Introduction
Silent brain infarction (SBI) is a cerebrovascular
disorder. The clinical and pathological aspects of SBI are distinct
from those of ischemic stroke; while vascular brain lesions are
evident using magnetic resonance imaging (MRI), SBI is clinically
asymptomatic (1,2). SBI and white matter lesions are
frequently observed using brain MRI in healthy elderly individuals
and are associated with an increased risk of stroke and dementia
(1–6). SBI is a risk factor for stroke
(7). SBI has been associated with
several cardiovascular risk factors. Previous studies strongly
suggest that SBI can be significantly influenced by multiple risk
factors, including hypertension, homocysteine levels, cigarette
smoking and metabolic syndrome (7–11).
However, relatively little is known about the genes involved in SBI
pathogenesis. The present study was a candidate gene association
study focusing on the association between the adrenoceptor-α2
(ADRA2) genes and SBI. The rationale was two-fold: i)
ADRA2 genes are responsible for blood flow vasoconstriction,
which is linked to thrombosis; ii) ADRA2 defects are
associated with an increased likelihood of ischemic stroke
(12,13).
There are three subtypes of α2-adrenoceptors
(α2-ARs), α2A, α2B and α2C, which are encoded by the ADRA2A,
ADRA2B and ADRA2C genes, respectively (10,11).
The α2-AR, a membrane receptor for norepinephrine and epinephrine,
mediates physiological responses to endogenous catecholamine. The
α2-AR is involved in blood pressure reduction, inhibition of
presynaptic neurotransmitter release, lipolysis and insulin
secretion, as well as augmentation of platelet aggregation
(13). The three α2-AR subtypes
exhibit similar affinities for endogenous catecholamines, but
differ in their pharmacological properties, tissue distribution and
sensitivity to receptor desensitization and phosphorylation
(13).
Although the clinical significance of the α2-AR
subtypes and their genetic variants for human disease has yet to be
fully elucidated, several case-control and population-based studies
have reported positive associations among genetic polymorphisms of
ADRA2A, ADRA2B and ADRA2C and several vascular
diseases as follows: i) ADRA2A 1780G>A polymorphism
(rs553668) and hypertension (14)
and diabetes mellitus (15), ii)
ADRA2B 301–303 insertion/deletion (I/D) polymorphism
(rs28365031) and hypertension and coronary arterial disease
(14,16); and iii) ADRA2C 322–325I/D
polymorphism (rs61767072) and congestive heart failure (17).
The objective of the present study was to
investigate associations of ADRA2A 1780G>A, ADRA2B
301–303I/D and ADRA2C 322–325I/D polymorphisms with SBI and
its risk factors.
Materials and methods
Subjects
The study population comprised 361 patients with SBI
(151 males, 210 females) and 448 control subjects (186 males, 262
females). The patients were enrolled from July 1, 2000 to February
28, 2008 in the Neurology Department at CHA Bundang Medical Center
(Seongnam, South Korea) by consecutive referral. Control subjects
were enrolled from individuals who came to CHA Bundang Medical
Center for health examinations.
Patients with a known history of stroke or
cardiovascular disease were excluded. MRI was performed with a
1.5-T superconducting magnet (Siemens Magnetom Symphony, Erlangen,
Germany). Transverse T1-weighted, T2-weighted, and fluid-attenuated
inversion recovery (FLAIR) images were obtained with a slice
thickness of 7 mm. SBI was diagnosed based on the following
criteria (17,18): i) Spotty areas ≥3 mm in diameter in
the area supplied by deep perforating arteries, showing high
intensity in the T2 and FLAIR images and low intensity in the T1
image; ii) absence of neurological symptoms corresponding to the
MRI lesions; iii) no history of clinical stroke, including
transient ischemic attack; iv) no prior ischemic heart disease; v)
≥40 years of age and vi) Korean descendance (residency in Seoul or
Kyeonggi, South Korea). Among the initial 1,000 participants
evaluated, 191 met the exclusion criteria, leaving 448 controls and
361 cases. The diagnosis of SBI was made when two independent
researchers agreed on the diagnosis. Small, punctate hyperintense
lesions (1–2 mm in diameter) were more likely to represent a
dilated perivascular space and were not considered in the present
study.
Control subjects were selected from gender- and age-
(within 4.5 years) matched individuals presenting at the hospital
for a health examination that included biochemical assessment, an
electrocardiogram and brain MRI during the same period, and who had
no history of cerebrovascular disease (including SBI) or of
myocardial infarction. A total of 448 age- and gender-matched
control subjects (mean age ± standard deviation, 64.07±11.30 years;
males, 41.5%) were included in the present study. The enrollment
was conducted by matching age and gender in the control group to
the mean age and frequency of gender in the stroke group. Baseline
demographic data and a history of conventional vascular risk
factors were obtained from each control subject. With regard to the
patient group, patients with a known history of stroke or
cardiovascular disease were excluded.
Hypertension was diagnosed as a high baseline blood
pressure (systolic blood pressure ≥140 mm Hg or diastolic blood
pressure ≥90 mm Hg) on more than one occasion or current treatment
with antihypertensive medication. Diabetes mellitus was defined as
high fasting plasma glucose levels (≥126 mg/dl) or current
treatment with an oral hypoglycemic agent or insulin.
Hyperlipidemia was defined as high fasting serum total cholesterol
levels (≥240 mg/dl) or a history of antihyperlipidemic
treatment.
Informed consent was obtained from all study
participants once a full explanation of the study had been
provided. The institutional review board of CHA Bundang Medical
Center approved the present genetic study in June 2000.
Assessment of homocysteine, vitamin B12
and folate concentrations
Blood was collected in a tube containing
anticoagulant after 12 h of fasting. The tube was centrifuged for
15 min at 1,000 × g and the plasma was separated from the whole
blood. The concentration of homocysteine in the plasma was measured
using a fluorescent polarizing immunoassay with IMx (Abbott
Laboratories, Abbott Park, IL, USA). Folate and vitamin B12 were
assessed in plasma using the ACS:180 radioassay (Bayer Industries,
Tarrytown, NY, USA).
Genotyping of ADRA2A 1780G>A, ADRA2B
301–303I/D and ADRA2C 322–325I/D
Genomic DNA was extracted from anticoagulant-treated
peripheral blood using the G-DEX blood extraction kit (Intron
Biotechnology, Seongnam, South Korea). ADRA2A 1780G>A
polymorphisms were determined by polymerase chain reaction
(PCR)-restriction fragment length polymorphism (RFLP) analysis with
the following primers, generating a 211-bp product: Forward, 5′-ACT
GGA CTA CAA GGG CAT GG-3′ and reverse, 5′-ACA TCA AAA CCA AGG CCA
AG-3′. The PCR product was incubated with 5 units DraI (New
England Biolabs, Beverly, MA, USA) at 37°C for 16 h. The ADRA2A
1780A allele was cut into two fragments of 113 and 98 bp,
whereas the ADRA2A 1780G allele was uncut.
ADRA2B and ADRA2C polymorphisms were
screened by PCR using the following primers: ADRA2B forward,
5′-AGG GTG TTT GTG GGG CAT CT-3′ and reverse, 5′-CAA GCT GAG GCC
GGA GAC ACT-3′; ADRA2C forward, 5′-AGC CGG ACG AGA GCA GCG
CA-3′ and reverse, 5′-AGG CCT CGC GGC AGA TGC CGT ACA-3′. The sizes
of ADRA2B and ADRA2C with the insertion polymorphisms
were 112 and 384 bp, whereas those of ADRA2B and
ADRA2C with deletion polymorphisms were 103 and 372 bp,
respectively. The products were electrophoretically resolved on a
4.5% agarose gel stained with ethidium bromide and visualized under
ultraviolet illumination. For three studied polymorphisms, 30% of
the PCR assays were randomly selected for a second PCR assay
followed by DNA sequencing to validate the RFLP findings.
Sequencing was performed using an ABI 3730xl DNA Analyzer (Applied
Biosystems, Foster City, CA, USA). The concordance of the quality
control samples was 100%.
Statistical analysis
To estimate the relative risk for SBI for the
various genotypes, an adjusted odds ratio (AOR) and 95% confidence
interval (CI) were calculated. Case and control groups were
compared using the Mann Whitney-test for continuous variables and
the χ2 test for categorical variables. For the
multivariate analysis, logistic regression analysis was used to
adjust for possible confounders, including age, gender,
hypertension, diabetes mellitus and hyperlipidemia. The analyses
were performed using GraphPad Prism 4.0 (GraphPad Software, Inc.,
San Diego, CA, USA), StatsDirect Statistical Software 2.4.4
(StatsDirect Ltd., Altrincham, UK) and MedCalc 12.0 (Frank
Schoonjans, Ostend, Belgium). Multiple hypothesis testing was
performed using the Benjamini-Hochberg method to control for false
discovery rate (FDR) in the logistic regression analysis (19). The calculation of the FDR is a
technique to address the problems associated with multiple
comparisons and provides a measure of the expected proportion of
false-positives among the data.
Results
The demographic characteristics of the 361 patients
with SBI and 448 controls are shown in Table I. The SBI and control populations
consisted of 41.8 and 41.5% males, respectively. The mean ages of
the SBI and control populations were 64.29±11.65 and 64.07±11.30
years, respectively. Few significant differences were observed
between the two groups. However, total plasma homocysteine levels
were significantly higher in patients with SBI than those in the
controls. Table II presents a
comparison of genotype frequencies of ADRA2A 1780G>A,
ADRA2B 301–303I/D and ADRA2C 322–325I/D polymorphisms
between the case and control groups. The frequency of the
ADRA2C 322–325I/D polymorphism was significantly associated
with an increased risk of SBI (AOR, 2.026; 95% CI, 1.335–3.075;
P=0.001).
 | Table IBaseline characteristics of controls
(n=488) and patients with SBI (n=361). |
Table I
Baseline characteristics of controls
(n=488) and patients with SBI (n=361).
| Characteristic | Controls | Patients with
SBI | P-value |
|---|
| Male, n (%) | 186 (41.5) | 151 (41.8) | 0.929 |
| Age in years (mean ±
SD) | 64.07±11.30 | 64.29±11.65 | 0.784 |
| tHCy in μmol/l,
mean ± SD (total n) | 10.11±4.21
(444) | 11.48±6.46
(359) |
<0.001 |
| Folate in nmol/l,
mean ± SD (total n) | 9.69±8.99
(329) | 9.07±5.94
(343) | 0.289 |
| Vitamin B12 in
pg/ml, mean ± SD (total n) | 751.86±733.84
(321) | 763.90±1419.53
(342) | 0.890 |
| Total cholesterol
in mg/dl, mean ± SD (total n) | 192.63±44.73
(433) | 204.76±41.91
(340) |
<0.001 |
| Triglyceride in
mg/dl, mean ± SD (total n) | 142.37±88.10
(433) | 159.20±121.87
(338) | 0.033 |
| Hypertension, n
(%) | 217 (48.4) | 184 (51.0) | 0.480 |
| Diabetes mellitus,
n (%) | 71 (15.8) | 53 (14.7) | 0.695 |
| Hyperlipidemia, n
(%) | 99 (22.1) | 92 (25.5) | 0.279 |
 | Table IIGenotype frequencies of
ADRA2A, ADRA2B and ADRA2C polymorphisms. |
Table II
Genotype frequencies of
ADRA2A, ADRA2B and ADRA2C polymorphisms.
|
Characteristics | Controls, n
(%) | Patients with SBI,
n (%) | AOR (95% CI) | β-Coefficient | Standard error | P-value | FDR-P | Statistical power
(%) |
|---|
| ADRA2A 1780
G>A |
| GG | 149 (33.3) | 123 (34.1) | 1.000 | | | | | |
| GA | 230 (51.3) | 180 (49.9) | 0.932
(0.683–1.272) | −0.070 | 0.159 | 0.659 | 0.659 | 6.4 |
| AA | 69 (15.4) | 58 (16.0) | 1.015
(0.660–1.560) | 0.015 | 0.219 | 0.946 | 0.946 | 5.1 |
| Dominant, GG vs.
GA+AA | | | 0.953
(0.710–1.280) | −0.048 | 0.151 | 0.750 | 0.750 | 5.7 |
| Recessive, GG+GA
vs. AA | | | 1.054
(0.720–1.544) | 0.053 | 0.195 | 0.787 | 0.787 | 5.7 |
| ADRA2B
301–303 I/D |
| II | 176 (39.3) | 149 (41.3) | 1.000 | | | | | |
| ID | 224 (50.0) | 157 (43.5) | 0.814
(0.602–1.099) | −0.206 | 0.154 | 0.179 | 0.269 | 23.7 |
| DD | 48 (10.7) | 55 (15.2) | 1.373
(0.878–2.145) | 0.317 | 0.228 | 0.165 | 0.329 | 27.3 |
| Dominant, II vs.
ID+DD | | | 0.913
(0.687–1.213) | −0.091 | 0.145 | 0.530 | 0.750 | 8.9 |
| Recessive, II+ID
vs. DD | | | 1.506
(0.994–2.283) | 0.410 | 0.212 | 0.054 | 0.107 | 48.1 |
| ADRA2C
322–325 I/D |
| II | 405 (90.4) | 298 (82.5) | 1.000 | | | | | |
| ID | 43 (9.6) | 63 (17.5) | 2.026
(1.335–3.075) | 0.706 | 0.213 | 0.001 | 0.003 | 90.7 |
To clarify the clinical significance of the
ADRA2 polymorphisms, interaction analyses were performed for
vascular risk factors hypertension, diabetes mellitus,
hyperlipidemia and total plasma homocysteine levels according to
the ADRA2 genotypes (Table
III). The ADRA2C 322–325I/D genotype was associated with
an increased risk for SBI in the presence of hypertension (AOR,
2.952; 95% CI, 1.561–5.584) and also in the presence of elevated
total plasma homocysteine levels (AOR, 9.223; 95% CI,
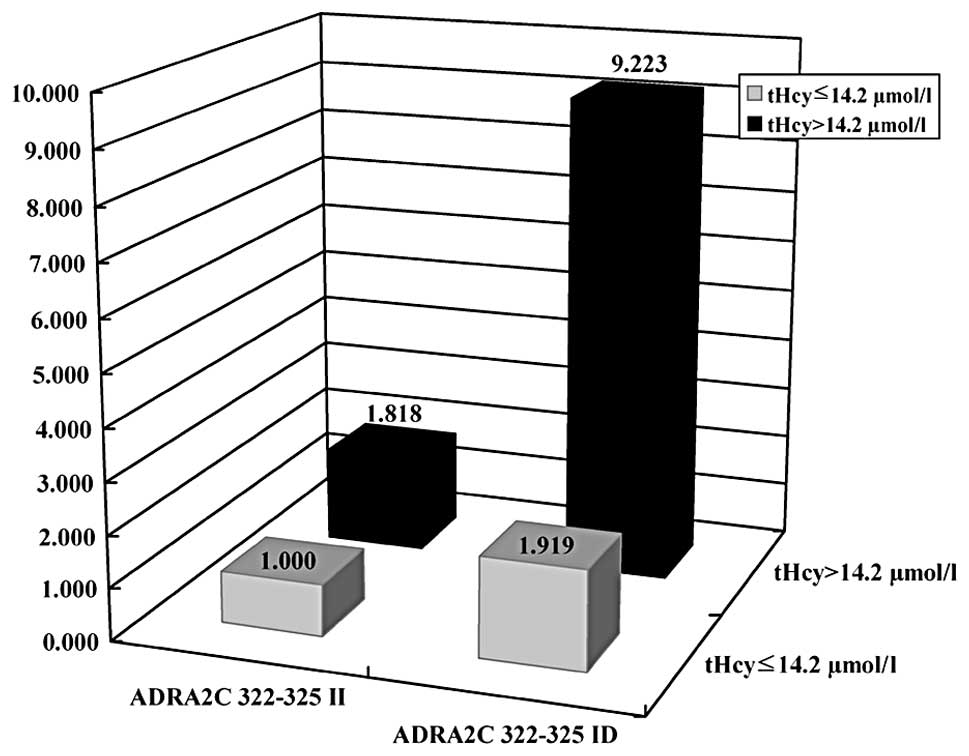
2.037–41.759). Fig. 1 shows the
combined effects of ADRA2C 322–325I/D and plasma
homocysteine levels.
 | Table IIICombinatorial effects of ADRA2
genotypes with individual vascular risk factors for silent brain
infarction occurrence. |
Table III
Combinatorial effects of ADRA2
genotypes with individual vascular risk factors for silent brain
infarction occurrence.
| ADRA2A 1780
G>A | ADRA2B
301–303 I>D | ADRA2C
322–325 I>D |
|---|
|
|
|
|
|---|
| Genotypes | GG+GA | AA | II+ID | DD | II | ID |
|---|
| Hypertension |
| (−) | 1.000
(reference) | 0.756
(0.444–1.288) | 1.000
(reference) | 0.860
(0.547–1.352) | 1.000
(reference) | 1.068
(0.782–1.458) |
| (+) | 1.042
(0.760–1.428) | 1.596
(0.891–2.860) | 0.752
(0.500–1.130) | 0.956
(0.627–1.457) | 1.656
(0.921–2.978) | 2.952
(1.561–5.584) |
| Diabetes
mellitus |
| (−) | 1.000
(reference) | 0.922
(0.611–1.392) | 1.000
(reference) | 0.804
(0.516–1.253) | 1.000
(reference) | 0.833
(0.536–1.296) |
| (+) | 0.760
(0.491–1.177) | 2.014
(0.711–5.705) | 1.474
(0.932–2.332) | 1.367
(0.544–3.435) | 2.102
(1.325–3.335) | 1.420
(0.560–3.601) |
| Hyperlipidemia |
| (−) | 1.000
(reference) | 0.865
(0.557–1.344) | 1.000
(reference) | 1.335
(0.932–1.910) | 1.000
(reference) | 1.449
(1.016–2.068) |
| (+) | 1.125
(0.783–1.616) | 2.124
(0.977–4.619) | 1.735
(1.074–2.802) | 1.334
(0.594–2.999) | 2.600
(1.616–4.181) | 1.130
(0.464–2.757) |
| tHCya |
| ≤14.2 μmol/l | 1.000
(reference) | 1.068
(0.707–1.615) | 1.000
(reference) | 1.815
(1.162–2.833) | 1.000
(reference) | 1.919
(1.228–3.000) |
| >14.2
μmol/l | 2.002
(1.248–3.213) | 1.982
(0.735–5.344) | 2.332
(1.467–3.708) | 1.212
(0.364–4.040) | 1.818
(1.145–2.887) | 9.223
(2.037–41.759) |
ADRA2 polymorphisms were associated with
plasma homocysteine and vitamin B12. The ADRA2A 1780AA
genotype was associated with elevated plasma homocysteine levels
(Table IV), while the
ADRA2B 301–303DD genotype was associated with lower vitamin
B12 levels (Table V).
 | Table IVGenetic associations of
ADRA2A, ADRA2B and ADRA2C polymorphisms with
plasma Hcy levels. |
Table IV
Genetic associations of
ADRA2A, ADRA2B and ADRA2C polymorphisms with
plasma Hcy levels.
| ADRA2A
1780G>A | ADRA2B
301–303 I/D | ADRA2C
322–325 I/D |
|---|
|
|
|
|
|---|
| Hcy decile
(μmol/l) | AA/GG+GA | AORtrend
(95% CI) |
P-valuetrend | DD/II+ID | AORtrend
(95% CI) |
P-valuetrend | ID/II | AORtrend
(95% CI) |
P-valuetrend |
|---|
| Hcy≤6.69 | 7/74 | 1.160
(1.025–1.313)a |
0.019a | 11/70 | 1.099
(0.960–1.258)a | 0.173a | 11/70 | 1.016
(0.889–1.160)a | 0.816a |
|
6.69<Hcy≤7.56 | 12/68 | | | 5/75 | | | 12/68 | | |
|
7.56<Hcy≤8.34 | 14/66 | | | 11/69 | | | 9/71 | | |
|
8.34<Hcy≤8.95 | 13/67 | | | 7/73 | | | 6/74 | | |
|
8.95<Hcy≤9.75 | 10/70 | | | 9/71 | | | 9/71 | | |
|
9.75<Hcy≤10.42 | 13/68 | | | 13/68 | | | 13/68 | | |
|
10.42<Hcy≤11.40 | 18/63 | | | 14/67 | | | 15/66 | | |
|
11.40<Hcy≤12.60 | 9/72 | | | 14/67 | | | 9/72 | | |
|
12.60<Hcy≤15.14 | 14/64 | | | 9/69 | | | 13/65 | | |
| Hcy>15.14 | 14/64 | | | 9/71 | | | 8/72 | | |
 | Table VGenetic associations of
ADRA2A, ADRA2B and ADRA2C polymorphisms with
VB12 levels. |
Table V
Genetic associations of
ADRA2A, ADRA2B and ADRA2C polymorphisms with
VB12 levels.
| ADRA2A
1780G>A | ADRA2B
301–303 I/D | ADRA2C
322–325 I/D |
|---|
|
|
|
|
|---|
| VB12 decile
(pg/ml) | AA/GG+GA | AORtrend
(95% CI) |
P-valuetrend | DD/II+ID | AORtrend
(95% CI) |
P-valuetrend | ID/II | AORtrend
(95% CI) |
P-valuetrend |
|---|
| VB12≤351 | 9/58 | 1.091
(0.957–1.245)a | 0.193a | 6/61 | 0.862
(0.750–0.991)a |
0.037a | 9/58 | 1.081
(0.937–1.246)a | 0.285a |
|
351<VB12≤435 | 5/62 | | | 11/56 | | | 7/60 | | |
|
435<VB12≤490 | 10/55 | | | 9/56 | | | 7/58 | | |
|
490<VB12≤547 | 13/54 | | | 17/50 | | | 10/57 | | |
|
547<VB12≤600 | 16/50 | | | 8/58 | | | 10/56 | | |
|
600<VB12≤669 | 14/52 | | | 11/55 | | | 8/58 | | |
|
669<VB12≤745 | 14/54 | | | 11/57 | | | 12/56 | | |
| 45<VB12≤880 | 11/55 | | | 8/58 | | | 9/57 | | |
|
880<VB12≤1123 | 6/59 | | | 9/56 | | | 11/54 | | |
| VB12>1123 | 11/55 | | | 3/63 | | | 8/58 | | |
Discussion
In the present study, the risk for SBI in the Korean
population was evaluated by investigating the associations between
SBI and the three polymorphisms of the ADRA2 gene family
alone and in combination with individual vascular risk factors. The
results indicate that the ADRA2C 322–325ID and ADRA2A
1780AA genotypes were associated with a risk of SBI. The
ADRA2C 322–325ID polymorphism was associated with increased
plasma homocysteine levels. Furthermore, the ADRA2A 1780AA
genotype was associated with higher plasma homocysteine levels.
Hyperhomocysteinemia is usually caused by a mutation
in the methylenetetrahydrofolate reductase gene and is linked with
vascular diseases associated with thrombosis, induced hypertension
and angiopathy. In addition, for the past decade, mildly elevated
plasma homocysteine levels have been recognized as a risk factor
for a number of occlusive vascular diseases. Hyperhomocysteinemia
is associated with an increased risk for SBI (8,9).
The ADRA2 gene family is well known to have
several vascular functions, including maintenance of basal cerebral
blood flow, cerebral vasodilation, vascular integrity and the
normal functions of vascular smooth muscle cells (20–23).
High levels of α2-AR activity are found in blood vessels, where
these receptors act as temperature receptors to mediate
cold-induced vasoconstriction. α2-AR stimulation causes calcium
mobilization in the vascular smooth muscle cells, which is involved
in the contraction and relaxation of blood vessels, affecting blood
pressure (24). However, the ADRA2
proteins are differentially distributed in various tissue types and
have different physiological functions and pharmacological
activities. mRNAs encoding three subtypes of α2-ARs have been
observed in the central nervous system; however, while the
distribution of α2B-ARs is limited to the thalamus, α2A- and
α2C-ARs are widely distributed throughout the brain (13). These data suggest that α2A- and
α2C-ARs are more likely than α2B-ARs to be associated with
physiological conditions of the whole brain.
In previous studies, the A allele of ADRA2A
1780G>A was associated with increased α2A-AR expression,
hypertension, diabetes mellitus and decreased insulin secretion
(14,15). The ADRA2B 301–303I/D
polymorphism has been associated with impaired agonist-dependent
phosphorylation, loss of desensitization and with sustained
signaling of α2B-AR, despite continued activation by α2-agonists
and subsequent prolonged vasoconstriction (25). Several studies have reported that
the ADRA2B 301–303I/D polymorphism is associated with
clinical vascular diseases, including cardiovascular diseases
(26,27). Although the clinical impact of the
ADRA2C 322–325I/D polymorphism on human disease is
inconclusive, the D allele of ADRA2C 322–325 has been
associated with cardiac disorder (17) and may be linked to blood flow or
vascular function as a result of its association with decreased
function in vitro (28).
In the present study, the importance of the
ADRA2C 322–325I/D polymorphism was demonstrated by several
associations. The interaction analysis of hypertension and the
ADRA2C 322–325I/D genotype showed a significant association.
It is suggested that there may be a positive interaction of the
ADRA2C 322–325I/D polymorphism with hypertension or cerebral
blood flow to increase the risk of SBI. Furthermore, the
ADRA2C 322–325I/D polymorphism was associated with elevated
plasma levels of homocysteine. It is proposed that the combination
of ADRA2C 322–325I/D and higher plasma levels of
homocysteine has a strong synergistic effect that increases the
risk for SBI. ADRA2C 322–325I/D and higher plasma levels of
homocysteine are risk factors that induce SBI. The interaction of
the two factors increases the risk of developing SBI. The
ADRA2A 1780G>A polymorphism was also associated with
elevated plasma levels of homocysteine, with the ADRA2A
1780AA genotype tending to be associated with higher levels.
Several limitations of the present study warrant
consideration. As the participation rate for the present study was
low, the recruitment phase was extended over a long time period.
Furthermore, the control subjects in the present study were not
completely healthy, since some of them had sought medical
attention. According to previous experience, recruitment of healthy
participants with imaging and laboratory tests would markedly
reduce the enrollment rate. However, enrollment of participants
without imaging and laboratory tests may produce another bias in
vascular risk factor assessment. Finally, the present study did not
collect sufficient data on smoking, a risk factor associated with
SBI. Therefore, no correlation test was performed for smoking. As
smoking is a risk factor for vascular diseases, including stroke
and white matter lesion, it is expected to be associated with SBI.
However, the ADRA2 gene family is known to affect vascular
disorders regardless of smoking (24). It is suggested that a single RFLP
approach of ADRA2C 322–325 I/D polymorphism may have
considerable clinical utility.
In conclusion, it was observed that the
ADRA2C 322–325I/D genotype increased the risk of SBI.
Furthermore, the combination of the ADRA2C 322–325I/D
genotype and high plasma levels of homocysteine had a strong
synergistic effect that increased the risk for SBI. It is suggested
that the ADRA2C 322–325I/D genotype is a novel genetic risk
marker for SBI among individuals with hyperhomocysteinemia. Further
studies using larger and more heterogeneous cohorts are required to
validate the association of ADRA2 polymorphisms with
SBI.
Acknowledgements
The present study was supported by grants from the
Basic Science Research Program (NRF-2012R1A1A2007033 and
NRF-2013R1A1A2008177) and the Priority Research Centers Program
(2009-0093821) through the National Research Foundation of Korea,
funded by the Ministry of Education, Science and Technology,
Republic of Korea.
References
|
1
|
Han IB, Kim OJ, Ropper AE, Kim HS, Cho YK,
Teng YD and Kim NK: Association between kinase insert
domain-containing receptor gene polymorphisms and silent brain
infarction: a Korean study. J Neurol Sci. 318:85–89. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Song J, Kim OJ, Kim HS, Bae SJ, Hong SP,
Oh D and Kim NK: Endothelial nitric oxide synthase gene
polymorphisms and the risk of silent brain infarction. Int J Mol
Med. 25:819–823. 2010.PubMed/NCBI
|
|
3
|
Kobayashi S, Okada K, Koide H, Bokura H
and Yamaguchi S: Subcortical silent brain infarction as a risk
factor for clinical stroke. Stroke. 28:1932–1939. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuller LH, Shemanski L, Manolio T, Haan M,
Fried L, Bryan N, Burke GL, Tracy R and Bhadelia R: Relationship
between ApoE, MRI findings, and cognitive function in the
Cardiovascular Health Study. Stroke. 29:388–398. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Barber R, Scheltens P, Gholkar A, Ballard
C, McKeith I, Ince P, Perry R and O’Brien J: White matter lesions
on magnetic resonance imaging in dementia with Lewy bodies,
Alzheimer’s disease, vascular dementia, and normal aging. J Neurol
Neurosurg Psychiatry. 67:66–72. 1999.
|
|
6
|
Vermeer SE, Koudstaal PJ, Oudkerk M,
Hofman A and Breteler MM: Prevalence and risk factors of silent
brain infarcts in the population-based Rotterdam Scan Study.
Stroke. 33:21–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vermeer SE, Hollander M, van Dijk EJ,
Hofman A, Koudstaal PJ and Breteler MM; Rotterdam Scan Study.
Silent brain infarcts and white matter lesions increase stroke risk
in the general population: the Rotterdam Scan Study. Stroke.
34:1126–1129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsui T, Arai H, Yuzuriha T, Yao H, Miura
M, Hashimoto S, Higuchi S, Matsushita S, Morikawa M, Kato A and
Sasaki H: Elevated plasma homocysteine levels and risk of silent
brain infarction in elderly people. Stroke. 32:1116–1119. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim NK, Choi BO, Jung WS, Choi YJ and Choi
KG: Hyperhomocysteinemia as an independent risk factor for silent
brain infarction. Neurology. 61:1595–1599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Eguchi K, Kario K, Hoshide S, Hoshide Y,
Ishikawa J, Morinari M, Hashimoto T and Shimada K: Smoking is
associated with silent cerebrovascular disease in a high-risk
Japanese community-dwelling population. Hypertens Res. 27:747–754.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kwon HM, Kim BJ, Lee SH, Choi SH, Oh BH
and Yoon BW: Metabolic syndrome as an independent risk factor of
silent brain infarction in healthy people. Stroke. 37:466–470.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oh SH, Min KT, Jeon YJ, Kim MH, Kim OJ,
Shin BS, Oh D and Kim NK: Association between common genetic
variants of α2A-, α2B-, and α2C-adrenergic receptors and ischemic
stroke. Clin Neurol Neurosurg. 115:26–31. 2013.
|
|
13
|
MacDonald E, Kobilka BK and Scheinin M:
Gene targeting - homing in on alpha 2-adrenoceptor-subtype
function. Trends Pharmacol Sci. 18:211–219. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lockette W, Ghosh S, Farrow S, MacKenzie
S, Baker S, Miles P, Schork A and Cadaret L: Alpha 2-adrenergic
receptor gene polymorphism and hypertension in blacks. Am J
Hypertens. 8:390–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosengren AH, Jokubka R, Tojjar D,
Granhall C, Hansson O, Li DQ, Nagaraj V, Reinbothe TM, Tuncel J,
Eliasson L, Groop L, Rorsman P, Salehi A, Lyssenko V, Luthman H and
Renström E: Overexpression of alpha2A-adrenergic receptors
contributes to type 2 diabetes. Science. 327:217–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobilka BK, Matsui H, Kobilka TS,
Yang-Feng TL, Francke U, Caron MG, Lefkowitz RJ and Regan JW:
Cloning, sequencing, and expression of the gene coding for the
human platelet alpha 2-adrenergic receptor. Science. 238:650–656.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Small KM, Wagoner LE, Levin AM, Kardia SL
and Liggett SB: Synergistic polymorphisms of beta1-and
alpha2C-adrenergic receptor and the risk of congestive heart
failure. N Engl J Med. 347:1135–1142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim OJ, Lee JH, Choi JK, Oh SH, Hong SH,
Oh D and Kim NK: Association between tumor necrosis factor-alpha
(-308G→A and -238G→A) polymorphisms and homocysteine levels in
patients with ischemic strokes and silent brain infarctions.
Cerebrovasc Dis. 30:483–490. 2010.
|
|
19
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: a practical and powerful approach to
multiple testing. J R Statist Soc B. 57:289–300. 1995.
|
|
20
|
Avery RA, Franowicz JS, Studholme C, van
Dyck CH and Arnsten AF: The alpha-2A-adrenoceptor agonist,
guanfacine, increases regional cerebral blood flow in dorsolateral
prefrontal cortex of monkeys performing a spatial working memory
task. Neuropsychopharmacology. 23:240–249. 2000. View Article : Google Scholar
|
|
21
|
Willems EW, Valdivia LF, Villalón CM and
Saxena PR: Possible role of alpha-adrenoceptor subtypes in acute
migraine therapy. Cephalalgia. 23:245–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaya S, Kolodjaschna J, Berisha F, Polska
E, Pemp B, Garhöfer G and Schmetterer L: Effect of the
α(2)-adrenoceptor antagonist yohimbine on vascular regulation of
the middle cerebral artery and the ophthalmic artery in healthy
subjects. Microvasc Res. 81:117–122. 2011.
|
|
23
|
Neumeister A, Charney DS, Belfer I, Geraci
M, Holmes C, Sharabi Y, Alim T, Bonne O, Luckenbaugh DA, Manji H,
Goldman D and Goldstein DS: Sympathoneural and adrenomedullary
functional effects of alpha2C-adrenoreceptor gene polymorphism in
healthy humans. Pharmacogenet Genomics. 15:143–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chotani MA, Mitra S, Su BY, Flavahan S,
Eid AH, Clark KR, Montague CR, Paris H, Handy DE and Flavahan NA:
Regulation of alpha(2)-adrenoceptors in human vascular smooth
muscle cells. Am J Physiol Heart Circ Physiol. 286:H59–H67. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Small KM, Brown KM, Forbes SL and Liggett
SB: Polymorphic deletion of three intracellular acidic residues of
the alpha 2B-adrenergic receptor decreases G protein-coupled
receptor kinase-mediated phosphorylation and desensitization. J
Biol Chem. 276:4917–4922. 2001. View Article : Google Scholar
|
|
26
|
Snapir A, Heinonen P, Tuomainen TP,
Alhopuro P, Karvonen MK, Lakka TA, Nyyssönen K, Salonen R, Kauhanen
J, Valkonen VP, Pesonen U, Koulu M, Scheinin M and Salonen JT: An
insertion/deletion polymorphism in the alpha2b-adrenergic receptor
gene is a novel genetic risk factor for acute coronary events. J Am
Coll Cardiol. 37:1516–1522. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Snapir A, Mikkelsson J, Perola M, Penttilä
A, Scheinin M and Karhunen PJ: Variation in the
alpha2B-adrenoceptor gene as a risk factor for prehospital fatal
myocardial infarction and sudden cardiac death. J Am Coll Cardiol.
41:190–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Small KM, Forbes SL, Rahman FF, Bridges KM
and Liggett SB: A four amino acid deletion polymorphism in the
third intracellular loop of the human alpha 2C-adrenergic receptor
confers impaired coupling to multiple effectors. J Biol Chem.
275:23059–23064. 2000. View Article : Google Scholar
|