gene appears to be a critical regulator of programmed cell
death in a variety of physiological and pathological contexts
(14). Additionally, the
expression of the Bcl-2 protein was reduced when the AGS cells were
exposed to higher doses (10–200 μmol/l) of capsaicin, which was
similar to results of the present study (12). These results suggest that capsaicin
is potentially involved in the modulation of proliferation and
apoptosis in human gastric cancer cells.
The ERK 1/2 pathway has been associated with cell
proliferation and differentiation (15). Findings of a recent study have
demonstrated that exposure to capsaicin clearly inhibited the
activation of ERK in breast cancer cell lines including BT-474 and
SK BR-3. In human fibrosarcoma cells, capsaicin suppressed the
EGF-induced phosphorylation of EGFR, Akt, Raf, ERK1/2 and p38 MAPK
(16), while blocking upstream ERK
1/2 phosphorylation reduced the amount of IL-8 produced by AGS
cells in response to H. pylori infection (17). In this study, capsaicin suppressed
the phosphorylation of ERK 1/2, JNK and p38 MAPK in human gastric
cancer cells. Thus, capsaicin may be useful for anti-inflammatory
or anti-carcinogenic activity in gastric cancer associated with
H. pylori infection.
However, as the pharmacokinetics of capsaicin in
human have not yet been investigated, predicting the amount of
chili peppers that would need to be consumed to achieve the
effective concentration of capsaicin observed is unclear.
Capsaicin reduced cell proliferation and increased
apoptosis in a concentration-dependent manner in human gastric
cancer cells. Cleaved caspase-3 was increased and Bcl-2 was reduced
by treatment with capsaicin in AGS cells. Capsaicin treatment
decreased the expression of phosphorylated ERK 1/2, p38 MAPK or JNK
in AGS cells.
In conclusion, results of this study suggest that
capsaicin may serve as an anti-tumorigenic agent in human gastric
cancer. However, the physiological capsaicin dose for the
therapeutic or chemopreventive agents is unclear and remains to be
determined in future investigations.
References
|
1
|
Monsereenusorn Y, Kongsamut S and Pezalla
PD: Capsaicin - a literature survey. Crit Rev Toxicol. 10:321–339.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bley K, Boorman G, Mohammad B, McKenzie D
and Babbar S: A comprehensive review of the carcinogenic and
anticarcinogenic potential of capsaicin. Toxicol Pathol.
40:847–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thoennissen NH, O’Kelly J, Lu D, et al:
Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and
-negative breast cancer cells by modulating the EGFR/HER-2 pathway.
Oncogene. 29:285–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi CH, Jung YK and Oh SH: Autophagy
induction by capsaicin in malignant human breast cells is modulated
by p38 and extracellular signal-regulated mitogen-activated protein
kinases and retards cell death by suppressing endoplasmic reticulum
stress-mediated apoptosis. Mol Pharmacol. 78:114–125. 2010.
View Article : Google Scholar
|
|
5
|
Kim YM, Hwang JT, Kwak DW, Lee YK and Park
OJ: Involvement of AMPK signaling cascade in capsaicin-induced
apoptosis of HT-29 colon cancer cells. Ann NY Acad Sci.
1095:496–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eling TE, Baek SJ, Shim M and Lee CH:
NSAID activated gene (NAG-1), a modulator of tumorigenesis. J
Biochem Mol Biol. 39:649–655. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CC, Lin JP, Yang JS, et al: Capsaicin
induced cell cycle arrest and apoptosis in human esophagus
epidermoid carcinoma CE 81T/VGH cells through the elevation of
intracellular reactive oxygen species and Ca2+
productions and caspase-3 activation. Mutat Res. 601:71–82. 2006.
View Article : Google Scholar
|
|
8
|
Chanda S, Erexson G, Frost D, Babbar S,
Burlew JA and Bley K: 26-Week dermal oncogenicity study evaluating
pure trans-capsaicin in Tg.AC hemizygous mice (FBV/N). Int J
Toxicol. 26:123–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Akagi A, Sano N, Uehara H, Minami T,
Otsuka H and Izumi K: Non-carcinogenicity of capsaicinoids in
B6C3F1 mice. Food Chem Toxicol. 36:1065–1071. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HM, Chuang SM, Su YC, Li YH and Chueh
PJ: Down-regulation of tumor-associated NADH oxidase, tNOX (ENOX2),
enhances capsaicin-induced inhibition of gastric cancer cell
growth. Cell Biochem Biophys. 61:355–366. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee IO, Lee KH, Pyo JH, Kim JH, Choi YJ
and Lee YC: Anti-inflammatory effect of capsaicin in Helicobacter
pylori-infected gastric epithelial cells. Helicobacter. 12:510–517.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lo YC, Yang YC, Wu IC, et al:
Capsaicin-induced cell death in a human gastric adenocarcinoma cell
line. World J Gastroenterol. 11:6254–6257. 2005.PubMed/NCBI
|
|
13
|
Kim JD, Kim JM, Pyo JO, et al: Capsaicin
can alter the expression of tumor forming-related genes which might
be followed by induction of apoptosis of a Korean stomach cancer
cell line, SNU-1. Cancer Lett. 120:235–241. 1997. View Article : Google Scholar
|
|
14
|
Weil M, Jacobson MD, Coles HS, et al:
Constitutive expression of the machinery for programmed cell death.
J Cell Biol. 133:1053–1059. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schlessinger J: Cell signaling by receptor
tyrosine kinases. Cell. 103:211–225. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang YP, Yun HJ, Choi JH, et al:
Suppression of EGF-induced tumor cell migration and matrix
metalloproteinase-9 expression by capsaicin via the inhibition of
EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling.
Mol Nutr Food Res. 55:594–605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keates S, Keates AC, Warny M, Peek RM Jr,
Murray PG and Kelly CP: Differential activation of
mitogen-activated protein kinases in AGS gastric epithelial cells
by cag+ and cag− Helicobacter pylori.
J Immunol. 163:5552–5559. 1999.PubMed/NCBI
|