Introduction
Due to latent onset, rapid progression and poor
prognosis, hepatocellular carcinoma (HCC) is the third-leading
cause of cancer-related mortality worldwide, particularly in Africa
and Asia (1,2). Despite the continuous introduction of
numerous new treatments, the overall five-year survival rate
remains low (3,4). As the majority of patients are in the
advanced stages and have metastasis when they are diagnosed with
HCC, they are not referred for surgical resection, liver
transplantation or radiofrequency ablation. These patients are only
able to be treated with chemotherapy and/or radiotherapy in
addition to palliative care. However, the development of cancer
cell resistance and severe side effects represent main obstacles to
the success of chemoradiotherapy. It is thus urgent to develop new
therapeutics with lower toxicity and higher efficiency, or new
adjuvant therapies that are able to improve the efficiency or
decrease the side effects of the current chemoradiotherapy to
improve patient survival and quality of life. Traditional Chinese
medicine (TCM) may be a promising candidate.
In accordance with the TCM principle, cancer occurs
when Xie is stronger than Zheng in the human body. Xie refers to
various pathogenic factors (including viruses, fungi and bacteria).
Zheng means the disease-fighting ability of the body, including
immune function. Fuzheng (improving Zheng) Quxie (eradicating Xie)
is thus the basic theory of fighting cancer in TCM. The clinical
manifestations of Zheng deficiency include weakness, night
sweating, dizziness, pallor, shortness of breath and anemia. Zheng
deficiency is common in patients following chemotherapy and/or
radiotherapy. These patients often present with symptoms of Xie,
including ache, hyperpyrexia, restlessness and a quick pulse rate.
Clearly, Zheng deficiency and Xie often coexist in patients with
malignancy. Therefore, doctors of TCM always prescribe Fuzheng
herbs and Quxie herbs together for cancer patients.
Fuzheng recipes are able to supplement the vital
energy, tonify kidney and nourish yin, including Ginseng and
Ganoderma. In clinical trials and basic experiments, these
herbs are found to possess the ability to modulate immunity,
enhance the efficiency of chemoradiotherapy and improve the quality
of life of patients (5–8). Quxie herbs are capable of
heat-clearing and detoxification, including Hedyotis Diffusa
Willd and Prunella vulgaris. Pharmacological studies have
demonstrated that they contain ingredients that directly induce
cancer cell apoptosis, suppress angiogenesis as well as cell
invasion and migration (9–12). The combination of Fuzheng and Quxie
herbs are not only able to improve the efficiency but also
alleviate the adverse effect of chemoradiotherapy.
Fuzheng Qingjie (FZQJ) recipe is a polyherbal
formula of Fuzheng and Quxie herbs, which includes Astragalus
membranaceus, Ligustrum lucidum, Ganoderma lucidum, Rhizoma
dioscorea, Hedyotis Diffusa Willd and Prunella vulgaris
(13). The first four are Fuzheng
herbs and the other two are Quxie herbs. They have been used for a
long time as adjuvant treatments for gastrointestinal malignancies
with proven clinical efficacy. Previously, we used an apoptosis
microarray (Spring Bioscience, Pleasanton, CA, USA) to screen the
pharmacological targets of FZQJ recipe in cancer cells and
identified that FZQJ could regulate numerous apoptosis-related
genes, including Bax, caspase-3 and -9 and P38 mitogen-activated
protein kinase (MAPK; unpublished data). Since the activation of
P38 MAPK can result in mitochondria-dependent apoptosis, we
investigated whether the activation of P38 MAPK is involved in the
apoptotic cell death induced by FZQJ.
Materials and methods
Preparation of water extract of FZQJ
herbs
Herbs were purchased from Tongchun Pharmaceutical
Co., Ltd (Fuzhou, Fujian, China). Their quality met the criteria
described in the Pharmacopoeia of the People’s Republic of China.
To prepare water extract, Astragalus membranaceus (60 g),
Ligustrum lucidum (60 g), Ganoderma lucidum (30 g),
Rhizoma dioscorea (30 g), Prunellae vulgaris (30 g)
and Hedyotis diffusa Willd (60 g) were pulverized into
extremely fine powders with a mortar and pestle and immersed in
distilled water, respectively. The mixture was simmered for 2 h and
filtered. The solution was concentrated so that it contained 2.66 g
of dry herbs per 1 ml and was stored at 4°C until use.
Cell culture
Human HepG2 hepatoma cells were purchased from the
Shanghai Institute of Life Science, Chinese Academy of Sciences
(Shanghai, China). Cells were maintained in RPMI-1640 culture
medium (Gibco-BRL, Carlsbad, CA, USA) with 100 ml/l of fetal calf
serum, 1×105 U/l of penicillin and 100 mg/l of
streptomycin (Gibco-BRL) in an incubator (Thermo Fisher Scientific,
Rockford, IL, USA) at 37°C with 5% CO2. Cell morphology
was observed using an inverted microscope (Olympus, Tokyo,
Japan).
MTT assay
Cells were cultured in the absence or presence of
water extract of FZQJ at the final concentrations of 0.5, 1, 1.5
and 2 mg/ml for 24, 48 and 72 h respectively. Then, 20 μl of 5
mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT; Invitrogen Life Technologies, Carlsbad, CA, USA) was added to
each well and incubated for another 4 h prior to discarding the
medium. The purple-blue formazan precipitate was dissolved in 100
μl of dimethyl sulfoxide (DMSO). The absorbance (OD) was measured
at 490 nm with a microplate reader (BioTek Instruments Inc.,
Winooski, VT, USA). The cell viability ratio was calculated
according to the following formula: Cell viability ratio (%) =
average ODtreatment group/average ODvehicle
group × 100%.
DAPI staining
In brief, following treatment with different
concentrations of water extract of FZQJ for 24 h, the cells were
fixed with 4% paraformaldehyde and then incubated with 1 μg/ml of
4,6-diamidino-2-phenylindole (DAPI) staining solution (Beyotime
Inc., Shanghai, China) at room temperature for 5 min and washed
with PBS 3 times. The morphology of nuclei in HepG2 cells was
observed under a fluorescence microscope (Olympus) at an excitation
wavelength of 350 nm.
Cell apoptosis and mitochondrial membrane
potential assays
Cells were incubated in 6-well plates for 24 h in
the absence or presence of different concentrations of FZQJ water
extract. Then cells were digested by trypsinase and washed twice
with cold PBS. A final concentration of 1×106 cells/ml
of single-cell suspension was prepared. Apoptosis and mitochondrial
membrane potential (Δψ) were measured by a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) with Annexin-V fluorescein
isothiocyanate (FITC) or
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide (JC-1; BD Biosciences). Cells (10,000) were acquired and
analyzed using CellQuest software (BD Biosciences). Each
determination was performed as three parallel assays.
Reverse transcription polymerase chain
reaction (RT-PCR)
Total cellular RNA was isolated using the TRIzol
one-step method according to the manufacturer’s instructions
(Invitrogen Life Technologies). Single-stranded cDNA was
synthesized using oligo (dT) primer (Takara, Dalian, China) in a 20
μl reaction mixture. Bcl-2 and Bax mRNA were evaluated using PCR.
The primer pairs of Bcl-2, Bax and GAPDH were as follows: Bcl-2,
forward 5′-CAG CTG CAC CTG ACG CCC TT-3′ and reverse 5′-GCC TCC GTT
ATC CTG GAT CC-3′; Bax, forward 5′-TGC TTC AGG GTT TCA TCC AGG-3′
and reverse 5′-TGG CAA AGT AGA AAA GGG CGA-3′; GAPDH, forward
5′-AGA AGG CTG GGG CTC ATT TG-3′ and reverse 5′-AGG GGC CAT CCA CAG
TCT TC-3′. DNA amplification was performed for 40 cycles following
an initial denaturation step at 94°C for 5 min in a thermo cycler
by using the following program: denaturation at 94°C for 30 sec,
annealing at 58°C for 30 sec, extension at 72°C for 30 sec and a
final extension at 72°C for 10 min. PCR reagent kit was obtained
from Takara. Finally, the amplified products were separated on a
1.2% agarose gel and examined using a Gel Doc 2000 Imaging System
(Bio-Rad, Hercules, CA, USA).
Western blot analysis
HepG2 cells were collected and lysed in lysis buffer
(Beyotime Inc.) for 10 min following treatment with different
concentrations of FZQJ water extract for 24 h. Following
centrifugation at 12,000 × g at 4°C for 20 min, the supernatant was
collected and the protein concentration determined using the
Bradford assay. Equal amounts of denatured protein were separated
on SDS-PAGE gels and transferred onto PVDF membranes. These
membranes were then put into blocking solution for 1 h and
incubated in solution with either monoclonal anti-human Bax or
Bcl-2 primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) overnight at 4°C with agitation and then in horseradish
peroxidase (HRP)-conjugated secondary antibody (Beyotime Inc.) for
at least 1 h. Protein was detected with ECL solution (Beyotime
Inc.) using a chemiluminescence imaging system (Bio-Rad).
Caspase-3 and -9 activation analysis
The activities of caspase-3 and -9 were examined
using caspase-3 and -9 colorimetric assay kits (KeyGen Biotech,
Nanjing, Jiangsu, China). Briefly, HepG2 cells were collected and
lysed on ice with 100 μl of lysis buffer (containing 1%
dithiothreitol) following treatment with different concentrations
of FZQJ water extract for 24 h. Following centrifugation at 11,000
× g for 1 min, the protein concentration of the supernatant was
determined using the Bradford assay. Equal amounts of protein were
incubated with specific substrates of caspase-3 or -9 at 37°C in
the dark for 4 h. Finally the samples were determined at 405 nm by
a microplate reader (BioTek Instruments Inc.). The activities of
caspase -3 or -9 were calculated according to the formula: average
ODtreatment/average ODvehicle.
Phosphorylated P38 MAPK assay
Phosphorylated P38 MAPK (p-P38 MAPK) was evaluated
using flow cytometry. The cells were digested and rinsed with PBS
following treatment with different concentrations of FZQJ water
extract for 48 h. Then cells were suspended, fixed with fixation
buffer for 10 min at 37°C, permeabilized on ice for 30 min and
stained with Alexa Fluor® 647 mouse anti-p-P38 MAPK
antibody (BD Biosciences). The cells were analyzed with the flow
cytometer.
Statistical analysis
Data were analyzed using the SPSS 16.0 statistical
package. All results are expressed as the mean ± standard deviation
(SD) of at least three experiments. The data for multiple
comparisons were performed by one-way ANOVA. P<0.05 was
considered to indicate a statistically significant difference.
Results
Water extract of FZQJ recipe inhibits
HepG2 cell proliferation
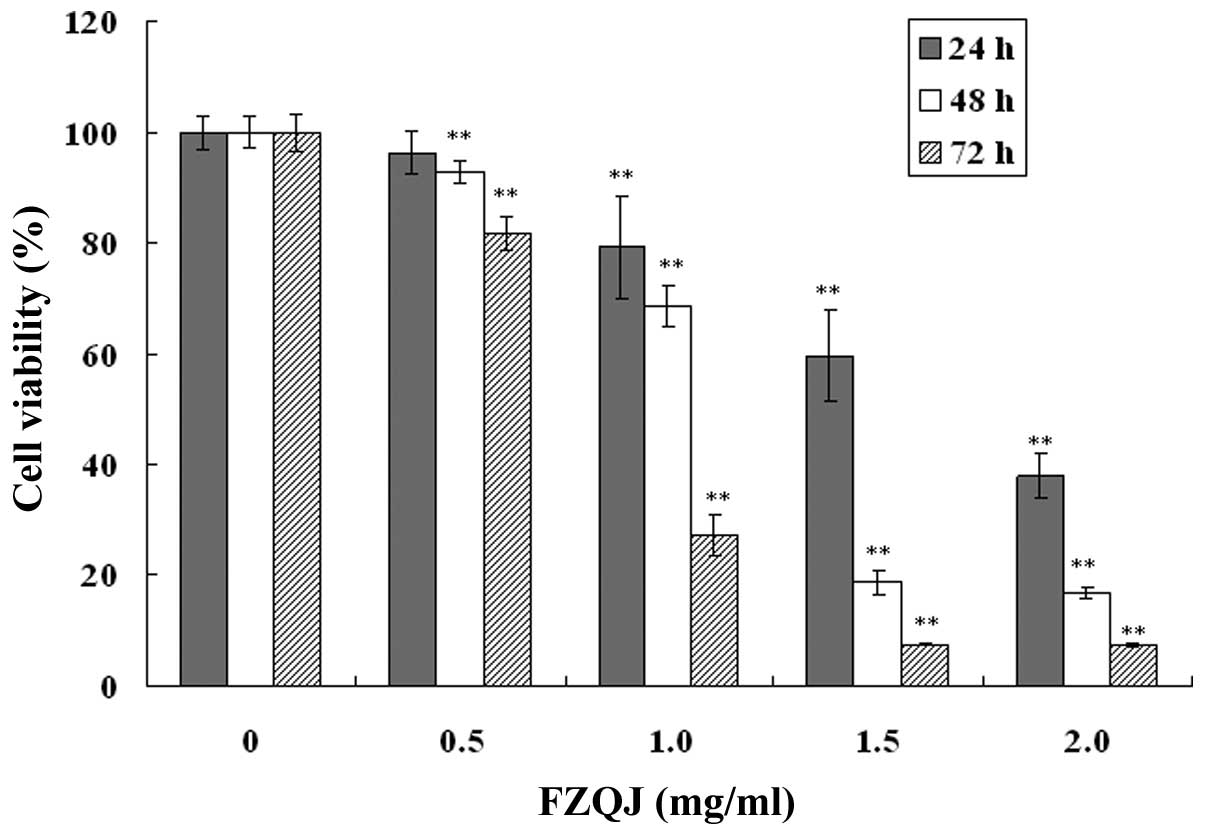
FZQJ made according to the patent (13). The growth of HepG2 cells was
evaluated using an MTT assay. As shown in Fig. 1, in the presence of indicated
concentrations of water extracts, cell proliferation was inhibited
in a dose- and time-dependent manner. The inhibitory rate of HepG2
cells was able to reach as high as 92.57% at the highest
concentration for 72 h. The results demonstrated that water extract
of FZQJ markedly inhibited the proliferation of HepG2 cells.
Water extract of FZQJ induces apoptosis
of HepG2 cells
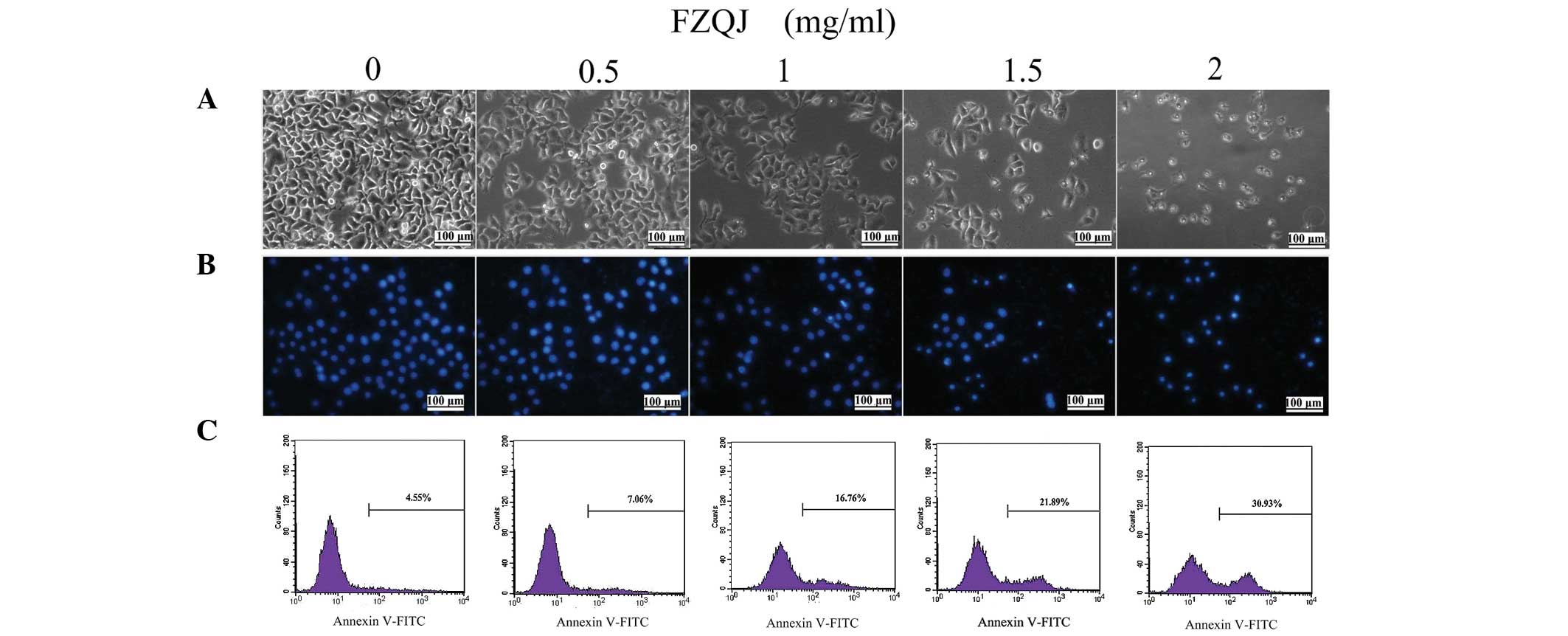
We first evaluated apoptosis of HepG2 cells treated
with indicated doses of FZQJ for 24 h through observing the
morphological changes using an inverted fluorescence microscope. As
shown in Fig. 2A, a decreased cell
number was associated with morphological changes of cells following
the addition of FZQJ to the medium; polygon- or spindle-shaped
cells became round, shrunk and collapsed. Following staining of the
nuclei with DAPI (a fluorescent DNA-binding agent), more brightened
nuclei were observed in the FZQJ-treated cells, with chromatin
pyknosis and fragmentation (Fig.
2B). Clearly, FZQJ water extract induced marked apoptotic
morphological alterations.
Next, cells were evaluated flow cytometrically by
Annexin-V staining. Annexin-V is a cellular protein used as a probe
to detect cells that have expressed phosphatidylserine on the cell
surface (an event identified in apoptosis). Therefore, Annexin-V
positive cells are considered to be apoptotic cells. As displayed
in Fig. 2C, the percentage of
apoptotic cells stained with Annexin V markedly increased in
FZQJ-treated cells in a dose-dependent manner. Taken together,
these data revealed that FZQJ extract induced apoptosis of HepG2
cells.
Water extract of FZQJ induces a decrease
in the mitochondrial membrane potential
Apoptosis is often accompanied by a decrease of Δψ.
Loss of Δψ is important in the early apoptotic process (14). JC-1 is a lipophilic fluorochrome
that is found to be sensitive to Δψ and is used as an indicator of
Δψ during apoptosis. JC-1 has two different formations, aggregates
and monomers. In normal cells with a polarized Δψ, JC-1 aggregates
stay in the mitochondria and emit red fluorescence (red channel)
and monomers stay in the cytoplasm and exhibit green fluorescence
(green channel). Therefore, JC-1-treated normal cells demonstrate
green and red channels on flow cytometers. When the mitochondria Δψ
depolarizes, JC-1 does not form aggregates in the mitochondria but
remains as monomers in the cytoplasm, resulting in increased
numbers of cells with reduced JC-1 fluorescence in the red channel.
As shown in Fig. 3, JC-1
fluorescence was observed in red and green channels (R2 region) in
the vehicle-treated cells, indicating that the majority of cells
were alive. In the presence of increasing concentrations of FZQJ,
there was a significant increase in the number of cells with
lowered red fluorescence (R3 region), indicative of a depolarized
Δψ. Thus, the data indicate that FZQJ-induced apoptosis was
associated with the depolarization of Δψ.
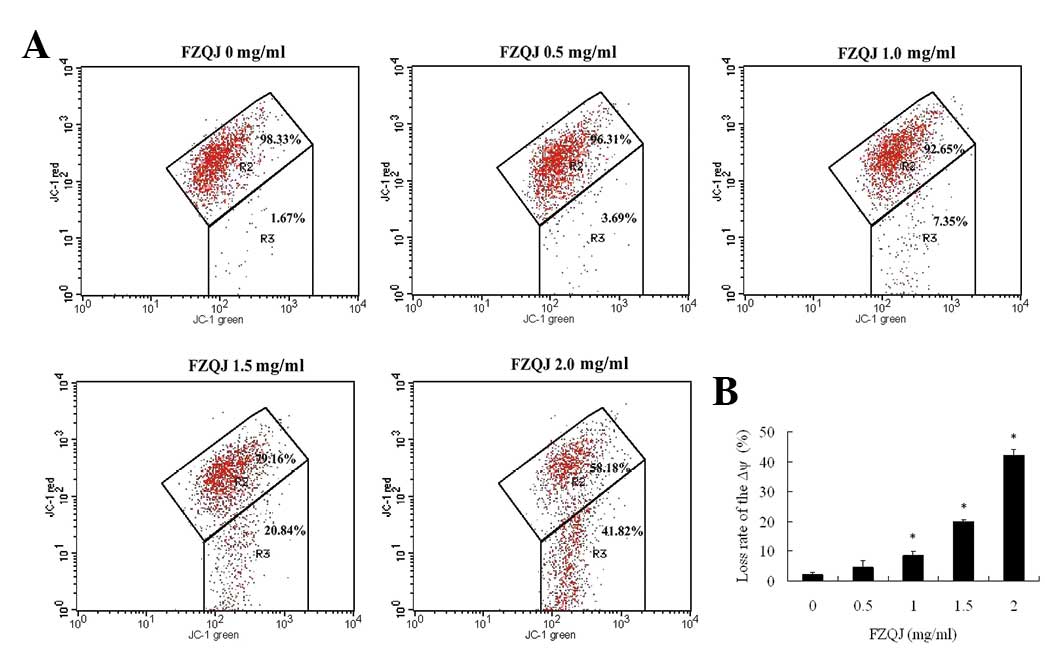 | Figure 3Water extract of FZQJ induces loss of
the Δψ. After the cells were treated with indicated concentrations
of FZQJ water extract for 24 h, Δψ was measured using JC-1
fluorescence dye by a flow cytometer. (A) A typical chart is shown.
(B) Loss rate of Δψ from three experiments. *P<0.05
and **P<0.01, compared with the vehicle control.
FZQJ, Fuzheng Qingjie; Δψ, mitochondrial membrane potential; JC-1,
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide. |
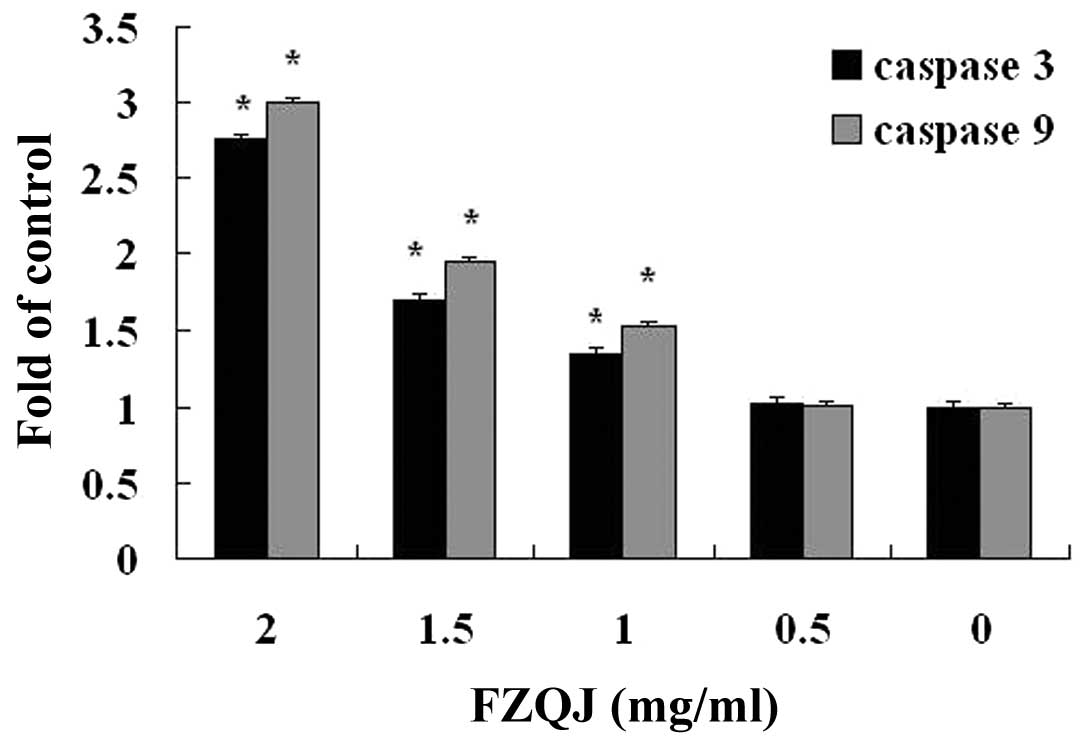
Water extract of FZQJ induces activation
of caspase-3 and-9 in HepG2 cells
The activities of caspase-3 and -9 are closely
associated with the mitochondria-dependent apoptosis pathway. We
next examined whether caspase-3 and -9 were involved in
FZQJ-induced depolarization of Δψ in HepG2 cells. The activities of
caspase-3 and -9 were examined by colorimetric assay. The results
demonstrated that the activities of caspase-3 and -9 were markedly
increased in a dose-dependent manner. Following treatment with 2
mg/ml of FZQJ extract for 24 h, 2.77-fold and 2.99-fold increases
were observed for caspase-3 and -9 activity compared with the
vehicle control, respectively (Fig.
4). The data suggested that water extract of FZQJ could decease
Δψ in HepG2 cells via the activation of caspase-3 and -9.
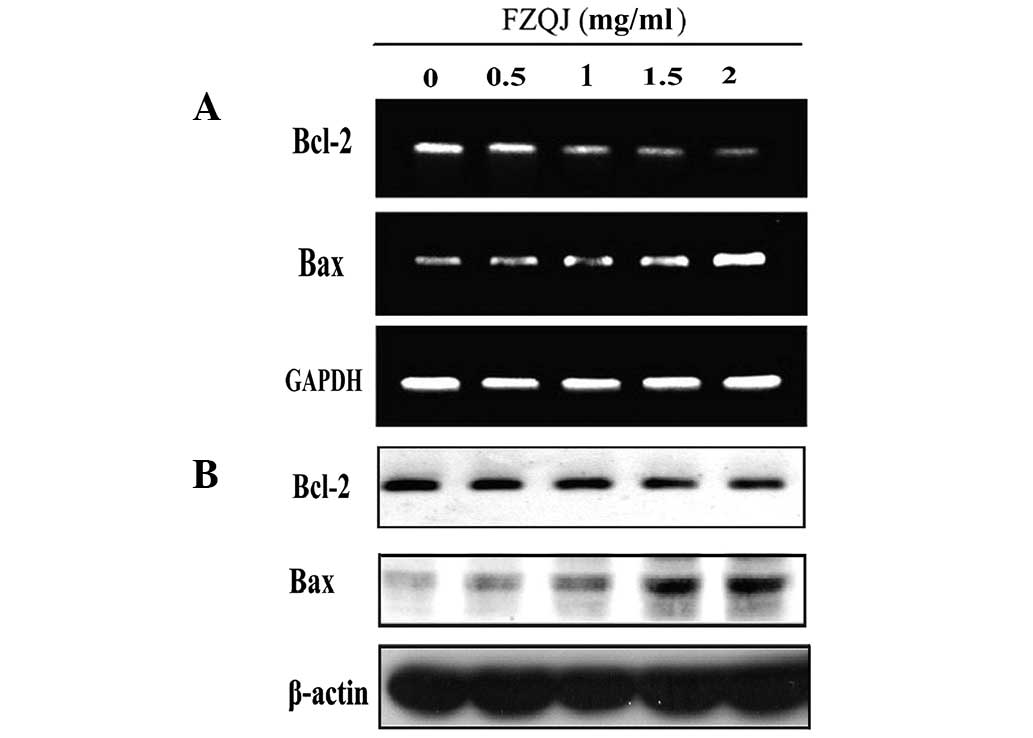
Water extract of FZQJ downregulates Bcl-2
and upregulates Bax
Antiapoptotic Bcl-2 and proapoptotic Bax are members
of the Bcl-2 family and are critical in apoptosis controlled by
mitochondria. To further investigate how FZQJ induces
mitochondria-dependent apoptosis, the expression of Bcl-2 and Bax
were evaluated in mRNA and protein levels by RT-PCR and western
blot analysis, respectively. As shown in Fig. 5A, compared with the vehicle
control, FZQJ decreased Bcl-2 mRNA and increased Bax mRNA
expression in a dose-dependent manner. Similar results were
observed in western blot analyses (Fig. 5B), indicating that FZQJ induced
depolarization of Δψ in HepG2 cells through regulating the
expression of members of the Bcl-2 family.
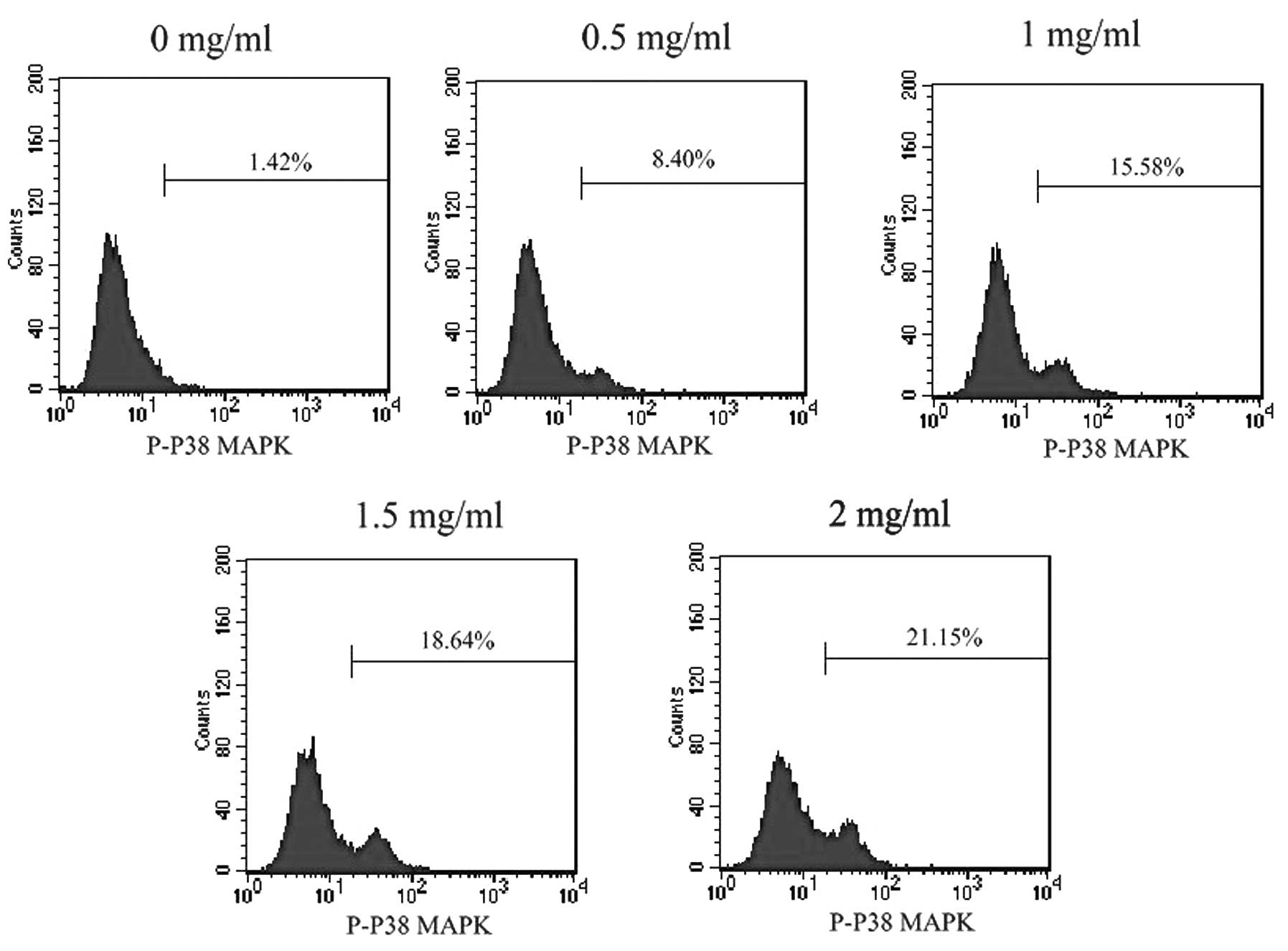
Water extract of FZQJ activates P38
MAPK
Activation of P38 MAPK has been demonstrated to lead
to cell death via stimulating mitochondrial Bax translocation and
activating caspase -9 and -3 (15). To investigate whether FZQJ induces
mitochondrial apoptosis via activation of P38 MAPK, phosphorylation
of P38 MAPK was evaluated. As shown in Fig. 6, FZQJ treatment caused a
dose-dependent increase of p-P38 MAPK level in HepG2 cells,
indicating that FZQJ could activate P38 MAPK.
Discussion
In previous years, TCMs have been used in an
increasing number of countries to treat tumors and the underlying
mechanisms are being investigated (16,17).
TCMs are drawing more and more attention from oncologists as, in
addition to direct inhibition on tumor growth, TCMs may improve the
anticancer response and reduce the side effects of chemotherapy.
Moreover, TCMs have few and mild adverse effects (18,19).
FZQJ recipe has been prescribed to treat cancer and
manage the side effects of chemotherapy in China. It is composed of
six medinical herbs. Our previous study demonstrated that these 6
herbs are commonly prescribed for patients with malignant tumors
according to cluster analysis (20). These herbs contain compounds with
anticancer activities, including polysaccharides, saponins,
flavones, anthraquinones and polyphenols. By molecular docking
simulation, we revealed that Bcl-xl, tumor necrosis factor (TNF)-α,
interleukin (IL)-2, and cyclooxygenase 2 are possible targets of
Hedyotis diffusa Willd and Prunella vulgaris in the
FZQJ recipe (21). Pharmacological
studies have demonstrated that compounds in herbs of FZQJ recipe
can inhibit the proliferation of tumor cells, induce apoptosis of
tumor cells, improve the sensitivity of cancer cells to
chemotherapy and modulate immune function. For instance,
Astragalus polysaccharides were demonstrated to enhance the
chemosensitivity of H22 hepatoma cells resistant to adriamycin
(22). Astragalus
polysaccharides can inhibit the proliferation of the basal-like
breast cancer cell line MDA-MB-468 via regulating p53/MDM2 positive
and negative feedback loops (23).
2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd
was revealed to be able to induce apoptosis in different tumor
cells through the modulation of MAPK pathways (24), the
Ca2+/calpain/caspase-4 pathway (25), as well as the alteration of
Fas/FasL and the activation of caspase-8 (26). Ganoderma lucidum
polysaccharides can enhance immunity via reducing the levels of
serum IL-6 and TNF-α and increasing those of serum IL-2, IL-4 and
IL-10 (27). Oleanolic acid from
Prunella Vulgaris was demonstrated to induce apoptosis of
lung adenocarcinoma cells through downregulating Bcl-2 expression
and upregulating Bax and Bad expression (28). An oleanolic acid-enriched extract
of Ligustrum Lucidum were found to have potential
immunomodulatory effects through enhancing the proliferative
activity of blood lymphocytes and upregulating the
CD4+CD8+ and CD4+CD8−
cell populations as well as regulating the expression of Th1- and
Th2-related cytokines (29). In
addition, a polysaccharide from Dioscorea opposita Thunb
roots was demonstrated to be able to enhance the immunological
activity via stimulating ConA-induced T lymphocyte proliferation
(30). Thus, it is possible the
recipe containing these herbs according to the TCM theory may have
additive anticancer effects.
Cysteine aspartyl-specific proteases (caspases), a
family of cysteine proteases, act in concert in a cascade-triggered
manner and are activated via three main pathways when cells receive
apoptotic signals (31). The three
pathways are death receptors signaling, the mitochondrial pathway
and the endoplasmic reticulum stress pathway. The second pathway is
an intrinsic pathway, in which mitochondria are the central
organelle governed by pro- and anti-apoptotic members of the Bcl-2
family (32). Numerous molecules
in mitochondria are found to be closely associated with cell
apoptosis, including cytochrome c (cyt-c), apoptosis-inducing
factor and reactive oxygen species (33). When a mitochondrion is damaged,
mitochondrial outer membrane permeabilization occurs and Δψ
collapse. Then cyt-c is released from the mitochondrial
intermembrane space into the cytoplasm. Subsequently, cyt-c couples
with apoptosis protease activating factor-1 and triggers
pro-caspase-9 assembly to promote production of active caspase-9
(34,35). Active caspase-9 further activates
the downstream proteases of the caspase cascade, including
caspase-3, -6 and -7 (36). These
proteases may lead to DNA mismatch repair dysfunction, and even
fragmentation, finally causing inevitable apoptotic cell death
(37). Caspase-3 and -9 are key
proteases responsible for the execution of apoptosis.
The mitochondria-dependent cell death cascade is
regulated by the Bcl-2 family. The members of the Bcl-2 family
include anti-apoptotic (Bcl-2) and pro-apoptotic members (Bax). Bax
can promote the openness of mitochondrial permeability transition
pores (MPTP) and release cyt-c, subsequently activating the caspase
cascade and triggering apoptosis. Inversely, Bcl-2 can inhibit the
openness of MPTP and protect cells from apoptosis. Previous studies
have provided evidence that overexpression of the Bcl-2 protein or
decreased expression of the Bax gene was associated with poor
prognosis in various diseases (38). In the present study, we
demonstrated that FZQJ may downregulate Bcl-2 expression and
upregulate Bax expression and activate caspase-3 and -9, indicating
that FZQJ-induced apoptosis of HepG2 cells is at least through the
mitochondria-dependent apoptotic cascade.
P38 MAPK is a member of the MAPK family. It is
crucial in regulating cellular proliferation, differentiation and
apoptosis (39–41). The upregulation of P38 MAPK
activity is essential for apoptotic induction in tumor cells.
Apoptosis mediated by Fas/Fas-ligand (42), c-myc (43), p53 (44) as well as c-jun and c-jos (41) are associated with the enhanced
activation of p38 MAPK. In addition, numerous studies also
demonstrated that P38 MAPK activity could activate the
mitochondria-dependent cell death pathway by stimulating Bax
translocation from the cytosol to the mitochondria, further
promoting the release of cyt-c and thereby triggering the cell
death cascade (45,46,15).
Our findings demonstrated that water extract of FZQJ could activate
P38 MAPK, which may be responsible for the activation of the
mitochondria-dependent cell death cascade.
In conclusion, our results demonstrate that the
anti-cancer effect of FZQJ recipe on HepG2 cells may involve the
activation of P38 MAPK and the subsequent mitochondria-dependent
apoptotic cascade. These data provide scientific basis for the
clinical application of FZQJ recipe as an adjunct for
chemoradiotherapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81102582) and the Natural Science
Foundation of Fujian Province (no. 2013J01379).
Abbreviations:
|
TCM
|
traditional chinese medicine
|
|
HCC
|
hepatocellular carcinoma
|
|
FZQJ
|
Fuzheng Qingjie
|
|
MAPK
|
mitogen-activated protein kinase
|
|
DAPI
|
4,6-diamidino-2-phenylindole
|
|
DMSO
|
dimethyl sulfoxide
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
RT-PCR
|
reverse transcription polymerase chain
reaction
|
|
Δψ
|
mitochondrial membrane potential
|
|
JC-1
|
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine
iodide
|
|
caspase
|
cysteine aspartyl-specific
protease
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
Rampone B, Schiavone B, Martino A, Viviano
C and Confuorto G: Current management strategy of hepatocellular
carcinoma. World J Gastroenterol. 15:3210–3216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chung YH, Song IH, Song BC, Lee GC, Koh
MS, Yoon HK, Lee YS, Sung KB and Suh DJ: Combined therapy
consisting of intraarterial cisplatin infusion and systemic
interferon-alpha for hepatocellular carcinoma patients with major
portal vein thrombosis or distant metastasis. Cancer. 88:1986–1991.
2000. View Article : Google Scholar
|
|
3
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Urruticoechea A, Alemany R, Balart J,
Villanueva A, Viñals F and Capellá G: Recent advances in cancer
therapy: an overview. Curr Pharm Des. 16:3–10. 2010. View Article : Google Scholar
|
|
5
|
Kang S and Min H: Ginseng, the ‘Immunity
Boost’: The Effects of Panax ginseng on Immune System. J Ginseng
Res. 36:354–368. 2012.
|
|
6
|
Choi J, Kim TH, Choi TY and Lee MS:
Ginseng for health care: a systematic review of randomized
controlled trials in Korean literature. PLoS One. 8:e599782013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang YH, Yang JS, Yang JL, Wu CL, Chang
SJ, Lu KW, Kuo CL, Hsia TC and Chung JG: Gandoderma lucidum extract
promotes immune responses in normal BALB/c mice in vivo. In Vivo.
23:755–759. 2009.PubMed/NCBI
|
|
8
|
Bao PP, Lu W, Cui Y, Zheng Y, Gu K, Chen
Z, Zheng W and Shu XO: Ginseng and Ganoderma lucidum use after
breast cancer diagnosis and quality of life: a report from the
Shanghai Breast Cancer Survival Study. PloS One. 7:e393432012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi Y, Wang CH and Gong XG:
Apoptosis-inducing effects of two anthraquinones from Hedyotis
diffusa WILLD. Biol Pharm Bull. 31:1075–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin J, Wei L, Shen A, Cai Q, Xu W, Li H,
Zhan Y, Hong Z and Peng J: Hedyotis diffusa Willd extract
suppresses Sonic hedgehog signaling leading to the inhibition of
colorectal cancer angiogenesis. Int J Oncol. 42:651–656. 2013.
View Article : Google Scholar
|
|
11
|
Kim SH, Huang CY, Tsai CY, Lu SY, Chiu CC
and Fang K: The aqueous extract of Prunella vulgaris suppresses
cell invasion and migration in human liver cancer cells by
attenuating matrix metalloproteinases. Am J Chin Med. 40:643–656.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen XZ, Cao ZY, Chen TS, Zhang YQ, Liu
ZZ, Su YT, Liao LM and Du J: Water extract of Hedyotis
Diffusa Willd suppresses proliferation of human HepG2 cells and
potentiates the anticancer efficacy of low-dose 5-fluorouracil by
inhibiting the CDK2-E2F1 pathway. Oncol Rep. 28:742–748. 2012.
|
|
13
|
Cai Jing, Cao Zhiyun, Chen Liwu, Chen
Xuzheng, Du Jian and Liu Zhizhen: Traditional Chinese medicine for
treating tumor of digestive tract, strengthening body, resistance
and removing summer-heat. CN Patent 201010130786. Filed March 23,
2010; issued August 4, 2010.
|
|
14
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar
|
|
15
|
Ghatan S, Larner S, Kinoshita Y, Hetman M,
Patel L, Xia Z, Youle RJ and Morrison RS: p38 MAP kinase mediates
bax translocation in nitric oxide-induced apoptosis in neurons. J
Cell Biol. 150:335–347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ernst E and Cassileth BR: The prevalence
of complementary/alternative medicine in cancer: a systematic
review. Cancer. 83:777–782. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boon H, Brown JB, Gavin A, Kennard MA and
Stewart M: Breast cancer survivors’ perceptions of
complementary/alternative medicine (CAM): making the decision to
use or not to use. Qual Health Res. 9:639–653. 1999.
|
|
18
|
Konkimalla VB and Efferth T:
Evidence-based Chinese medicine for cancer therapy. J
Ethnopharmacol. 116:207–210. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Posadzki P, Watson LK and Ernst E: Adverse
effects of herbal medicines: an overview of systematic reviews.
Clin Med. 13:7–12. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Chen S, Cai J, Zhang E, Lan L,
Zheng J, Liao L, Yang X, Zhou C and Du J: Traditional Chinese
medicine syndrome-related herbal prescriptions in treatment of
malignant tumors. J Tradit Chin Med. 33:19–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LW, Zheng CS and Du J: Study on
antitumor mechanism of Qingre xiaozheng drink by molecular docking
method. Chin J Clin Pharmacol Ther. 12:324–328. 2007.(In
Chinese).
|
|
22
|
Tian QE, De Li H, Yan M, Cai HL, Tan QY
and Zhang WY: Effects of Astragalus polysaccharides on
P-glycoprotein efflux pump function and protein expression in H22
hepatoma cells in vitro. BMC Complement Altern Med. 12:942012.
|
|
23
|
Ye MN, Chen HF, Zhou RJ and Liao MJ:
Effects of Astragalus polysaccharide on proliferation and
Akt phosphorylation of the basal-like breast cancer cell line.
Zhong Xi Yi Jie He Xue Bao. 9:1339–1346. 2011.(In Chinese).
|
|
24
|
Wang N, Li DY, Niu HY, Zhang Y, He P and
Wang JH: 2-hydroxy-3-methylanthraquinone from Hedyotis
diffusa Willd induces apoptosis in human leukemic U937 cells
through modulation of MAPK pathways. Arch Pharm Res. 36:752–758.
2013.
|
|
25
|
Liu Z, Liu M, Liu M and Li J:
Methylanthraquinone from Hedyotis diffusa WILLD induces
Ca(2+)-mediated apoptosis in human breast cancer cells. Toxicol In
Vitro. 24:142–147. 2010.
|
|
26
|
Wang JH, Shu LH, Yang LL, Zhang M and He
P: 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa
WILLD induces apoptosis via alteration of Fas/FasL and activation
of caspase-8 in human leukemic THP-1 cells. Arch Med Res.
42:577–583. 2011.
|
|
27
|
Pan K, Jiang Q, Liu G, Miao X and Zhong D:
Optimization extraction of Ganoderma lucidum polysaccharides
and its immunity and antioxidant activities. Int J Biol Macromol.
55:301–306. 2013.
|
|
28
|
Feng L, Au-Yeung W, Xu YH, Wang SS, Zhu Q
and Xiang P: Oleanolic acid from Prunella Vulgaris L induces
SPC-A-1 cell line apoptosis via regulation of Bax, Bad and Bcl-2
expression. Asian Pac J Cancer Prev. 12:403–408. 2011.
|
|
29
|
Wang J, Shan A, Liu T, Zhang C and Zhang
Z: In vitro immunomodulatory effects of an oleanolic acid-enriched
extract of Ligustrum lucidum fruit (Ligustrum lucidum
supercritical CO2 extract) on piglet immunocytes. Int
Immunopharmacol. 14:758–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao G, Kan J, Li Z and Chen Z: Structural
features and immunological activity of a polysaccharide from
Dioscorea opposita Thunb roots. Carbohydr Polym. 61:125–131.
2005. View Article : Google Scholar
|
|
31
|
Hakem R, Hakem A, Duncan GS, et al:
Differential requirement for caspase 9 in apoptotic pathways in
vivo. Cell. 94:339–352. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferri KF and Kroemer G: Organelle-specific
initiation of cell death pathways. Nat Cell Biol. 3:E255–E263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hajnóczky G, Csordás G, Das S,
Garcia-Perez C, Saotome M, Sinha Roy S and Yi M: Mitochondrial
calcium signalling and cell death: approaches for assessing the
role of mitochondrial Ca2+ uptake in apoptosis. Cell
Calcium. 40:553–560. 2006.PubMed/NCBI
|
|
34
|
Zou H, Li Y, Liu X and Wang X: An APAF-1.
cytochrome cmultimeric complex is a functional apoptosome that
activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Acehan D, Jiang X, Morgan DG, Heuser JE,
Wang X and Akey CW: Three-dimensional structure of the apoptosome:
implications for assembly, procaspase-9 binding, and activation.
Mol Cell. 9:423–432. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baliga B and Kumar S: Apaf-1/cytochrome c
apoptosome: an essential initiator of caspase activation or just a
sideshow? Cell Death Differ. 10:16–18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu X, Wang L, Acehan D, Wang X and Akey
CW: Three-dimensional structure of a double apoptosome formed by
the Drosophila Apaf-1 related killer. J Mol Biol. 355:577–589.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yin C, Knudson CM, Korsmeyer SJ and Van
Dyke T: Bax suppresses tumorigenesis and stimulates apoptosis in
vivo. Nature. 385:637–640. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brenner B, Koppenhoefer U, Weinstock C,
Linderkamp O, Lang F and Gulbins E: Fas- or ceramide-induced
apoptosis is mediated by a Rac1-regulated activation of Jun
N-terminal kinase/p38 kinases and GADD153. J Biol Chem.
272:22173–22181. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ichijo H, Nishida E, Irie K, ten Dijke P,
Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K and Gotoh
Y: Induction of apoptosis by ASK1, a mammalian MAPKKK that
activates SAPK/JNK and p38 signaling pathways. Science. 275:90–94.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schwenger P, Bellosta P, Vietor I,
Basilico C, Skolnik EY and Vilcek J: Sodium salicylate induces
apoptosis via p38 mitogen-activated protein kinase but inhibits
tumor necrosis factor-induced c-Jun N-terminal
kinase/stress-activated protein kinase activation. Proc Natl Acad
Sci USA. 94:2869–2873. 1997. View Article : Google Scholar
|
|
42
|
Kornmann M, Ishiwata T, Kleeff J, Beger HG
and Korc M: Fas and Fas-ligand expression in human pancreatic
cancer. Ann Surg. 231:368–379. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Stoneley M, Chappell SA, Jopling CL,
Dickens M, MacFarlane M and Willis AE: c-Myc protein synthesis is
initiated from the internal ribosome entry segment during
apoptosis. Mol Cell Biol. 20:1162–1169. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cuadrado A, Lafarga V, Cheung PC, Dolado
I, Llanos S, Cohen P and Nebreda AR: A new p38 MAP kinase-regulated
transcriptional coactivator that stimulates p53-dependent
apoptosis. EMBO J. 26:2115–2126. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang X, Yao J, Luo Y, Han Y, Wang Z and Du
L: P38 MAP kinase mediates apoptosis after genipin treatment in
non-small-cell lung cancer H1299 cells via a mitochondrial
apoptotic cascade. J Pharmacol Sci. 121:272–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Owens TW, Valentijn AJ, Upton JP, Keeble
J, Zhang L, Lindsay J, Zouq NK and Gilmore AP: Apoptosis commitment
and activation of mitochondrial Bax during anoikis is regulated by
p38MAPK. Cell Death Differ. 16:1551–1562. 2009. View Article : Google Scholar : PubMed/NCBI
|