Introduction
Inguinal hernia is the most common type of hernia
(1). The development of novel
biological materials has led to a change in the therapeutic
strategies for inguinal hernia (2,3).
Open tension-free hernia surgery with applied mesh has decreased
the recurrence rate of inguinal hernias and reduced the
rehabilitation period compared with that of sutured repairs
(4). Several different
tension-free techniques have been developed and the use of mesh has
become increasingly common (5).
However, all hernioplasty techniques may present
postoperative complications associated with the scrotum and testis,
including hematomas, atrophy, sterility, tumefaction, ecchymosis
and hydrocele (6–8). Previous studies have shown that the
use of open tension-free hernioplasty with mesh is correlated with
spermatogenesis reduction and infertility (9,10).
Furthermore, the increase of hernia correction by means of
prosthesis and the interest in fertility later in life, added to
the fact that the mesh (foreign body) provokes a strong
fibroblastic reaction in the cicatrisation process of the posterior
wall of the inguinal canal whose effects on the structures and
function of the spermatic funiculus, epididium and testis are well
known (11).
A previous study conducted in the United States of
America found that tension-free repair of inguinal hernias resulted
in infertility after 0.5–3 years with vas deferens blockage.
It was proposed that it was correlated with the application of
polypropylene (PP) mesh (12).
Peiper et al (13) compared
tension-free hernia repair to the traditional method using pig and
rabbit experimental animal models. It was demonstrated that
following hernia repair, spermatic artery perfusion decreased and
spermatic vein thrombosis occurred in one-third of pigs, while the
traditional method induced no abnormal symptoms indicating that
spermatic vein thrombosis was closely associated with mesh
implantation. Furthermore, LeBlanc et al (14) found implanted mesh can cause
testicular venous congestion. The interaction between the spermatic
cord and hernia mesh can impact the structure of the inguinal canal
and sperm quality.
Hypoxia-inducible factor-1α (HIF-1α) is widely
involved in adaptive responses to hypoxia in mammalian cells. It is
an important regulatory factor involved in blood supply, oxidation
and energy metabolism (15). As
the mesh applied in (endoscopic application) inguinal hernia repair
is placed close to the vas deferens and spermatic vessels,
the mesh-induced inflammatory reaction may lead to dysfunction of
these structures. Therefore, HIF-1α expression was examined to
determine the effects of hernia mesh application.
A previous study identified that serum antisperm
antibodies (AsAbs) were found in patients following vasectomy, and
in monkeys a correlation between granuloma formation and the
development of sperm autoantibodies after vasectomy has been
reported (16). In addition,
researchers have demonstrated that tension-free hernia repair can
lead to occlusion of the vas deferens and sperm granuloma
formation (17); however, whether
the pathological changes result in an autoimmune reaction have not
been fully elucidated.
In the present study, hernia meshes of different
materials were applied, including heavy PP, expanded
polytetrafluoroethylene (e-PTFE) and lightweight PP (UP) meshes,
into adult male Spague-Dawley rats to examine the effects on
spermatic structure, testicular structure, semen change, the number
of spermatogenic cells, HIF-1α expression levels and serum
concentration of AsAbs. This study aimed to compare the hernia
meshes of different materials in order to determine the most
appropriate application and a theoretical basis for reducing the
negative effects of hernia mesh on the reproductive function.
Materials and methods
Experimental animals and meshes
Forty male Sprague-Dawley rats (Rattus
norvegicus) (age, 90 days; weight, 300–320 g) were obtained
from the Experimental Animal Center of Guangdong Medical College
(Zhanjiang, China). The animals were housed in a colony room under
a 12/12 h light/dark cycle at 21±2°C and had free access to water
and a complete diet ad libitum. The animals were randomly
divided into five groups as follows: The normal control (NC) group,
the sham-operated (FO) group, the e-PTFE mesh group, the PP mesh
group and the UP mesh group. The PP, UP and e-PTFE meshes were
purchased from C.R. Bard, Inc. (MarlexH; St. Louis, MO, USA),
Ethicon, Inc. (Ultrapro; Amersfoort, The Netherlands) and W.L. Gore
& Associates, Inc. (Flagstaff, AZ, USA), respectively. The PP
mesh is a monofilament, non-absorbable, inert, sterile and porous
mesh with a thickness of ~0.44 mm. The UP mesh is partially
absorbable with 3–4-mm pores consisting of non-absorbable PP
filaments and polyglactin filaments absorbed by hydrolysis. The
e-PTFE mesh is a 1-mm thick mesh made from strong, soft inert and
conformable e-PTFE with a structure that ensures early fixation to
the host tissue with minimal foreign body reaction. This study
conformed with the Guide for the Care and Use of Laboratory Animals
published by the USA National Institutes of Health (NIH Publication
no. 85–23, Revised 1985), and has been approved by the Animal Care
and Use Committee of Guangdong Medical College (Xiashan,
China).
Surgical procedure
The rats were anesthetized by intraperitoneal
injection of ketamine hydrochloride (50 mg/kg) and xylazine (10
mg/kg). The skin was shaved and disinfected with povidone-iodine.
The three different meshes (PP, UP and e-PTFE) were inserted
surrounding the vas deferens and spermatic vessels with a
4–0 nonabsorbable silk fixed needle. The skin was closed using a
nylon 4.0 needle in a continuous suture. The FO group was
established by the simple closure of the midline laparatomy by
continuous nylon 4.0 sutures. The NC group received no surgery. All
animals were sacrificed by the method of air embolism according to
the requirements of the Guangdong Medical Experimental Animal
Center 90 days after mesh implantation. During the 90 day
observation period all animals were observed daily to assess local
and systemic (wound)-complications.
Postoperative observations
The adhesion formation was evaluated by the
classification of adhesions as loose, firmly attached or
integrated. Quantification was performed by determining the surface
area of each prosthesis covered by adhesions as previously
described (18). The spermatic
cord tissue located on the side of implanted mesh was obtained and
fixed in 4% neutral formalin solution for 24 h. Tissues were
embedded in paraffin for hematoxylin and eosin staining to observe
the pathological changes in the inner diameter thickness and
cross-sectional area of the vas deferens, and to grade the
spermatogenic cells in the cross-sectional areas of individual
tissue slices using the Johnsen score method (19).
Percentage of spermatogenic cells
Enzymatic digestion of testicular tissue was
performed. Briefly, the tissue was divided into small pieces and
washed three times in D-Hank’s solution (Senbeijia, Nanjing, China)
to eliminate blood cells. Tissue was minced using ophthalmic
scissors (Stronger Medical Instrument Co., Ltd., Suzhou, China)
until a semi-liquid state was achieved. Following the addition of
0.25% trypsin (50-fold the amount of tissue), tissues were
transferred into a small glass flask and digested for 30 min at
37°C. Trypsin was then removed and the suspension was washed three
times with D-Hank’s solution at 560 × g for 5 min. Collagenase
(0.1%) was added and incubated for 30 min under similar conditions.
The cell suspension was centrifuged and pelleted cells were
resuspended in D-Hank’s solution and propidium iodide nucleic acid
was added to stain for 30 min. The cells were then analyzed using
flow cytometry (FACSCanto II flow cytometer; BD Biosciences,
Shanghai, China).
Sperm motility detection
The epididymis located on the side of implanted mesh
was removed and washed three times with Dulbecco’s
phosphate-buffered saline (D-PBS) to remove surface blood. It was
then placed in a sterile petri dish with 5 ml of preheated (37°C)
D-PBS. Deep, longitudinal sections were sheared with ophthalmic
scissors from the tail of the epididymis and placed in an incubator
at 37°C for 10 min, then 20 ml sperm suspension was pipetted onto a
clean, prewarmed sperm counter (Huafang Shenhuo Technology Co.,
Ltd., Beijing, China). Motility parameters were measured with an
automatic sperm analyzer (Hamilton Thorn Motility Analyzer, version
7.2; Hamilton Thorne Research, Beverly, MA, USA). At least five
widely spaced fields were examined to provide an estimated
percentage of motile cells. The sperm motility was evaluated using
the WHO classification system (WHO, 1999), with grades a (rapid
progressive or linear motility), b (slow progressive or curvilinear
motility), c (not progressive or in loco motility) and d (absent
motility) (20).
Immunohistochemistry (IHC) of HIF-1α in
testicular tissue
IHC was performed according to the methods described
previously. The tissue sections were pretreated, heated, blocked
and incubated with polyclonal rabbit anti-human HIF-1α (clone
H-206; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Negative controls, in which the primary antibody was substituted
with antibody diluent (Yajikit, Shanghai, China) were also
analyzed. The sections were imaged with a Nikon Eclipse 55i
microscope equipped with a Nikon DS-5M camera (Nikon, Tokyo,
Japan), using Image-Pro Plus acquisition software. Staining
intensity was assessed and five random fields of each slide were
analyzed with Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD,
USA) to calculate the mean optical density analysis value (mean
IOD).
ELISA to measure AsAb concentration
Venous blood of 4–5 ml was obtained from the rats
chest and centrifuged at 560 × g for 5 min. The supernatant was
collected in 1.5 ml eppendorf tubes and stored at −20°C prior to
analysis. Using ELISA, microtiter plates were coated with equal
quantities of the serum sample (50 μg) in each well and the
standard protocol of ELISA was followed using primary mouse
anti-human AsAb monoclonal IgG and secondary alkaline phosphatase
conjugated goat anti-mouse IgG (Chemicon, Temecula, CA, USA). The
results were measured at 405 nm using an ELISA reader (Quanta
Biotech, Surrey, UK). Standard curves for each of the substances
analyzed were included.
Statistical analysis
The measurement data were compared by one-way
analysis of variance. A least significant difference test (t-test)
was used to compare the mean values between groups. If the data did
not meet the normality and homogeneity, the Kruskal-Wallis rank sum
test was used. All data were analyzed using SPSS software, version
16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
General observations
The breathing and heart rate of the rats in each
group, and recovering activity, including drinking and eating, were
normal 6 h after surgery. There were two cases of wound infection
in the PP and e-PTFE groups, respectively; however, infection was
decreased seven days after treatment with iodophor disinfectant and
the mesh was not removed. No subcutaneous hematoma, effusion and
empyema were observed in each group. There were no fatalities
during this experiment.
Hernia meshes and spermatic cord
adhesion
Adhesions were graded according to an adhesion
scoring system (grade 0–3). No adhesions were observed in the NC
group and mild adhesions were identified in the groin area of the
FO group. Spermatic cord adhesions in the FO and e-PTFE groups were
thin and filmy. The adhesion area in the e-PTFE group was <25%
with a mean score of 1.0 at 3 months (P<0.05 compared with the
FO group). Adhesions in the UP group were dense at 3 months and the
adhesion area was >50% with a mean adhesion score of 2.5.
However, adhesions in the PP group were more dense and extensive,
having a mean score of 3 (P<0.05 compared with the FO group) and
an adhesion area of >50% (Table
I).
 | Table IDegree of adhesion between different
hernia meshes and the spermatic cord. |
Table I
Degree of adhesion between different
hernia meshes and the spermatic cord.
| Group | No. of cases | Degree of
adhesion |
|---|
| NC | 8 | 0.0 (0.0) |
| FO | 8 | 0.5 (0.0–1.0) |
| e-PTFE | 8 | 1.0 (0.0–1.0)a |
| PP | 8 | 3.0 (2.5–3.0)a |
| UP | 8 | 2.5 (2.0–3.0)a |
Testicular pathological changes and
spermatogenic cell classification
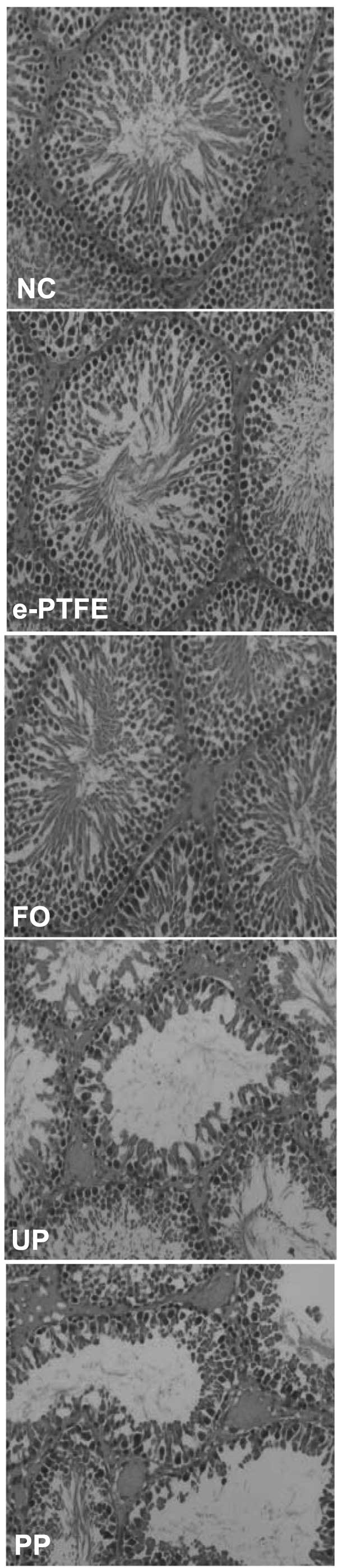
Histological analysis demonstrated that the process
of spermatogenesis was normal in the NC group with increased layers
of spermatogenic cells, higher sperm count, and fewer lumen of the
seminiferous tubules were obstructed by necrotic tissue. The FO and
e-PTFE groups were similar to the NC group, but a low sperm count
and less seminiferous tubules were observed. However, in the PP and
UP groups, an abnormal spermatogenesis process with disordered
seminiferous tubules, damaged germinal epithelium and reduced
spermatogenic cell layers was detected. A large number of sperm
cells were observed; however, few sperm were observed in the
seminiferous tubules and a section of the seminiferous tubule
cavity was congested by necrotic tissue (Fig. 1). Therefore, the Johnsen score
method was used to obtain the grade of spermatogenic cells in each
group (Table II).
 | Table IIGrade of spermatogenic cells in each
group. |
Table II
Grade of spermatogenic cells in each
group.
| Group | No. of cases | Scoring of
spermatogenic cell |
|---|
| NC | 8 | 10.0 (9.0–10.0) |
| FO | 8 | 9.0
(9.0–10.0)a |
| e-PTFE | 8 | 9.0
(9.0–10.0)a,b |
| PP | 8 | 8.0 (7.0–9.0)a,b |
| UP | 8 | 8.0 (8.0–9.0)a,b |
Influence of the different meshes on
sperm motility
The influence of PP, UP and e-PTFE meshes on sperm
motility was evaluated using the WHO classification system (WHO,
1999) by calculating the percentage of sperm with motility of
grades a+b. The percentage of sperm with motility of grades c+d was
eliminated due to the presence of apoptotic and necrotic
spermatozoa. Ninety days after surgery, no significant difference
was observed in the percentage of grade a+b sperm in the FO and
e-PTFE groups compared with that of the NC group (P>0.05). While
the percentage of sperm with a+b grade motility significantly
decreased compared with that of the NC and FO groups (P<0.05).
The percentage of sperm with grade a+b motile forms are shown in
Table III.
 | Table IIISperm percentage of a+b motile forms
in each group. |
Table III
Sperm percentage of a+b motile forms
in each group.
| Group | No. of cases | Grade a+b sperm
(%) |
|---|
| NC | 8 | 80.32±4.33 |
| FO | 8 | 69.83±8.83a |
| e-PTFE | 8 | 63.90±6.23a,b |
| PP | 8 | 37.25±11.54c–d |
| UP | 8 | 37.97±10.24c–f |
Spermatogenic cells detected by flow
cytometry
As C-values refer to the DNA content and ploidy
refers to the number of chromosomes, the detected cells were
classified as spermatogonia (2C), diploid primary spermatocytes
(4C) and haploid round spermatids (1C). Flow cytometry was used to
analyze the testicular germ cell population in rats. As shown in
Table IV, the difference in the
percentage of spermatogenic cells between the FO, e-PTFE and NC
groups were not statistically significant (P>0.05). The number
of 1C cells were significantly reduced in the PP and UP groups,
while the 2C and 4C cells markedly increased compared with that of
the NC, FO and e-PTFE groups.
 | Table IVPercentage of the different
populations of spermatogenic cells (mean ± standard deviation). |
Table IV
Percentage of the different
populations of spermatogenic cells (mean ± standard deviation).
| Group | No. of cases | 1C | 2C | 4C |
|---|
| NC | 8 | 56.66±5.59 | 24.09±4.97 | 16.25±3.51 |
| FO | 8 | 58.25±4.05a | 24.09±3.58a | 17.66±2.83a |
| e-PTFE | 8 | 56.85±4.22a,b | 23.95±4.08a,b | 19.20±3.95a,b |
| PP | 8 | 45.26±4.01b,d | 29.49±3.56b,d,e | 25.25±3.03b,d,e |
| UP | 8 | 44.08±5.00b,d–f | 30.26±4.16b,d–f | 25.66±3.04b,d–f |
Pathological changes in the vas
deferens
The cross-sectional area of the vas deferens
showed that the mucous membranes of the NC group were complete and
smooth, the lumen was unobstructed and sperm were detected in the
cavity. The FO and e-PTFE groups showed no significant changes
compared with the NC group. By contrast, the mucous membrane in the
cross-sectional area of the lumen in the PP and UP groups was
significantly reduced. The mucous membrane was disordered, sparse
or even absent (Fig. 2).
HIF-1α expression in testicular
tissue
To determine whether mesh affects the expression of
HIF-1α in the testis, IHC was performed and the mean IOD was
calculated. The expression of HIF-1α in the NC and FO groups was at
a minimal level (0.0023±0.0006 and 0.0026±0.0007, respectively).
Although the expression in the e-PTFE group showed a marginal
increase, the difference was not statistically significant compared
with the NC and FO groups. However, the expression levels of HIF-1α
in the PP and UP groups were significantly augmented (Table V and Fig. 3).
 | Table VExpression levels of HIF-1α in
testicular tissue of each group (mean ± standard deviation). |
Table V
Expression levels of HIF-1α in
testicular tissue of each group (mean ± standard deviation).
| Group | No. of cases | HIF-1α
expression |
|---|
| NC | 8 | 0.0023±0.0006 |
| FO | 8 |
0.0026±0.0007a |
| e-PTFE | 8 |
0.0042±0.0027a,c |
| PP | 8 |
0.0858±0.0209b,d,e |
| UP | 8 |
0.0936±0.0123b,d–f |
AsAb detection in the rat serum
The AsAb concentration in the NC group was expressed
at a minimal level. No statistically significant difference was
identified between the FO, e-PTFE and NC groups (P>0.05).
Compared with the NC, FO and e-PTFE groups, the concentration of
AsAbs was significantly increased and the differences were
statistically significant in the PP and UP groups (P<0.05)
(Table VI).
 | Table VIAsAb concentration in the serum of
each group (mean ± standard deviation). |
Table VI
AsAb concentration in the serum of
each group (mean ± standard deviation).
| Group | No. of cases | AsAb (pg/ml) |
|---|
| NC | 8 | 1.67±1.97 |
| FO | 8 | 6.81±3.44a |
| e-PTFE | 8 | 7.50±3.67a,c |
| PP | 8 |
114.72±11.57b,d,e |
| UP | 8 | 74.58±9.22b,d–f |
Discussion
At present, the incidence of male inguinal hernia is
~3% (21). In 1989, Lichtenstein
was the first to introduce the concept of tension-free hernia
repair with PP mesh and demonstrated its advantages, including low
recurrence and infection rates (22,23).
In the past few decades, the method of tension-free hernioplasty
with PP mesh has gradually been accepted by the majority of
surgeons and the number of applications is rapidly increasing.
Several studies have focused on the effects of
tension-free inguinal hernia repair in patients, particularly in
China (24). These studies have
shown excellent results with indices of low recidivism. Similar to
other surgical procedures, complications are always a risk factor
that must be considered. The application of hernia mesh to repair
inguinal hernia may induce long-term tissue reactions depending on
the type of mesh and location, and may be considered an etiological
factor, although the specific causes of tissue reaction remain
unknown. Considering the high incidence of inguinal hernia repairs
among male patients examined at infertility clinics, rats were
selected as models of inguinal hernia as their inguinal region is
similar to that of humans.
In this study, meshes of different materials
(e-PTFE, PP and UP) were used for hernia surgery. There was one
case of wound infection observed in the PP and e-PTFE groups;
however, administration of iodophor disinfectant healed the wound
infection after seven days and prevented mortality. The macroporous
biomaterials, PP and UP, induced a higher degree of adhesion
formation. By contrast, the expanded e-PTFE mesh achieved total
integration with newly formed surrounding tissue and increased the
resistance to adhesions with the spermatic cord. Furthermore,
e-PTFE did not cause significant changes to the vas
deferens, testicular tissue and AsAb concentration; therefore,
demonstrating effective protection of testicular function. In the
PP and UP groups, the pathological changes between adherence with
mesh in the spermatic funiculus was detected in all the animals,
including the jeopardizing of the deferent duct. Similar aspects
were also found in other studies (6,25).
The reduction of the lumen of the deferent duct on the mesh side
could also be induced by application of the PP and UP mesh.
HIF-α expression levels were significantly increased
in the PP and UP groups compared with the FO group. In addition,
basement membrane distortions in the rats seminiferous tubule,
disordered germinal epithelial and reduced spermatogenic cell
layers with rare sperms were observed in the PP and UP groups. Flow
cytometry also confirmed a decrease in the total number of germ
cells with reduced 1C and increased 4C. As HIF-α is a vital
regulatory factor associated with the blood supply, oxidation,
energy metabolism and gene transcription, it was hypothesized that
increased testicular tissue ischemia and hypoxia damage to the
basement membrane of endothelial and sertoli cells in the
seminiferous tubules may lead to the destruction of the
blood-testis barrier, activating autoimmune reaction to produce
AsAbs and result in infertility by direct interaction with sperm or
indirect change to the local microenvironment.
The vas deferens is the only channel for
mature sperm to be transported out of the epididymis (26); changes to the inner environment
results in infertility. In this study, examination of the vas
deferens showed that the cross-sectional area of the PP and UP
groups were significantly reduced with sparse mucosal folds 90 days
after surgery. In the e-PTFE group, no significant changes were
observed in the vas deferens compared with the NC and FO
groups. This may be due to the smooth surface and anti-adhesion
nature of e-PTFE mesh, which exhibited protective effects between
the vas deferens and spermatic vessels. It has been reported
that inguinal hernia tension-free repair can lead to sperm
granuloma (17); however, this
phenomenon was not observed in our experiment.
In conclusion, in this study >50% of mesh
adherence to the spermatic cord in the PP and UP groups was
identified, while no adhesion or mild adhesion was observed in the
NC, FO and e-PTFE groups. In the UP and PP groups, marked
congestion of necrotic tissue in the seminiferous tubule cavity, a
significant reduction of grade a+b sperm percentage and a
considerable statistical increase in the mean level of AsAbs and
HIF-α. By contrast, the e-PTFE mesh demonstrated only marginal
affects on the reproductive function of rats. Thus, it was
concluded that e-PTFE mesh had less impact on the reproductive
function compared with that of the PP and UP meshes. Therefore,
e-PTFE mesh is more suitable for tension-free hernioplasty and
should be selected for clinical application in patients with
inguinal hernia.
References
|
1
|
Eklund A, Carlsson P, Rosenblad A, et al:
Long-term cost-minimization analysis comparing laparoscopic with
open (Lichtenstein) inguinal hernia repair. Br J Surg. 97:765–771.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
López Cano M, Armengol Carrasco M, Quiles
Pérez MT and Arbós Vía MA: Biological implants in abdominal wall
hernia surgery. Cir Esp. 91:217–223. 2013.(In Spanish).
|
|
3
|
Yazdankhah Kenary A, Afshin SN, Ahmadi
Amoli H, et al: Randomized clinical trial comparing lightweight
mesh with heavyweight mesh for primary inguinal hernia repair.
Hernia. 17:471–477. 2013.PubMed/NCBI
|
|
4
|
Nathan JD and Pappas TN: Inguinal hernia:
an old condition with new solutions. Ann Surg. 238:S148–S157. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Birch C and Fynes MM: The role of
synthetic and biological prostheses in reconstructive pelvic floor
surgery. Curr Opin Obstet Gynecol. 14:527–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shin D, Lipshultz LI, Goldstein M, et al:
Herniorrhaphy with polypropylene mesh causing inguinal vasal
obstruction: a preventable cause of obstructive azoospermia. Ann
Surg. 241:553–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suradom C and Palaphun J: The usage of two
umbrella made-mesh plugs in herniorrhaphy: comparative study with
Bassini and Lichtenstein method. J Med Assoc Thai. 94:1373–1379.
2011.PubMed/NCBI
|
|
8
|
Kogan BA: Communicating hydrocele/hernia
repair in children. BJU Int. 100:703–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peiper C, Junge K, Klinge U, Strehlau E,
Ottinger A and Schumpelick V: Is there a risk of infertility after
inguinal mesh repair? Experimental studies in the pig and the
rabbit. Hernia. 10:7–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aydede H, Erhan Y, Sakarya A, Kara E,
Ilkgül O and Can M: Effect of mesh and its localisation on
testicular flow and spermatogenesis in patients with groin hernia.
Acta Chir Belg. 103:607–610. 2003.PubMed/NCBI
|
|
11
|
Goldenberg A and Paula JF: Effects of the
polypropylene mesh implanted through inguinotomy in the spermatic
funiculus, epididium and testis of dogs. Acta Cir Bras. 20:461–467.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kolbe T and Lechner W: Influence of
hernioplastic implants on male fertility in rats. J Biomed Mater
Res B Appl Biomater. 81:435–440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peiper C, Junge K, Bühner A, Bassalay P
and Schumpelick V: Load on the inguinal region under standard
conditions in pigs. Eur J Surg. 167:356–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeBlanc KA, Booth WV, Whitaker JM and
Baker D: In vivo study of meshes implanted over the inguinal ring
and external iliac vessels in uncastrated pigs. Surg Endosc.
12:247–251. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fernández-Braña M: Hypoxia: a therapeutic
target. Clin Transl Oncol. 7:475–476. 2005.(In Spanish).
|
|
16
|
Anderson DJ and Alexander NJ: Consequences
of autoimmunity to sperm antigens in vasectomized men. Clin Obstet
Gynaecol. 6:425–442. 1979.PubMed/NCBI
|
|
17
|
Silich RC and McSherry CK: Spermatic
granuloma. An uncommon complication of the tension-free hernia
repair. Surg Endosc. 10:537–539. 1996.PubMed/NCBI
|
|
18
|
Walker AP, Henderson J and Condon RE:
Double-layer protheses for repair of abdominal wall defects in
rabbit model. J Surg Res. 55:32–37. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnsen SG: Testicular biopsy score count
- a method for registration of spermatogenesis in human testes:
normal values and results in 335 hypogonadal males. Hormones.
1:2–25. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xue J, Yang J, Yan J, et al: Abnormalities
of the testes and semen parameters in clinical varicocele. Nan Fang
Yi Ke Da Xue Xue Bao. 32:439–442. 2012.PubMed/NCBI
|
|
21
|
Zendejas B, Ramirez T, Jones T, et al:
Incidence of inguinal hernia repairs in Olmsted County, MN: a
population-based study. Ann Surg. 257:520–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bierca J, Kosim A, Kołodziejczak M, Zmora
J and Kultys E: Effectiveness of Lichtenstein repairs in planned
treatment of giant inguinal hernia - own experience. Wideochir Inne
Tech Malo Inwazyjne. 8:36–42. 2013.PubMed/NCBI
|
|
23
|
Pielaciński K, Szczepanik AB, Misiak A and
Wróblewski T: Randomized clinical trial comparing inguinal hernia
repair with Lichtenstein technique using non-absorbable or
partially absorbable mesh. Preliminary report. Wideochir Inne Tech
Malo Inwazyjne. 6:190–206. 2011.
|
|
24
|
Wang WJ, Chen JZ, Fang Q, Li JF, Jin PF
and Li ZT: Comparison of the effects of laparoscopic hernia repair
and lichtenstein tension-free hernia repair. J Laparoendosc Adv
Surg Tech A. 23:301–305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Junge K, Binnebösel M, Kauffmann C, et al:
Damage to the spermatic cord by the Lichtenstein and TAPP
procedures in a pig model. Surg Endosc. 25:146–152. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Da Silva N, Silberstein C, Beaulieu V, et
al: Postnatal expression of aquaporins in epithelial cells of the
rat epididymis. Biol Reprod. 74:427–438. 2006.PubMed/NCBI
|