Introduction
Cutaneous wounds, known as ulcers, are an extremely
common clinical problem and often arise following acute or chronic
mechanical causes, physical or chemical burns, frostbite,
infections, and disorders, including rheumatism, diabetes,
peripheral vascular disease, lipodermatosclerosis and malignant
tumors (1,2). Furthermore, cutaneous wound healing
may be significantly delayed due to the aforementioned factors. At
present, delayed cutaneous wounds are one of major burdens for
health care, and lead to a reduced quality of life in patients who
suffer from this type of wound (3,4).
Wound healing is a complicated biological process
involving a series of dynamic events, including hemostasia,
inflammation, cell proliferation and differentiation,
neovascularization, granulation tissue formation, collagen
synthesis, epithelialization, and wound contraction. Increasing
evidence has established the hypothesis that growth factors,
including vascular endothelial growth factor (VEGF) and basic
fibroblast growth factor (bFGF), are key regulators of normal and
abnormal angiogenesis, and tissue repair in animals and humans
(5–8). VEGF promotes
angiogenesis/vasculogenesis and vascular permeability, and enhances
endothelial cell proliferation and migration as well as the
adhesion of leukocytes (5,9). Further research revealed that VEGF
stimulates hydrogen sulfide synthesis and release from endothelial
cells, thus leading to subsequent endothelial cell growth,
migration and permeability, microvessel formation, and wound
healing (10). In addition, VEGF
promotes epithelialization and collagen deposition in the wound
(11). FGF-2, known as bFGF, is a
member of a large FGF family and induces angiogenesis, endothelial
cell and fibroblast proliferation, and wound healing (12–14).
Recent data indicated that bFGF-mediated angiogenesis refers to
endothelial cell proliferation, migration and tube formation by
activating c-Jun N-terminal kinase/stress-activated protein kinase
signaling (15). Notably, the
expression of VEGF and bFGF was increased following skin injury,
particularly in the early stages of healing, showing greatest
intensity at the center of the wound, with progressive decline in
intensity towards the periphery and almost no VEGF or bFGF in
uninjured skin (16,17). VEGF and bFGF, however, are
decreased in delayed cutaneous wounds, including diabetic wounds
and chronic ulcers (17–19). Inhibition of VEGF and bFGF by
neutralizing antibodies results in a decrease in the migration of
fibroblasts and a delay in wound healing (20), while treatment with recombinant
bFGF and VEGF, or overexpression of VEGF accelerates wound healing
(14,21,22).
Therefore, it is a potentially clinically beneficial to increase
the levels of VEGF and bFGF in cutaneous wounds, particularly in
delayed wound healing.
In the past few decades, moist exposed burn ointment
(MEBO), a Chinese burn ointment with a USA patented formulation
since 1995, which was developed at the China National Science and
Technology Centre in Beijing in 1989 (23,24),
has been used to treat thickness burns in clinical practice and has
achieved beneficial efficacy (25–28).
MEBO contains sesame oil, β-sitosterol, berberine, and other small
quantities of plant ingredients from Chinese herbal remedies,
including Coptis chinensis Franch., Scutellaria
baicalensis Georgi, Phellodendron Chinese Schneid.,
Pheretima aspergillum (E Perrier) and Papaver
somniferum L. (29). Further
research indicated that β-sitosterol exhibits anti-inflammatory
effects (30) and that berberine
exhibits antimicrobial effects (31). Clinical and experimental studies
have shown that MEBO exhibits analgesic and antimicrobial effects,
and reduces the treatment time of burns (25–28,32,33).
Furthermore, MEBO induces debridement and epithelial repair,
associated with improved scar quality and reduced costs of
treatment for patients with burns (25,27,28,33).
MEBO has been observed to promote chronic ischemic and neurogenic
ulcer healing, and reduces the time of wound healing in patients
(34,35); however, the underlying mechanisms
remain unclear.
The aim of the present study was to investigate the
effects of MEBO on cutaneous excisional wounds in experimental rats
and to explore the underlying mechanisms.
Materials and methods
Reagents
MEBO was purchased from Shantou MEBO Pharmaceuticals
Co., Ltd. (Shantou, China). Recombinant bovine bFGF (rb-bFGF) was
purchased from Zhuhai Yisheng Biological Pharmaceutical Co.
(Zhuhai, China). Rabbit polyclonal antibodies against VEGF and bFGF
were purchased from Abcam (Cambridge, MA, USA), and secondary mouse
anti-rabbit peroxidase-conjugated monoclonal antibody was obtained
from Sigma-Aldrich (St. Louis, MO, USA). TRIzol reagent was
purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). A
bicinchoninic acid (BCA) protein assay kit was purchased from
Thermo Fisher Scientific (Waltham, MA, USA).
Wound preparation and experimental
design
Sixty male Sprague-Dawley rats (age, 8-weeks;
weight, 220–250 g) were purchased from the Animal Experimental
Center of Guangxi Medical University (Nanning, China;
SCXKGui2009–0002). The study was approved by the Ethics Committees
of Youjiang Medical University for Nationalities (Baise, China) and
Guangxi University of Chinese Medicine. Rats were housed in a
temperature- and humidity-controlled room with a 12-h ligh/dark
cycle and had free access to food and water. Following 1-week
acclimatization, cutaneous full-thickness excisional wounds were
prepared as previously described with some modifications (36). Briefly, the rat hair was shaved on
the dorsal side following anesthesia with pentobarbital sodium (39
mg/kg), and the skin was cleaned with 70% ethanol. A 2.4-cm
diameter full-thickness skin defect was created on the back by skin
punch biopsy under aseptic conditions. Two wounds were created in
each rat. Next, the rats were randomly divided into three groups
based on different treatments: Model (n=20), MEBO (n=20) and
rb-bFGF (n=20). The wounds in the model group were covered with a
single dressing soaked with physiological saline and double dry
sterile dressings; wounds in the MEBO group were covered with dry
sterile dressings following direct coating of the wound with 1 mm
thick MEBO; and wounds in the rb-bFGF group were covered with dry
sterile dressings following by spraying of the wound with rb-bFGF.
The sterile dressings were changed daily following clearing of
wound liquefaction with sterile dressings. All procedures were in
accordance with internationally accepted principles for laboratory
animal use and care, as found in the European Community Guidelines
(EEC Directive of 1986; 86/609/EEC) and the US guidelines (NIH
publication #85–23, revised in 1985).
Histopathological observation
Rats were euthanized using pentobarbital sodium.
Granulation tissue was collected from eight-day-old wounds not
containing healthy skin margin. Each tissue was fixed using 10%
formalin, processed for paraffin embedding and stored at 4°C. The
following parameters were evaluated with hematoxylin and eosin
(H&E) staining on multiple serial sections (4–5 μm):
Neovascularization, fibroblast proliferation and inflammatory cell
infiltration. All analysis was performed at ×200 magnification.
Western blot analysis
The protein expression of VEGF and bFGF in
granulation tissue from eight-day-old wounds was determined by
western blotting, as previously described with certain
modifications (37). Briefly,
tissue homogenates from wound granulation were prepared using RIPA
lysis buffer. Insoluble material was removed by centrifugation
(12,000 × g for 20 min; 4°C; Thermo Forma, Osteroden, Germany), and
the protein concentrations of the supernatants were determined
using a BCA protein assay kit. Equal quantities of protein (50 μg)
were separated via SDS-PAGE. The protein was electrophoretically
transferred to polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The blots were incubated with the primary
antibody overnight at 4°C and then with the secondary antibody for
1–2 h at room temperature. The protein bands were detected by an
Enhanced Cheminluminescent Plus detection reagent kit (Amersham
Pharmacia Biotech, Amersham, UK).
Quantification of mRNA levels by reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from eight-day-old wound
granulation tissue using TRIzol reagent according to the
manufacturer’s instructions, and subsequently transcribed into
cDNA. cDNA was then transcribed into mRNA by RT-PCR. The product
was separated via agarose gel electrophoresis. The sequences of
primers used were as follows: Forward: 5′-CGGAAGATTAGGGAGTTTTG-3′
and reverse: 5′-AGGGATGGGTTTGTCGTGT-3′ for VEGF; forward:
5′-GCGTCCGGGAGAAGAGCGAC-3′ and reverse: 5′-GCCAGGTACCGGTTCGCACA-3′
for bFGF; forward: 5′-CAGTGCCAGCCTCGTCTCAT-3′ and reverse:
5′-AGGGGCCATCCACAGTCTTC-3′ for GAPDH; and forward:
5′-CACCCGCGAGTACAACCTTC-3′ and reverse: 5′-CCCATACCCACCATCACACC-3′
for β-actin.
Statistical analysis
The data are presented as the mean ± standard
deviation. Statistical analysis was performed using the SPSS 17.0
for Windows (SPSS Inc., Chicago, IL, USA). Significant differences
among groups were analyzed by one-way analysis of variance, and
differences between means were determined by Fisher’s least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
MEBO promotes the formation of
granulation tissue and wound healing
MEBO was initially successfully designed to treat
burns. Previously, MEBO was observed to promote chronic ulcer
healing (34,35); however, the underlying mechanisms
remain to be fully elucidated. In the current study, a
full-thickness skin defect was induced by skin punch biopsy, and
treatment with MEBO for eight days significantly promoted the
formation of granulation tissue when compared with the model group.
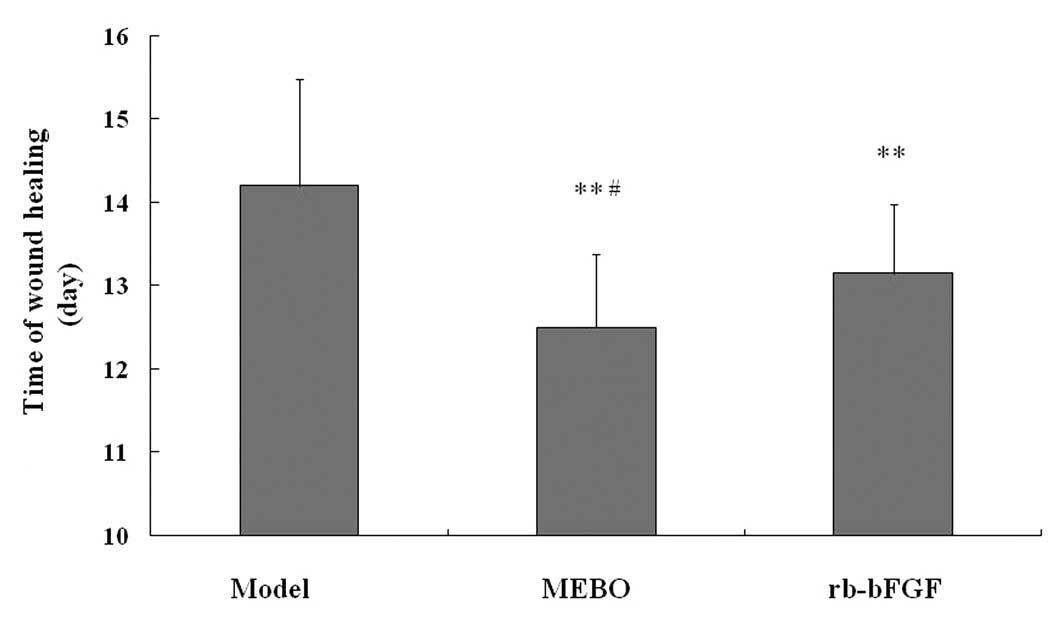
Furthermore, the time of wound healing in the MEBO group was
shorter compared with that of the model group (P<0.01; Fig. 1). In addition, rb-bFGF was designed
as a positive control medicine for MEBO. Consistent with MEBO,
rb-bFGF also caused the formation of granulation tissue and
shortened the time of wound healing when compared with the model
group (P<0.01); however, the time of wound healing in the MEBO
group was also shorter than that of the rb-bFGF group (P<0.05).
The data indicate that MEBO promotes cutaneous excisional wound
healing, similar to that of rb-bFGF, indicating that there is a
similarity in the effects of MEBO and rb-bFGF in the treatment of
wounds.
MEBO increases neovascularization and
fibroblasts in granulation tissue
To determine the mechanisms underlying the effects
of MEBO, the histology of granulation tissue in three groups was
observed following wound treatment for eight days. As shown in
Fig. 2, there were numerous novel
capillaries and fibroblasts in granulation tissue in the MEBO
group, which were more abundant compared with the model group and
rb-bFGF group, when observed at ×200 magnification. In addition,
rb-bFGF treatment for eight days resulted in marked increases in
the numbers of capillaries and fibroblasts in granulation tissue
when compared with the model group. These data suggest that MEBO
promotes neovascularization and enhances fibroblast proliferation
and/or migration.
Effects of MEBO on the protein expression
of VEGF and bFGF
MEBO promoted neovascularization and increased
fibroblasts in granulation tissue. As previously described, VEGF
and bFGF levels are closely correlated with neovascularization and
fibroblast proliferation (5–8). To
further determine the association between MEBO and VEGF/bFGF,
western blotting was used to determine the growth factor levels.
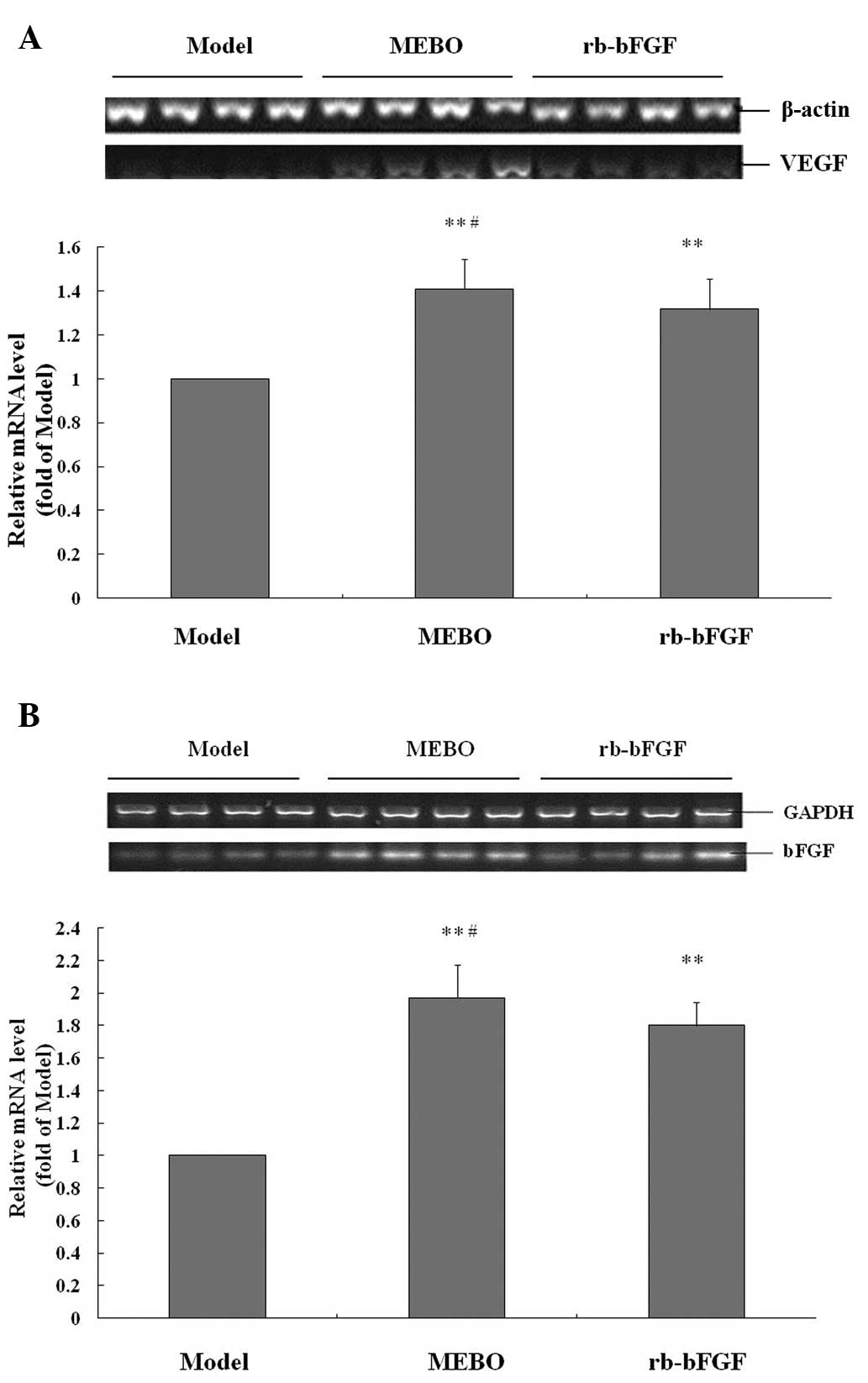
Local administration of MEBO for eight days markedly increased the
levels of VEGF and bFGF by ~77.5 and 90.8%, respectively (all
P<0.01; Fig. 3), when compared
with the model group. VEGF and bFGF protein expression in the
rb-bFGF group were higher compared with the model group (all
P<0.01). Notably, the levels of VEGF and bFGF in the MEBO group
were higher compared with the rb-bFGF group (P<0.05 and
P<0.01, respectively). The results indicate that MEBO at least
partially increases the protein expression levels of VEGF and bFGF
to promote wound healing.
Effects of MEBO on the gene expression of
VEGF and bFGF
qPCR analysis (Fig.
4) indicated that MEBO treatment for eight days led to
increases in the mRNA expression of VEGF and bFGF by 40.9 and
97.1%, respectively, when compared with the model group (all
P<0.01). The rb-bFGF treatment also increased the gene
expression of VEGF and bFGF (all P<0.01). Furthermore, the mRNA
levels of VEGF and bFGF genes in the MEBO group were higher
compared with the rb-bFGF group (all P<0.05).
Discussion
In China and other Asian countries, traditional
Chinese medicine has been widely used to treat wounds in clinical
practice due to its beneficial effects; however, convincing
evidence is lacking, and the mechanisms remain unclear.
In the present study, MEBO, a Chinese herbal
ointment, promotes granulation tissue formation and reduces the
time of wound healing in cutaneous excisional wounds, suggesting
that it is effective for wound healing, which is consistent with
the results of previous studies (34,35,38).
It is well established that excisional wounds invariably destroy
tissue integrity, and lead to vascular injury and
fibrin-fibronectin clot formation, thus leading to platelet
recruitment, and subsequently upregulation of growth factors and
cytokines, including VEGF and bFGF (5,39),
which triggers the formation of granulation tissue and wound
healing. The wound healing process involves migration and
proliferation of cells, including vascular endothelial cells and
fibroblasts. Further investigation has demonstrated that MEBO
increases neovascularization in the granulation tissue, implicating
the beneficial effects of MEBO on vascular endothelial cell
proliferation. Furthermore, MEBO also increased fibroblasts in the
granulation tissue. Increases in the number of fibroblasts
primarily arise due to the proliferation of resident fibroblasts in
response to growth factors, including bFGF, and fibroblasts
migrating from the surrounding connective tissue into the wound
site (40). It was observed that
recombinant bFGF accelerates the wound healing process (14,41),
thus, rb-bFGF was used as a positive control for MEBO. In the
current study, rb-bFGF promoted the formation of granulation
tissue, increased neovascularization and the number of fibroblasts
in the granulation tissue, and reduced the time of wound healing,
which were all consistent with the effects of MEBO.
It is generally accepted that VEGF induces
endothelial cell proliferation and migration, promotes vascular
permeability and angiogenesis, increases collagen deposition
(5,9–11),
and that bFGF mediates angiogenesis, promotes the proliferation and
migration of endothelial cells and fibroblasts proliferate and
migration (12–15,42).
Therefore, it was inferred that MEBO promotion of wound healing in
rats involves growth factors, including VEGF and bFGF. In order to
confirm this hypothesis, western blot analysis and RT-PCR were
respectively used to analyze the protein and mRNA expression of
VEGF and bFGF. Notably, MEBO enhanced the protein expression of
VEGF and bFGF and also elevated their mRNA expression. A previous
study reported that MEBO enhanced α-smooth muscle actin in
fibroblasts, indicating that MEBO activates fibroblasts (43). It is hypothesized that leukocytes,
including fibroblasts and endothelial cells are the primary origin
of VEGF and bFGF (41).
Furthermore, bFGF upregulates the expression of VEGF (9,41).
Decreased levels of VEGF and/or bFGF, or inhibition of their
activity leads to the limited migration of fibroblasts and the
delay of wound healing (20,44).
In the present study, rb-bFGF enhanced the protein and gene
expression of VEGF and bFGF, which is similar to the results of a
previous study (45). It is well
known that the expression of VEGF and bFGF is increased following
skin injury, particularly in the early stage of healing. A previous
report showed that the acute wound fluid, which is rich in VEGF and
bFGF, increases the mRNA levels of VEGF and bFGF (45). Since a similar effect between MEBO
and rb-bFGF in treating cutaneous excisional wounds was observed,
it was concluded that MEBO promotes wound healing by increasing
VEGF and bFGF production.
Furthermore, compared with rb-bFGF, MEBO took less
time to promote wound healing, and led to a higher production of
VEGF and bFGF. In addition, increasing evidence indicates that MEBO
promotes wound contraction and improves scar quality of wounds
(43,46). Therefore, MEBO is hypothesized to
be an ideal therapy for cutaneous wounds.
In conclusion, MEBO promotes cutaneous excisional
wound healing by at least partially enhancing the production of
VEGF and bFGF, which reveals part of the underlying mechanism and
suggests the use of MEBO for the treatment of delayed healing
cutaneous wounds.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 30860356) and the
Guangxi Natural Science Foundation (grant no.
2013GXNSFDA019020).
References
|
1
|
Ndip A, Ebah L and Mbako A: Neuropathic
diabetic foot ulcers - evidence-to-practice. Int J Gen Med.
5:129–134. 2012.PubMed/NCBI
|
|
2
|
Herouy Y, Kreis S, Mueller T, et al:
Inhibition of angiogenesis in lipodermatosclerosis: Implication for
venous ulcer formation. Int J Mol Med. 24:645–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Letizia M, Uebelhor J and Paddack E:
Providing palliative care to seriously ill patients with nonhealing
wounds. J Wound Ostomy Continence Nurs. 37:277–282. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang Y, Huang S, Fu X, et al:
Epidemiology of chronic cutaneous wounds in China. Wound Repair
Regen. 19:181–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Soyer T, Ayva S, Aliefendioğlu D, et al:
Effect of phototherapy on growth factor levels in neonatal rat
skin. J Pediatr Surg. 46:2128–2131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ching YH, Sutton TL, Pierpont YN, Robson
MC and Payne WG: The use of growth factors and other humoral agents
to accelerate and enhance burn wound healing. Eplasty.
11:e412011.PubMed/NCBI
|
|
7
|
Shi L, Chang Y, Yang Y, et al: Activation
of JNK signaling mediates connective tissue growth factor
expression and scar formation in corneal wound healing. PLoS One.
7:e321282012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Vascular endothelial growth factor (VEGF)-part 1: in
physiology and pathophysiology. Endokrynol Pol. 62:444–455.
2011.PubMed/NCBI
|
|
9
|
Nissen NN, Polverini PJ, Koch AE, et al:
Vascular endothelial growth factor mediates angiogenic activity
during the proliferative phase of wound healing. Am J Pathol.
152:1445–1452. 1998.PubMed/NCBI
|
|
10
|
Papapetropoulos A, Pyriochou A, Altaany Z,
et al: Hydrogen sulfide is an endogenous stimulator of
angiogenesis. Proc Natl Acad Sci USA. 106:21972–21977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bao P, Kodra A, Tomic-Canic M, et al: The
role of vascular endothelial growth factor in wound healing. J Surg
Res. 153:347–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nissen NN, Polverini PJ, Gamelli RL and
DiPietro LA: Basic fibroblast growth factor mediates angiogenic
activity in early surgical wounds. Surgery. 119:457–465. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bikfalvi A, Klein S, Pintucci G and Rifkin
DB: Biological roles of fibroblast growth factor-2. Endocr Rev.
18:26–45. 1997.
|
|
14
|
Yan L, Wu W, Wang Z, et al: Comparative
study of the effects of recombinant human epidermal growth factor
and basic fibroblast growth factor on corneal epithelial wound
healing and neovascularization in vivo and in vitro. Ophthalmic
Res. 49:150–160. 2012. View Article : Google Scholar
|
|
15
|
Kaikai S, Yuchen S, Lili J and Zhengtao W:
Critical role of c-Jun N-terminal kinase in regulating bFGF-induced
angiogenesis in vitro. J Biochem. 150:189–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu TM, Niu XT and Guo H: Clinical
observation on changes of fibroblast growth factor in burn wounds.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 14:261–263. 2000.(In
Chinese).
|
|
17
|
Lauer G, Sollberg S, Cole M, et al:
Expression and proteolysis of vascular endothelial growth factor is
increased in chronic wounds. J Invest Dermatol. 115:12–18. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zandifar E, Sohrabi Beheshti S, Zandifar A
and Haghjooy Javanmard S: The effect of captopril on impaired wound
healing in experimental diabetes. Int J Endocrinol.
2012:7852472012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peplow PV and Baxter GD: Gene expression
and release of growth factors during delayed wound healing: a
review of studies in diabetic animals and possible combined laser
phototherapy and growth factor treatment to enhance healing.
Photomed Laser Surg. 30:617–636. 2012. View Article : Google Scholar
|
|
20
|
Lee EY, Xia Y, Kim WS, et al:
Hypoxia-enhanced wound-healing function of adipose-derived stem
cells: increase in stem cell proliferation and up-regulation of
VEGF and bFGF. Wound Repair Regen. 17:540–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nauta A, Seidel C, Deveza L, et al:
Adipose-derived stromal cells overexpressing vascular endothelial
growth factor accelerate mouse excisional wound healing. Mol Ther.
21:445–455. 2013. View Article : Google Scholar
|
|
22
|
Anghel A, Mut-Vitcu B, Savu L, et al:
Clinical improvement after treatment with VEGF(165) in patients
with severe chronic lower limb ischaemia. Genomic Med. 1:47–55.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rongxiang X: On the Principles of
Treatment of Burn Wound. Beijing: China National Science and
Technology Centre for Burns, Wounds and Ulcers; 1994
|
|
24
|
Atiyeh BS, Dham R, Kadry M, et al:
Benefit-cost analysis of moist exposed burn ointment. Burns.
28:659–663. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ang ES, Lee ST, Gan CS, et al: The role of
alternative therapy in the management of partial thickness burns of
the face - experience with the use of moist exposed burn ointment
(MEBO) compared with silver sulphadiazine. Ann Acad Med Singapore.
29:7–10. 2000.PubMed/NCBI
|
|
26
|
Allam AM, Mostafa W, Zayed E and El-Gamaly
J: Management of the acute partial-thickness burned hand; moist
exposed burn ointment or silver sulphadiazine cream both combined
with a polyethylene bag. Ann Burns Fire Disasters. 20:144–148.
2007.PubMed/NCBI
|
|
27
|
Hirsch T, Ashkar W, Schumacher O, et al:
Moist exposed burn ointment (MEBO) in partial thickness burns - a
randomized, comparative open mono-center study on the efficacy of
dermaheal (MEBO) ointment on thermal 2nd degree burns compared to
conventional therapy. Eur J Med Res. 13:505–510. 2008.
|
|
28
|
Carayanni VJ, Tsati EG, Spyropoulou GC,
Antonopoulou FN and Ioannovich JD: Comparing oil based ointment
versus standard practice for the treatment of moderate burns in
Greece: a trial based cost effectiveness evaluation. BMC Complement
Altern Med. 11:1222011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yong YL: Analysis of MEBO cream. Institute
of Science and Forensic Medicine, Department of Scientific
Services, Health Science Division; Singapore: 1999, Report no.
99033191.
|
|
30
|
Valerio M and Awad AB: β-Sitosterol
down-regulates some pro-inflammatory signal transduction pathways
by increasing the activity of tyrosine phosphatase SHP-1 in J774A.1
murine macrophages. Int Immunopharmacol. 11:1012–1017. 2011.
|
|
31
|
Zou Q, Li Y, Zhang L, et al: Antibiotic
delivery system using nano-hydroxyapatite/chitosan bone cement
consisting of berberine. J Biomed Mater Res A. 89:1108–1117. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jewo PI, Fadeyibi IO, Babalola OS, et al:
A comparative study of the wound healing properties of moist
exposed burn ointment (MEBO) and silver sulphadiazine. Ann Burns
Fire Disasters. 22:79–82. 2009.PubMed/NCBI
|
|
33
|
Jurjus A, Atiyeh BS, Abdallah IM, et al:
Pharmacological modulation of wound healing in experimental burns.
Burns. 33:892–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang QS, Tang QL, Zhang L, et al: MEBO for
treating 47 cases of chronic ischemic ulcer in lower limber. Chin J
Burns Wound Surface Ulcer. 17:296–297. 2005.(In Chinese).
|
|
35
|
Li JH, Zhang L, Tang QL, et al: Clinical
observation on effect of MEBO on neurogenic ulcer. Liaoning J
Tradit Chin Med. 39:1095–1096. 2012.(In Chinese).
|
|
36
|
Hong JW, Lee WJ, Hahn SB, Kim BJ and Lew
DH: The effect of human placenta extract in a wound healing model.
Ann Plast Surg. 65:96–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng XT, Wang TZ, Chen Y, et al: Pollen
Typhae total flavone improves insulin-induced glucose uptake
through the β-arrestin-2-mediated signaling in C2C12 myotubes. Int
J Mol Med. 30:914–922. 2012.
|
|
38
|
Cho KY, Lee SJ, Burm JS and Park EA:
Successful combined treatment with total parenteral nutrition fluid
extravasation injuries in preterm infants. J Korean Med Sci.
22:588–594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Furie B and Furie BC: Molecular and
cellular biology of blood coagulation. N Engl J Med. 326:800–806.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Desmoulière A, Chaponnier C and Gabbiani
G: Tissue repair, contraction, and the myofibroblast. Wound Repair
Regen. 13:7–12. 2005.PubMed/NCBI
|
|
41
|
Lee E, Kim DY, Chung E, et al:
Transplantation of cyclic stretched fibroblasts accelerates the
wound-healing process in streptozotocin-induced diabetic mice. Cell
Transplant. Feb 4–2013.(Epub ahead of print).
|
|
42
|
Kanazawa S, Fujiwara T, Matsuzaki S, et
al: bFGF regulates PI3-kinase-Rac1-JNK pathway and promotes
fibroblast migration in wound healing. PLoS One. 5:e122282010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
El Kahi CG, Atiyeh BS, Abdallah Hajj
Hussein I, et al: Modulation of wound contracture alpha-smooth
muscle actin and multispecific vitronectin receptor integrin
alphavbeta3 in the rabbit’s experimental model. Int Wound J.
6:214–224. 2009.PubMed/NCBI
|
|
44
|
Lee HJ, Kwon JY, Shin SW, et al: Effects
of sevoflurane on collagen production and growth factor expression
in rats with an excision wound. Acta Anaesthesiol Scand.
54:885–893. 2010.PubMed/NCBI
|
|
45
|
Koenen P, Spanholtz TA, Maegele M, et al:
Acute and chronic wound fluids inversely influence adipose-derived
stem cell function: molecular insights into impaired wound healing.
Int Wound J. Mar 13–2013.(Epub ahead of print).
|
|
46
|
Atiyeh BS, Amm CA and El Musa KA: Improved
scar quality following primary and secondary healing of cutaneous
wounds. Aesthetic Plast Surg. 27:411–417. 2003. View Article : Google Scholar : PubMed/NCBI
|