Introduction
Lung cancer is the most frequent cancer-related
cause of death throughout the world, with a poor (<15%) 5-year
survival rate (1). More effective
approaches to the treatment and prevention of lung carcinoma depend
on a better understanding of the cellular and molecular mechanisms
that control lung tumor growth. During carcinogenesis, alterations
of the nuclear structure usually manifest as deformations of the
nuclear matrix architecture (2–4).
Changes in nuclear structure, which are largely determined by the
nuclear matrix, have long been recognized to correlate with tumor
growth and progression, prompting their use as biomarkers for the
diagnosis of cancer (5).
The nuclear matrix consists of the nuclear lamins,
RNA, and a fibrogranular network of proteins that include >200
nuclear matrix proteins (NMPs) (6). Numerous NMPs are involved in
important cellular functions, including gene transcription, steroid
hormone binding, and RNA processing. Functional changes in these
proteins have been associated with carcinogenesis (7–9), and
many NMPs have been identified as unique ‘fingerprint’ markers for
cancers of the colon (2), bladder
(10), kidney (11), prostate (12) and breast (13). For example, Partin et al
found that the level of the NMP PC-1 is specifically elevated in
prostatic cancer tissue compared to benign prostatic hyperplasia
and normal prostatic tissue (14).
Getzenberg et al found that BLCA-4 expression was detectable
before tumors were identified, highlighting the diagnostic
significance of this NMP in transitional cell carcinoma (10).
The human protein THO complex 1 (Thoc1), also called
hHpr1/p84, was originally identified as a nuclear matrix component
protein that binds to the tumor suppressor retinoblastoma protein
(pRb) (5). High levels of Thoc1,
observed in breast and lung cancer cells, have been associated with
tumor size and aggressiveness (15,16).
Thoc1 is expressed in most tissues throughout the cell cycle,
except for the G0 phase (17).
Overexpression of Thoc1 can induce p53-independent apoptosis that
is inhibited by binding of Thoc1 to pRb (18,19).
It was additionally reported that depletion of Thoc1 sensitizes
cancer cells to the cytotoxic effects of DNA damage (20).
This study was undertaken to evaluate the effect of
Thoc1 on lung cancer cell proliferation, cell cycle and apoptosis
by creating stable transfectants and evaluating their in
vitro growth potential. Furthermore, we examined whether the
effects of Thoc1 may be also observed in vivo, using nude
mice. We show, for the first time to the best of our knowledge,
that overexpression of Thoc1 inhibits lung cancer cell growth.
Materials and methods
Cell cultures
Human lung cancer cell lines SPC-A1 and NCI-H1975
were obtained from the Shanghai Cell Bank (Shanghai, China). Cells
were cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
containing 10% fetal bovine serum in a humidified atmosphere with
5% CO2 at 37°C.
Generation of stable cell lines
To generate stable cell lines overexpressing Thoc1,
the cDNA encoding the human full-length Thoc1 gene was amplified by
PCR using the following primers: forward,
5′-TTCCTCGAGATGTCTCCGACGCCGC-3′ and reverse,
5′-CCGGATCCACTATTTGTCTCATTGTC-3′. Next, the full-length cDNA was
cloned into the linearized plasmid vector pcDNA3 (Clontech
Laboratories, Inc., Mountain View, CA, USA) between the XhoI
and BamHI restriction sites. The resulting expression vector
pcDNA3/Thoc1 and the control empty vector pcDNA3 were transfected
in SPC-A1 and NCI-H1975 cells using Lipofectamine 2000 (Invitrogen
Life Technologies, Carlsbad, CA, USA). Stable clones were selected
in medium containing 800 or 500 μg/ml G418 (Sigma-Aldrich, St.
Louis, MO, USA). Individual clones were isolated and grown for
further characterization.
Western blot analysis
Cells were lysed with sodium dodecyl sulfate (SDS)
buffer (80 mM Tris-HCl, 2% SDS, 300 mM NaCl and 1.6 mM EDTA).
Proteins were separated using 10% SDS-polyacrylamide gel
electrophoresis, transferred onto a polyvinylidene difluoride
membrane and blocked with 5% skimmed milk. Membranes were next
incubated with antibodies targeting β-actin, Thoc1 (Abcam Inc.,
Cambridge, MA, USA), cyclin A1, cyclin B1, cyclin D1, Bax (Cell
Signaling Technology, Inc., Danvers, MA, USA), Bcl-2 (EMD Millipore
Corp., Billerica, MA, USA), caspase-3 (Abcam Inc.) and then
incubated with HRP-conjugated anti-mouse or -rabbit IgG antibodies
(Cell Signaling Technology, Inc.). Protein bands were visualized
using an enhanced chemiluminescence (ECL) solution (EMD Millipore
Corp.).
Quantitative PCR (qPCR)
Total RNA was extracted using TRIzol (Gibco-BRL,
Grand Island, NY, USA) and treated with RNase-free DNase I to
remove genomic DNA (Roche Diagnostics, Indianapolis, IN, USA). cDNA
was prepared from 1 μg of total RNA using M-MLV reverse
transcriptase (MBI; Fermentas, Waltham, MA, USA). Amplifications
were performed on an ABI Prism 7300 PCR system (Applied Biosystems,
Foster City, CA, USA) and reactions were prepared using the
SYBR® Premix Ex Taq™ kit (Takara Bio, Dalian, China).
The sequences of the primers for amplification of the human genes
Thoc1 and β-actin (considered as housekeeping) were as follows:
Thoc1 forward, 5′-CTGTGGACGGATTCAGCTCT-3′ and reverse,
5′-AGAGCACGTTGTTGGAGCTT-3′; β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′.
Standard curves were generated for each gene. The amplification was
90–110% efficient. Relative quantification of gene expression was
determined by comparison of threshold cycle values. The expression
level of all genes was normalized to that of β-actin.
Cell proliferation assay
Cell proliferation was measured with the MTT assay.
Cells (5×103) were plated in 24-well plates and 1 ml of
culture medium was added to each well. The cells were incubated at
37°C for 1, 2, 3, 4, 5, 6 or 7 days (d), and incubated with 500 μl
of MTT solution (1 g/l; Sigma-Aldrich) for an additional 2 h. The
reaction was stopped by the addition of 500 μl dimethylsulfoxide
(Sigma-Aldrich) and the absorbance of samples was then measured at
570 nm. A growth curve was plotted for each sample as the
absorbance vs. time. Three independent experiments were performed,
and the results were used for calculating the relative growth rate
± SD.
Flow cytometry analysis of cell cycle and
apoptosis
For cell cycle analysis, after 24 h of culture,
cells were collected and digested with trypsin, and fixed overnight
with 75% ice-cold ethanol at 4°C. Cells (1×106) were
centrifuged at 106 × g for 5 min, and the cells were resuspended
and incubated in 500 μl propidium iodide (50 μg/ml; Sigma-Aldrich)
for 30 min in the dark before analysis. The cell cycle profiles
were assessed by measurements, at 488 nm, on a FC500 flow cytometer
(Beckman Coulter, Brea, CA, USA), and the data were analyzed using
the Multicycle software (Phoenix Flow Systems, Inc., San Diego, CA,
USA). For the analysis of apoptosis, cells were collected and
digested with trypsin, processed as described in the Annexin V-FITC
Apoptosis Detection kit (BD Biosciences, San Diego, CA, USA) and
analyzed on a FC500 flow cytometer.
In vivo studies
Four-week-old female nude specific pathogen-free
(SPF) BALB/c mice were obtained from the Laboratory Animal Center
of Soochow University, and kept in a room at constant temperature
(23±2°C) and humidity (50–70%) with a 12 h light-dark cycle. SPC-A1
cells and their transfectants were trypsinized and harvested,
washed with phosphate-buffered saline (PBS) and resuspended in 0.2
ml PBS (1×107 cells/0.2 ml). They were subcutaneously
injected into the oxter of the nude mice. Each study group
comprised six nude mice. Every three or four days, the tumor
diameter was measured, and the volume was calculated according to
the formula V = (W2 × L)/2, where W denotes tumor width
and L tumor length. The growth curve of each tumor was plotted and
the tumor growth ratio was calculated. Five weeks after injection
of the cells, the mice were sacrificed, and tumors were collected
for histological analysis. The animal treatment protocol used in
this study was approved by the Institutional Animal Care and Use
Committee of Soochow University (Suzhou, China).
Immunofluorescence microscopy
analysis
Tumors were analyzed using immunofluorescence
analysis. The tumor xenografts were removed and fixed in 10%
phosphate-buffered formaldehyde at room temperature for 48 h,
embedded in paraffin, and sectioned at 5 μm. The sections were
deparaffinized, rehydrated, antigens were retrieved in Tris/EDTA
buffer at 100°C for 15 min, and left in the buffer for 10 min after
boiling. Following a rinse in distilled water and PBS, the sections
were treated with 0.03% hydrogen peroxide for 5 min to block
endogenous peroxidase activity, then incubated with mouse
anti-human anti-Thoc1 (1:100; Abcam Inc.) and -Ki-67 antibody
(1:100; Cell Signaling Technology, Inc.) overnight at 4°C,
Following a rinse in PBS, the sections were incubated with Alexa
Fluor 488/633-conjugated anti-rabbit antibody (1:1,000; Cell
Signaling Technology, Inc.) for 30 min at room temperature. Next,
they were rinsed in PBS, and the cell nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) for 5 min at room temperature.
The sections were finally prepared for confocal microscopy, and
images were recorded and analyzed with the TCS SP2 software (Leica,
Wetzlar, Germany).
Statistical analysis
Each experiment was repeated 3 times. Results are
expressed as mean ± SD. A P-value <0.05 was considered to
indicate statistically significant differences. Statistical
analyses were performed using the SPSS 17.0 software (IBM, Armonk,
NY, USA).
Results
Expression of Thoc1 in lung breast cancer
cell lines and generation of stable cell lines
To investigate the potential role of Thoc1 in human
lung cancer, we first detected the expression of Thoc1 in a panel
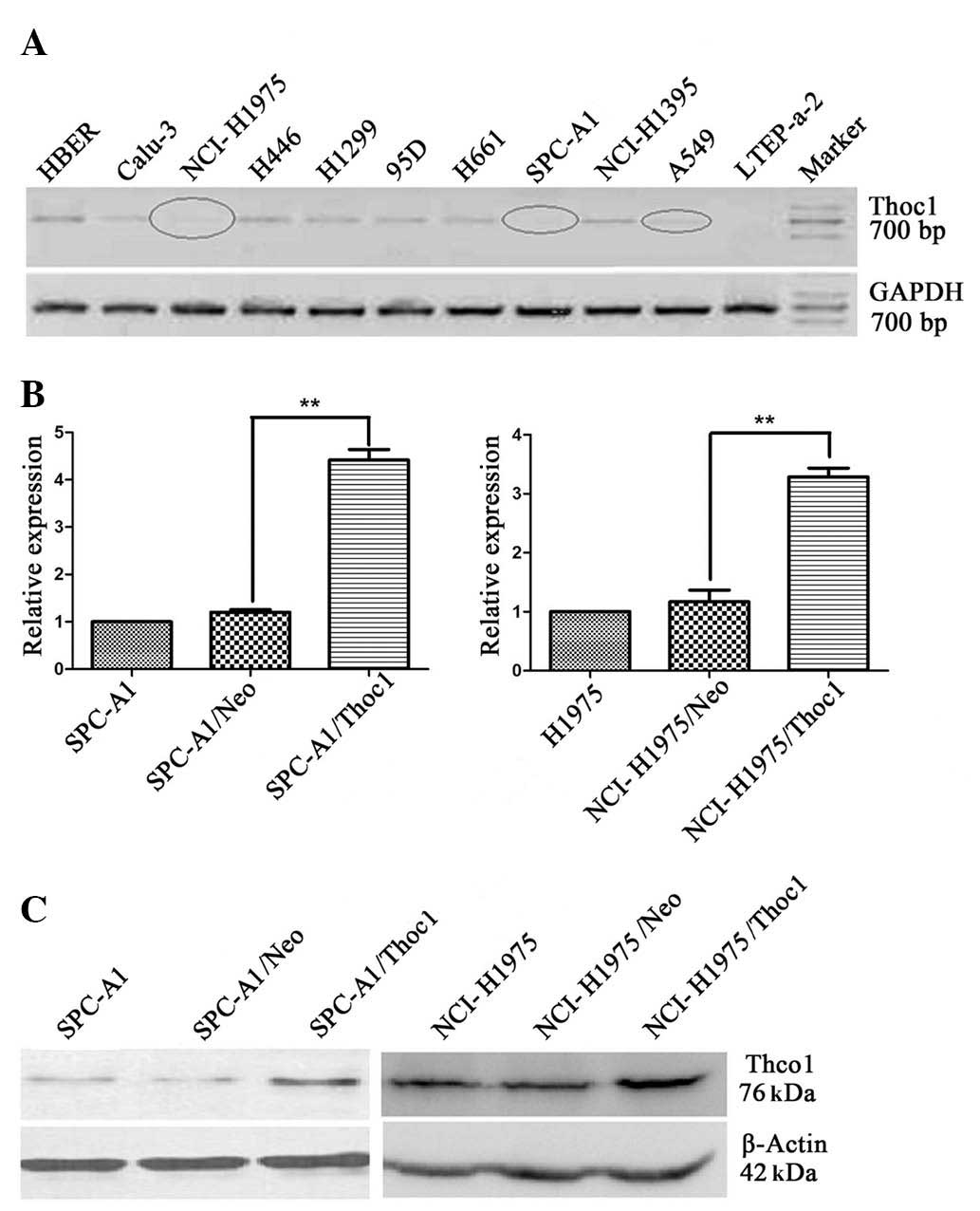
of human lung cancer cell lines. The mRNA level of Thoc1 in these
cell lines was determined by qPCR. As shown in Fig. 1A, the lowest Thoc1 mRNA level was
observed in SPC-A1 and NCI-H1975 cells. To further explore the role
of Thoc1 in lung cancer cells, SPC-A1 and NCI-H1975 cells were
transfected with recombinant vector expressing Thoc1 (pcDNA3/Thoc1)
or a control empty vector (pcDNA3/Neo). G418-resistant mix clones
were selected for further experiments. The Thoc1 mRNA and protein
levels in SPC-A1 and NCI-H1975 cells were measured by qPCR
(Fig. 1B) and western blot
analysis (Fig. 1C), respectively.
When compared to control cells, Thoc1 expression was significantly
increased in the cells transfected with the pcDNA3/Thoc1 sense
vector (SPC-A1/Thoc1 and NCI-H1975/Thoc1 cells).
Overexpression of Thoc1 inhibits lung
cancer cell proliferation
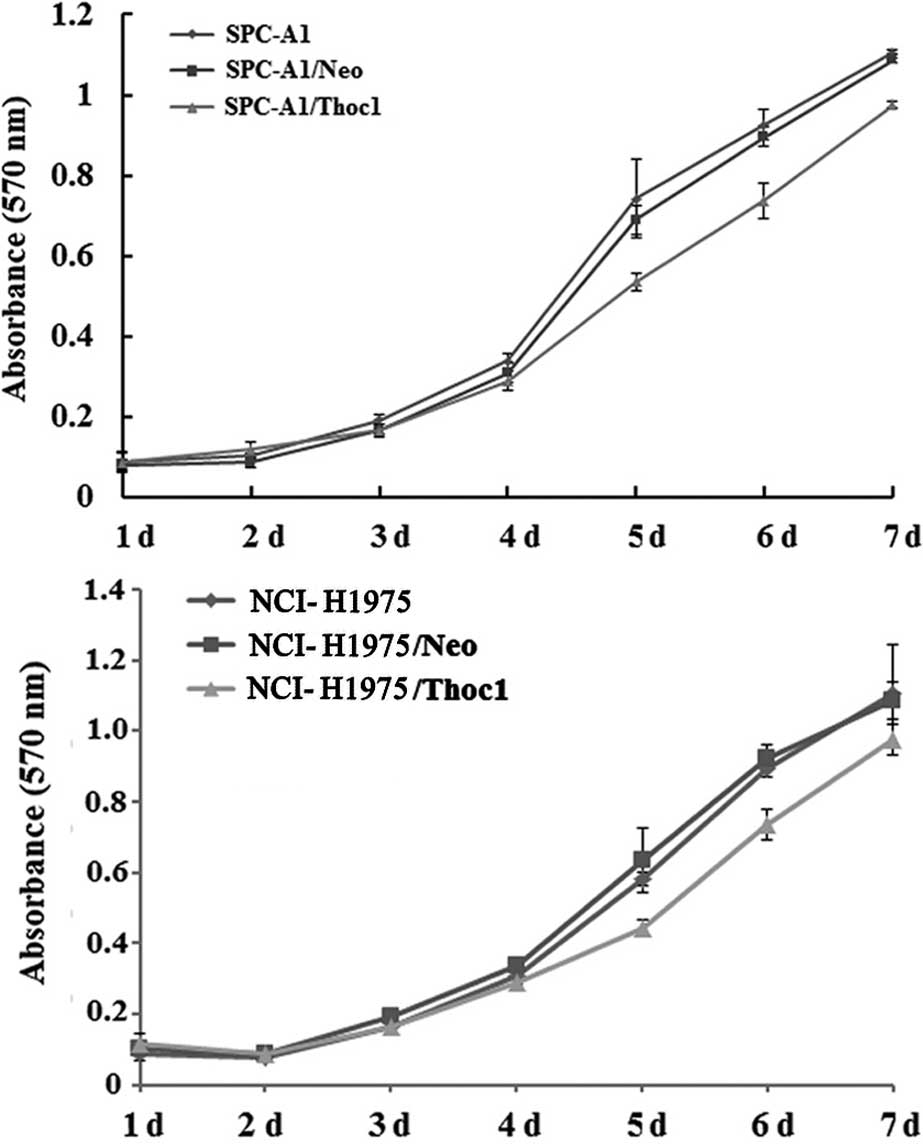
First, we addressed the question whether Thoc1 is
involved in regulation of lung cancer cell proliferation, using the
MTT assay to determine cell proliferation. The untransfected SPC-A1
or NCI-H1975 cells, the control cells (transfected with empty
vector), as well as the SPC-A1/Thoc1 or NCI-H1975/Thoc1 cells were
grown in culture for 7 days. The proliferative ability of
SPC-A1/Thoc1 and NCI-H1975/Thoc1 cells decreased compared to
untransfected and control cells, in a time-dependent manner
(Fig. 2).
Overexpression of Thoc1 induces G2/M cell
cycle arrest in lung cancer cell lines
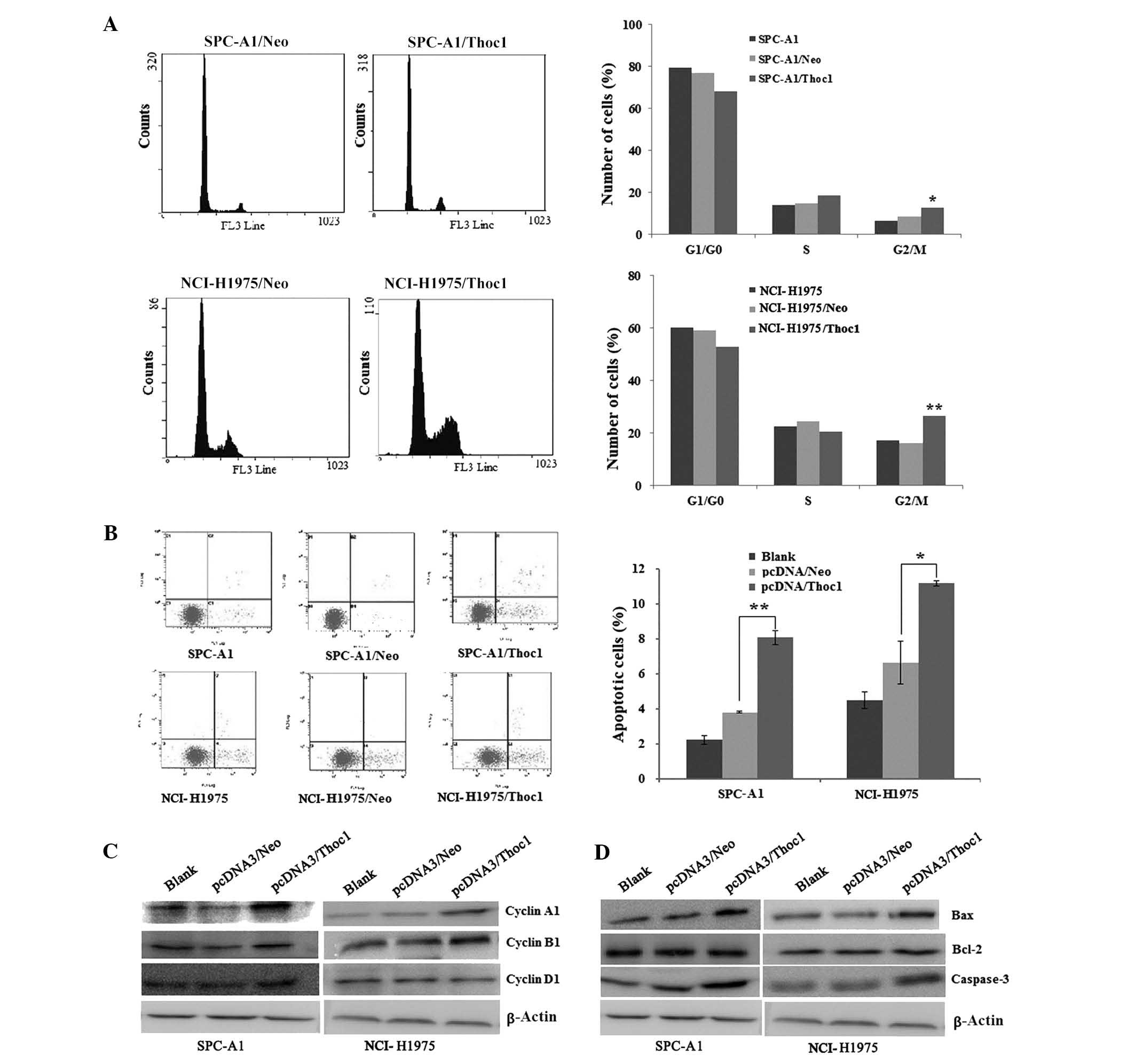
Because overexpression of Thoc1 led to inhibition of
lung cancer cell proliferation, we asked whether it can also
influence progression of the cell cycle. To address this question,
we conducted a cell cycle analysis and assessed the expression of
proteins related to the cell cycle before and after transfection
with the Thoc1 expression vector. Notably, increased amounts of
cells at the G2/M phase were observed in SPC-A1/Thoc1 and
NCI-H1975/Thoc1 cells compared to the controls and the
untransfected cells. In addition, a shift at the G1 phase was
detected (Fig. 3A).
To further characterize the molecular mechanism
underlying the G2/M cell cycle arrest, we studied the expression of
the main proteins related to the cell cycle before and after
transfection with the Thoc1 expression vector. As shown in Fig. 3C, overexpression of Thoc1 led to a
significant increase in the cyclin B1 and A1 expression levels,
which correlates with the increase in the population of
G2/M-arrested cells.
Overexpression of Thoc1 promotes cell
apoptosis in lung cancer cell lines and changes in Bcl-2, Bax and
caspase-3 expression
The extent of apoptosis was investigated by
estimating the number of cells stained with Annexin V, which is a
marker of early-stage apoptosis. In this assay, the percentage of
apoptotic cells was higher for the SPC-A1/Thoc1 and NCI-H1975/Thoc1
cells compared to control or untransfected cells (Fig. 3B). To further characterize the
molecular mechanism underlying the induction of cell apoptosis, we
detected the expression levels of anti-apoptotic factor Bcl-2 and
pro-apoptotic factors Bax and caspase-3 using western blot
analysis. As shown in Fig. 3D,
overexpression of Thoc1 increased the expression of the
pro-apoptotic proteins Bax and caspase-3 and did not affect Bcl-2
expression in a significant manner in SPC-A1/Thoc1 and
NCI-H1975/Thoc1 cells. These results indicated that the promotion
of cell apoptosis by Thoc1 is most likely mediated by Bcl-2, Bax
and caspase-3 proteins in lung cancer cells.
Overexpression of Thoc1 inhibits
xenograft formation and growth in vivo
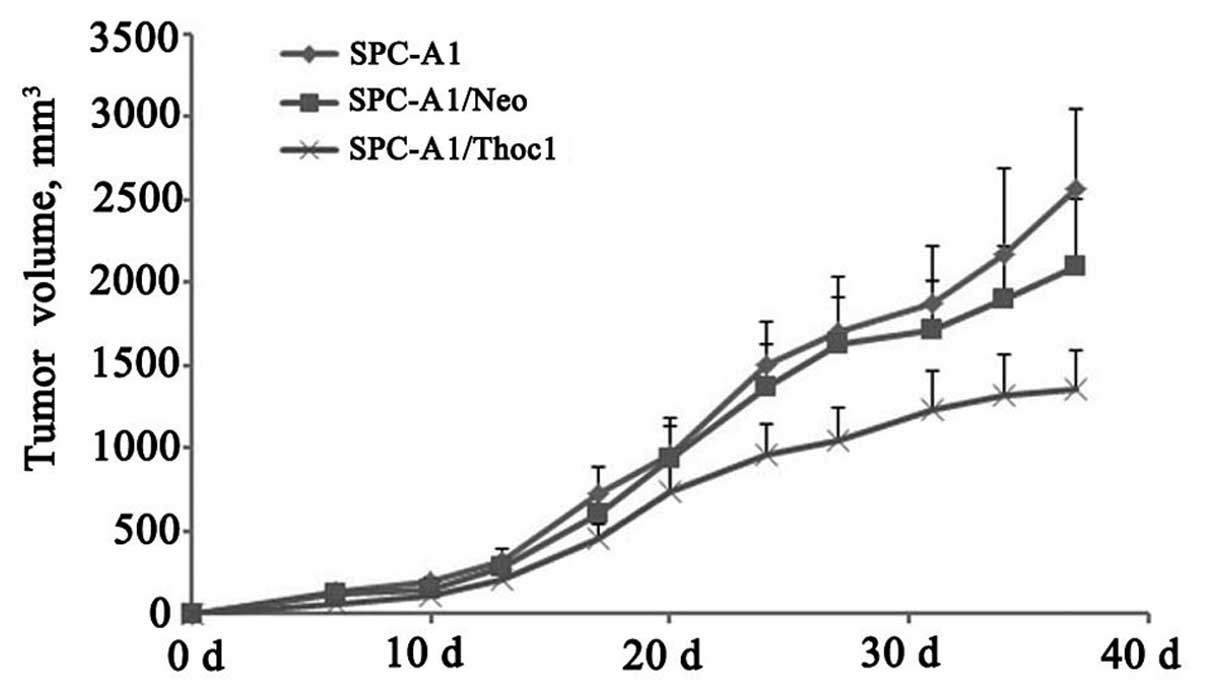
In vitro experiments with the SPC-A1 and
NCI-H1975 cells showed that the overexpression of Thoc1 can induce
G2/M cell cycle arrest and apoptosis, and inhibit cell growth.
Hence, we examined whether this effect could be also observed in
vivo. The transfected (pcDNA3/Thoc1 or negative control
pcDNA3/Neo) and untransfected SPC-A1 cells were subcutaneously
injected into nude mice (n=6 per group). After five weeks of
growth, the tumor masses obtained from the SPC-A1/Thoc1 cell
xenografts were markedly smaller than those from the control mice
(Fig. 4, P<0.05).
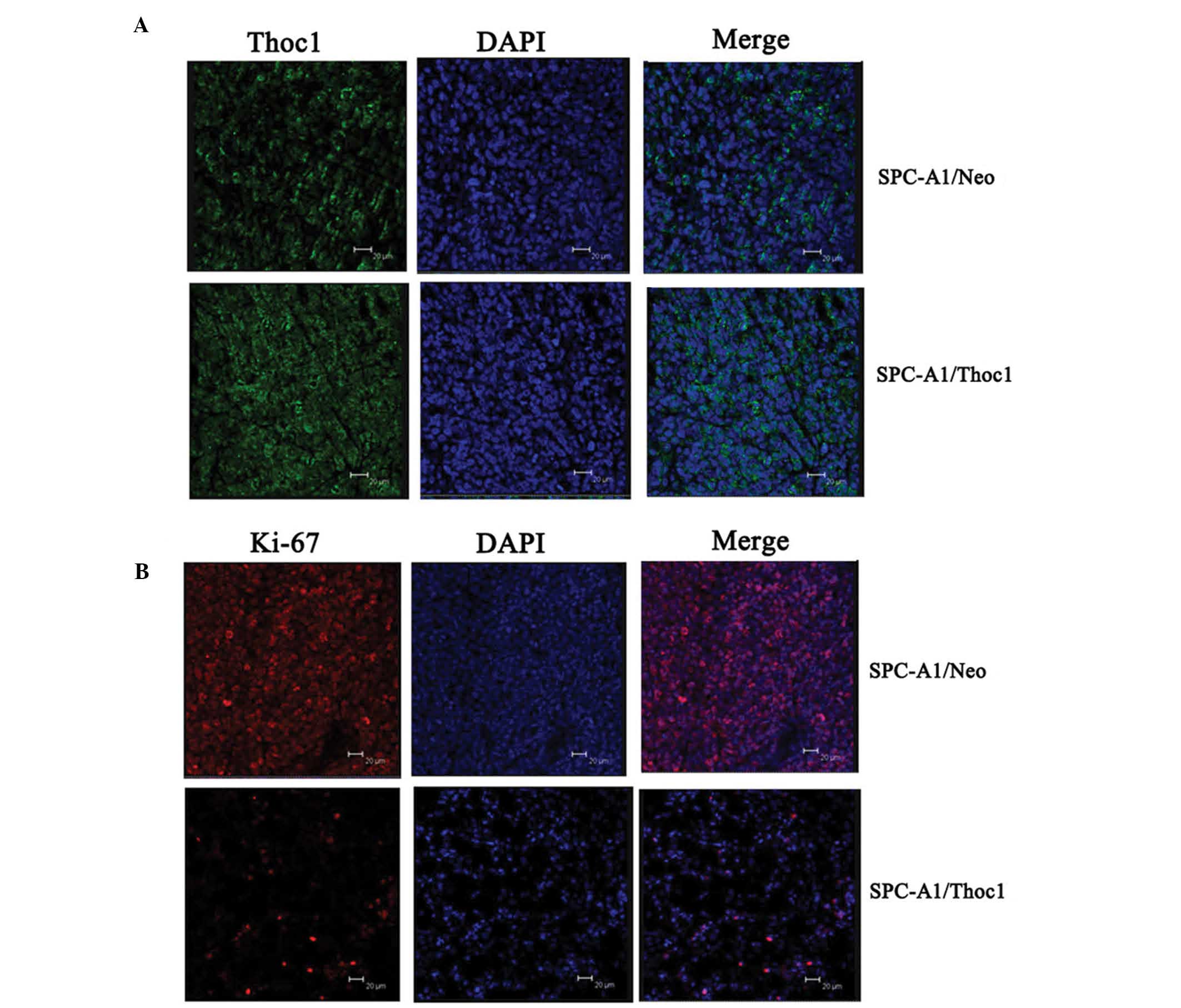
Immunofluorescence staining showed that Thoc1 expression was higher
in the SPC-A1/Thoc1 cell tumors (Fig.
5A), and that the protein localized on the nuclear membrane and
appeared to be concentrated in localized spots without the nucleus.
By contrast, Ki-67 appeared less expressed in the SPC-A1/Thoc1 cell
tumors (Fig. 5B) and localized on
the nuclear membrane. These results strongly supported the in
vitro observations and indicated that Thoc1 might play an
important role in lung cancer cell growth.
Discussion
Thoc1 has been identified as a human nuclear matrix
protein for more than a decade (5). In a previous study, Thoc1 expression
tended to associate with poor survival in subgroups of patients who
were at an early tumor stage, had tumors of squamous cell type, or
had a family history of lung cancer (16). The exact role of Thoc1 in lung
cancer has however not been elucidated. In this study, we
investigated the role of Thoc1 in growth of lung cancer cells. The
Thoc1 expression vector induced overexpression of the Thoc1 mRNA
and protein in lung cancer cells, which further showed reduced
proliferation, G2/M cell cycle phase arrest and apoptosis.Nude mice
injected with Thoc1-overexpressing cells also showed reduced
xenograft formation and in vivo growth. Our findings show
that Thoc1 plays a role in lung cancer cell growth. Furthermore, in
an attempt to elucidate the mechanisms underlying the observed
effects, we obtained evidence that Thoc1 may regulate key genes of
cell cycle and apoptosis.
Cell cycle deregulation resulting in uncontrolled
cell proliferation is one of the most frequent alterations that
occur during tumor development. The G2/M checkpoint regulators
cyclin A1 and B1 are markers of G2/M cell cycle arrest induced by
DNA damage. The expression level of cyclin A1 and B1 is minimal at
the initiation of S phase and peaks at the G2/M checkpoint
(21,22). In this study, we found that
overexpression of Thoc1 can affect cell cycle distribution in the
lung cancer cell lines SPC-A1 and NCI-1975, induce G2/M phase
arrest and reduce the population of cells at the G1/G0 phase.
Furthermore, we found that cyclin A1 and B1 protein levels
increased in response to Thoc1 overexpression in the lung cancer
cell lines SPC-A1 and NCI-H1975, indicating that this increase
might cause the increase in the population of G2/M-arrested cells.
The cyclin D1 protein level did not significantly change in
response to Thoc1 overexpression.
Apoptosis has been considered the major form of
cancer cell death, and occurs through two pathways: The extrinsic
or cytoplasmic pathway, and the intrinsic or mitochondrial one.
Bcl-2 family proteins are in part controlling the intrinsic pathway
(23,24). This family is composed of various
pro- and anti-apoptotic proteins that heterodimerize and modulate
each other’s function. Thus, the relative concentration of each
Bcl-2 family member is thought to determine whether programmed cell
death will occur. The ratio of anti-apoptotic Bcl-2 to
pro-apoptotic Bax is a critical determinant of apoptosis, as Bcl-2
heterodimerizes with Bax, blocking apoptosis (25). In this study, we demonstrated that
overexpression of Thoc1 induces lung cancer cell apoptosis,
accompanied by a rise in Bax and caspase-3 levels, while no
significant changes in the level of Bcl-2 were observed. These
results indicate that the inhibitory effect of Thoc1 on cell
apoptosis most likely involves a reduction in the Bcl-2/Bax ratio.
Bax upregulation was reported to result in loss of the
mitochondrial transmembrane potential and cytochrome C release,
followed by activation of the caspase cascade (26).
An important finding of the present study is that
Thoc1 inhibited lung cancer cell growth in vitro and in
vivo, as confirmed by our animal model. The growth rate of
established Thoc1-overexpressing xenografts was slower compared to
the empty vector control group. The Ki-67 protein is a marker of
proliferation, and is strongly linked to cell cycle control. During
mitosis, phosphorylation and dephosphorylation of Ki-67 occur at
the breakdown and the reorganization of the nucleus, two hallmark
events of the cell cycle (27,28).
Molecular functions proposed for Ki-67 include organization and
maintenance of the DNA architecture and synthesis of ribosomes
during mitosis (29,30). There is a positive correlation
between Ki-67 protein expression, cell proliferation rate, and the
active phase of the cell cycle in invasive breast carcinoma
(31,32). In this study, immunofluorescence
staining indicated that Ki-67 expression was lower in the
SPC-A1/Thoc1 cell tumors, and that the protein localized on the
nuclear membrane. These data suggest that Thoc1-mediated inhibition
of tumor growth may involve a decrease in the in vivo
expression of Ki-67.
In summary, our study, investigating the role of
Thoc1 in lung cancer cell growth, showed that this protein induces
cell phase arrest at G2/M, accompanied by an accumulation of cell
cycle-related proteins, changes in the expression level of several
proteins that directly relate to cell apoptosis, and a reduction in
both in vitro and in vivo cell growth. These findings
provide new insights into the role of Thoc1 in lung cancer and may
have important implications in the development of targeted
therapies for lung cancer.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81071906 and 81172127) and
the Priority Academic Program Development (PAPD) of Jiangsu Higher
Education Institutions.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Brunagel G, Vietmeier BN, Bauer AJ, Schoen
RE and Getzenberg RH: Identification of nuclear matrix protein
alterations associated with human colon cancer. Cancer Res.
62:2437–2442. 2002.PubMed/NCBI
|
|
3
|
Dey P: Chromatin pattern alteration in
malignant cells: an enigma. Diagn Cytopathol. 32:25–30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zink D, Fischer AH and Nickerson JA:
Nuclear structure in cancer cells. Nat Rev Cancer. 4:677–687. 2004.
View Article : Google Scholar
|
|
5
|
Durfee T, Mancini MA, Jones D, Elledge SJ
and Lee WH: The amino-terminal region of the retinoblastoma gene
product binds a novel nuclear matrix protein that co-localizes to
centers for RNA processing. J Cell Biol. 127:609–622. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nickerson J: Experimental observations of
a nuclear matrix. J Cell Sci. 114:463–474. 2001.PubMed/NCBI
|
|
7
|
Ruh MF, Dunn R 2nd and Ruh TS:
Interrelationships between nuclear structure and ligand-activated
intracellular receptors. Crit Rev Eukaryot Gene Expr. 6:271–283.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martelli AM, Bareggi R, Bortul R, Grill V,
Narducci P and Zweyer M: The nuclear matrix and apoptosis.
Histochem Cell Biol. 108:1–10. 1997. View Article : Google Scholar
|
|
9
|
Barboro P, Repaci E, D’Arrigo C and Balbi
C: The role of nuclear matrix proteins binding to matrix attachment
regions (Mars) in prostate cancer cell differentiation. PLoS One.
7:e406172012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Getzenberg RH, Konety BR, Oeler TA,
Quigley MM, Hakam A, Becich MJ and Bahnson RR: Bladder
cancer-associated nuclear matrix proteins. Cancer Res.
56:1690–1694. 1996.PubMed/NCBI
|
|
11
|
Konety BR, Nangia AK, Nguyen TS, Veitmeier
BN, Dhir R, Acierno JS, Becich MJ, Hrebinko RL and Getzenberg RH:
Identification of nuclear matrix protein alterations associated
with renal cell carcinoma. J Urol. 159:1359–1363. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Getzenberg RH, Pienta KJ, Huang EY and
Coffey DS: Identification of nuclear matrix proteins in the cancer
and normal rat prostate. Cancer Res. 51:6514–6520. 1991.PubMed/NCBI
|
|
13
|
Luftner D and Possinger K: Nuclear matrix
proteins as biomarkers for breast cancer. Expert Rev Mol Diagn.
2:23–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Partin AW, Briggman JV, Subong EN, Szaro
R, Oreper A, Wiesbrock S, Meyer J, Coffey DS and Epstein JI:
Preliminary immunohistochemical characterization of a monoclonal
antibody (PRO:4-216) prepared from human prostate cancer nuclear
matrix proteins. Urology. 50:800–808. 1997. View Article : Google Scholar
|
|
15
|
Guo S, Hakimi MA, Baillat D, Chen X,
Farber MJ, Klein-Szanto AJ, Cooch NS, Godwin AK and Shiekhattar R:
Linking transcriptional elongation and messenger RNA export to
metastatic breast cancers. Cancer Res. 65:3011–3016.
2005.PubMed/NCBI
|
|
16
|
Yang J, Li Y, Khoury T, Alrawi S, Goodrich
DW and Tan D: Relationships of hHpr1/p84/Thoc1 expression to
clinicopathologic characteristics and prognosis in non-small cell
lung cancer. Ann Clin Lab Sci. 38:105–112. 2008.PubMed/NCBI
|
|
17
|
Gasparri F, Sola F, Locatelli G and Muzio
M: The death domain protein p84N5, but not the short isoform
p84N5s, is cell cycle-regulated and shuttles between the nucleus
and the cytoplasm. FEBS Lett. 574:13–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doostzadeh-Cizeron J, Yin S and Goodrich
DW: Apoptosis induced by the nuclear death domain protein p84N5 is
associated with caspase-6 and NF-kappa B activation. J Biol Chem.
275:25336–25341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doostzadeh-Cizeron J, Terry NH and
Goodrich DW: The nuclear death domain protein p84N5 activates a
G2/M cell cycle checkpoint prior to the onset of apoptosis. J Biol
Chem. 276:1127–1132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Wang X, Zhang X and Goodrich DW:
Human hHpr1/p84/Thoc1 regulates transcriptional elongation and
physically links RNA polymerase II and RNA processing factors. Mol
Cell Biol. 25:4023–4033. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tyagi AK, Singh RP, Agarwal C, Chan DC and
Agarwal R: Silibinin strongly synergizes human prostate carcinoma
DU145 cells to doxorubicin-induced growth inhibition, G2-M arrest,
and apoptosis. Clin Cancer Res. 8:3512–3519. 2002.
|
|
22
|
Singh RP, Dhanalakshmi S and Agarwal R:
Phytochemicals as cell cycle modulators - a less toxic approach in
halting human cancers. Cell Cycle. 1:156–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nahta R and Esteva FJ: Bcl-2 antisense
oligonucleotides: a potential novel strategy for the treatment of
breast cancer. Semin Oncol. 30:143–149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kymionis GD, Dimitrakakis CE,
Konstadoulakis MM, Arzimanoglou I, Leandros E, Chalkiadakis G,
Keramopoulos A and Michalas S: Can expression of apoptosis genes,
bcl-2 and bax, predict survival and responsiveness to chemotherapy
in node-negative breast cancer patients? J Surg Res. 99:161–168.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Endl E and Gerdes J: The Ki-67 protein:
fascinating forms and an unknown function. Exp Cell Res.
257:231–237. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scholzen T and Gerdes J: The Ki-67
protein: from the known and the unknown. J Cell Physiol.
182:311–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bridger JM, Kill IR and Lichter P:
Association of pKi-67 with satellite DNA of the human genome in
early G1 cells. Chromosome Res. 6:13–24. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
MacCallum DE and Hall PA: The location of
pKi67 in the outer dense fibrillary compartment of the nucleolus
points to a role in ribosome biogenesis during the cell division
cycle. J Pathol. 190:537–544. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ruiz C, Seibt S, Al Kuraya K, Siraj AK,
Mirlacher M, Schraml P, Maurer R, Spichtin H, Torhorst J, Popovska
S, et al: Tissue microarrays for comparing molecular features with
proliferation activity in breast cancer. Int J Cancer.
118:2190–2194. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Irigoyen MA, Garcia FV, Iturriagagoitia
AC, Beroiz BI, Martinez MS and Grima FG: Molecular subtypes of
breast cancer: prognostic implications and clinical and
immunohistochemical characteristics. An Sist Sanit Navar.
34:219–233. 2011.(In Spanish).
|