1. Risk factors of breast cancer
Breast cancer is a common disease estimated to occur
in 1 in 9 women over their lifetime. Thus, a large population is
available for epidemiological, genetic and molecular studies. The
aetiology of human breast cancer is unknown; however, it is a
complex disease, characterized by a progressive multistep process
caused by interactions of both genetic and non-genetic factors.
Epidemiological research has identified a number of
risk factors for breast cancer. However, racial and ethnic
differences in breast cancer mortality rates have been difficult to
ascertain (1). Possible
explanations include differences in the biological characteristics
of the tumor (2), patient
characteristics such as obesity that may affect prognosis,
frequency of mammography examinations (3), timeliness and completeness of breast
cancer diagnosis and treatment (4,5),
social factors such as education, literacy and cultural beliefs,
and economic factors (6–8).
Age is the strongest demographic risk factor for
most human malignancies, including breast cancer (9). Approximately 80% of all breast cancers
occur in women >50 years of age; the 10-year probability of
developing invasive breast cancer increases from ~1.5% at age 40,
to ~3% at age 50 and to ~4% by the age of 70, resulting in a
cumulative lifetime risk of 13.2% (1 in 8) and a near 9-fold higher
incidence rate in women >50 years of age as compared to their
younger counterparts (10).
Fig. 1A shows the increase in the
incidence of breast cancer in Arica, Chile, from 1997 to 2007,
particularly in 2005, reaching 55.1% per 100,000 women. This
percentage decreased in 2006 and 2007, but was higher than the
previous years. Fig. 1B shows the
distribution of patients by age in relation to the incidence of
breast cancer. A greater percentage, reaching ~25.6%, was noted
among individuals between 46 and 65 years of age. The Indian
population, Aymara, showed only a 13.9% incidence as noted in
Fig. 1C. Fig. 1D shows that the incidence of breast
cancer in patients with no family history was ~88%, with or without
Indian background.
2. Cancer biomarkers
Tumor biology is altered with aging, and at the
cellular level it has been linked to increased genomic instability,
global and promoter-specific epigenetic changes and altered
expression of genes involved in cell division and extracellular
matrix remodelling (11–16). A number of associations have led to
the hypothesis that cancer in older individuals results from
cumulative mutations, increased epigenetic gene silencing, telomere
dysfunction and altered estromal milieu (17). Younger age at diagnosis (≤45 years
of age) is associated with more aggressive breast cancer
biomarkers, including overexpression of the ERBB2 growth factor
receptor (18), abnormal p53
expression (19), estrogen receptor
(ER) negativity (18–20), higher nuclear grade and higher Ki-67
proliferation index (19,20).
Even though breast cancer biomarkers are
interdependent, ER expression in particular, is inversely
correlated with abnormal p53 (19),
overexpression of ERBB2 (19), a
high Ki-67 and nuclear grade and poor prognosis (21). In general, the normal mammary gland
ER content as well as the proportion of ER-expressing (ER-positive)
in ductal epithelial cells increase with each decade of age and
reach a plateau with menopause at age ~50 years (22,23).
By contrast, breast cancer ER expression continues to increase
beyond menopause, reaching an ~25-fold difference between normal
and malignant mammary gland ER expression by the age 70 years
(22). Notably, the expression of
certain ER-inducible gene markers, such as progesterone receptor
(PR), pS2, Bcl2 and cathepsin D, do not show any significant
relationship with age at diagnosis (18,22),
while other markers show an increased expression in breast cancers
arising earlier in life (24).
Of importance is the age-related change in PR
co-expression within ER-positive breast cancers, since PR has been
used extensively as a clinical indicator for a functioning ER
pathway in tumors likely to respond to endocrine therapy (25). Among all ethnic patient groups,
ER-positive/PR-negative breast cancers showed the greatest
age-related increase in incidence after the age of 40 (26). Potentially relevant to this
ER-positive/PR-negative phenotype is the fact that
growth-factor-activated pathways down-regulate PR expression
(26) and that the inverse
correlation between overexpression of the ERBB2 growth factor
receptor and positive PR is only noted in breast cancers arising
after the age of 40 (27). The
age-adjusted breast cancer incidence rates for women of
racial/ethnic minority groups are substantially lower than those
for Caucasian women. In addition, African-American women are likely
to be diagnosed at a more advanced stage (28) and to have larger tumors, usually
ER-negative (29,30) and of high grade (29), than those found in Caucasian women.
African-American women also have higher breast cancer mortality
(31). On the other hand, the
association of oral contraceptives with risk of breast cancer was
similar in Hispanic and non-Hispanic white women in certain studies
(32). African-American (33,34),
Hispanic (33,35), native American (35,36),
Hawaiian and Filippino (33,37,38)
women living in the US are more likely to be diagnosed with
advanced stages of breast cancer and to have poorer survival after
diagnosis compared to non-Hispanic white women. Alternatively,
Japanese and Chinese women exhibit no difference with respect to
breast cancer stage and survival (33), they also present less advanced
stages and have better survival when compared to non-Hispanic
whites (37,38).
Differences in socio-demographic, cultural and
behavioral characteristics have been postulated. Stage and survival
differences may also be related to the differential expression of
breast tumor characteristics that have been independently shown to
be related to mortality. Specifically, hormone receptor-negative
breast tumors (39) are associated
with poorer survival, whereas those tumors that have a lobular
histology are associated with better survival (40). Previous studies suggested that
African-American women are more likely to have ER-negative,
PR-negative (41) and medullary
(42) breast tumors. One study
found that Asian women were more likely to have ER-/PR-negative
breast tumors than non-Hispanic white women (43). African-American and Hispanic white
women appear to have decreased risks of lobular carcinoma and
increased risks of medullary carcinoma compared to non-Hispanic
whites (41,42,44). A
more pronounced expression of cell cycle- and
proliferation-associated genes has emerged as a strong defining
feature of ER-positive breast cancers arising in younger women,
perhaps even leading to the earlier clinical appearance. This
observation is consistent with the more aggressive clinical nature
of early-age-onset breast cancer.
3. Hormonal replacement therapy
The use of hormone replacement therapy is associated
with an increased risk of lobular tumors (45). Breast cancer incidence rates rose
throughout the 1980s and 1990s in the US and declined during 2004.
This decline was attributed to the reduction in menopausal hormone
use (46). However, data gathered
from 1995 to 2004 did not consider either the histological type or
race/ethnicity which may have influenced these trends.
Invasive ductal carcinoma and invasive lobular
carcinoma incidence rates fell steadily from 1998 to 2004. Declines
in overall breast cancer rates and invasive ductal carcinoma were
limited to women who were 50 years of age, non-Hispanic white and
Asian/Pacific Islanders, and declines in the rates of invasive
lobular carcinoma were primarily limited to non-Hispanic white
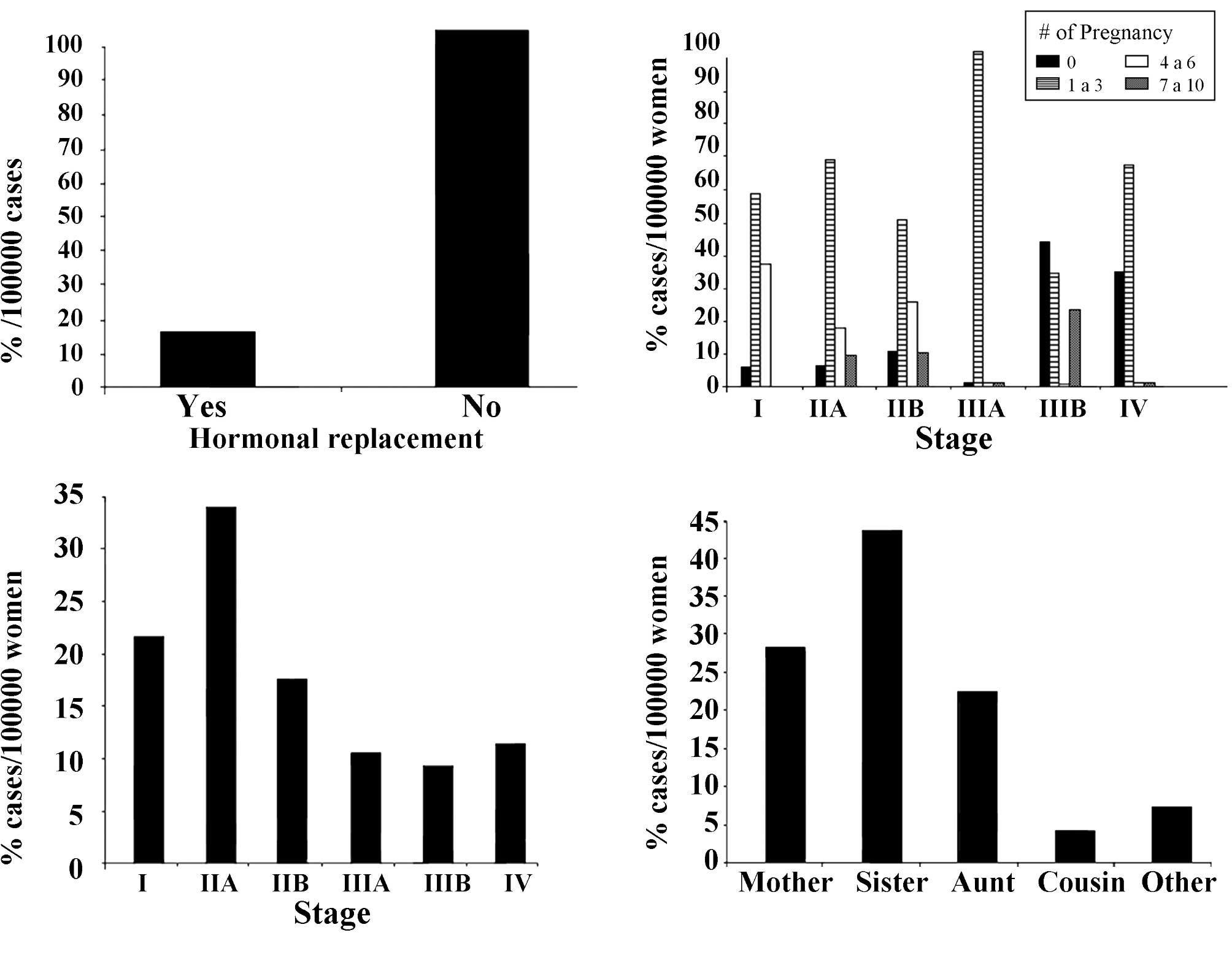
women. Fig. 2A shows the incidence
of breast cancer in Arica, Chile, from 1997 to 2007, in relation to
hormonal replacement. Of these women, 98.4% did not have such
treatment.
Fig. 2B shows the
incidence during the same years in relation to stage of the disease
and number of pregnancies. Results showed an increase in the
progression of the disease that reached 100% from stage I to stage
III in women that had 1–3 pregnancies. Reports (47) have confirmed that women of
childbearing age experience an increased breast cancer risk
associated with a completed pregnancy. For younger women, the
increase in breast cancer risk was transient, and within a decade
after parturition a crossover effect resulted in an ultimate
protective benefit. The post-partum peak of increased risk was
greater in women with advanced maternal age. Furthermore, their
lifetime risk for developing breast cancer remained elevated for a
number of years, with the crossover to protection occurring decades
later or not at all.
Fig. 2C indicates
that 20.9 and 33.2% of the breast cancer patients who received
tamoxifen treatment were in stages I and IIA, respectively.
Fig. 2D shows the incidence of
breast cancer in Arica, Chile, in relation to family history, with
the results indicating that the incidence of breast cancer reached
42.4% when the patient had a sister with the disease.
4. Breast cancer and family history
Ethnic variation in the incidence rate of breast
cancer is considerable. Non-Hispanic white women, women of various
races/ethnicities living in the US, including African-Americans,
native Americans, Filippinos, Chinese, Koreans, Vietnamese,
Indians/Pakistanis, Mexicans, South/Central Americans and Puerto
Ricans, were found to have a greater risk of presenting with breast
cancer with characteristics associated with a poor prognosis. A
combination of biological, genetic, environmental and lifestyle
differences across these populations is likely to account for these
variations. One of the strongest risk factors for breast cancer is
a family history of the disease (48), although this factor varies among
ethnic groups. In general, ethnicity has been equated with minority
status. Thus, studies on the topic are evaluations of incidence,
mortality and survival of black people, Asians (primarily Chinese,
Japanese and Filipino), Hispanics, American Indians, native
Hawaiians and Alaska natives. Migrant studies (49) have demonstrated that variability in
incidence is attributed to differences in risk factor levels.
Similarly, geographic variation within a country may also be partly
influenced by population risk factor differences (50).
Breast cancer risk is also influenced by host
differences in the genetic variation or predisposition to the
disease. Such risk has been attributed to ethnic variation in the
frequency of specific susceptibility genes. A combination of BRCA1
and BRCA2 gene mutations appears to be responsible for 20–30% of
the cases with familial breast cancer. The prevalence of BRCA1/2
pathogenic mutations largely varies within different populations;
in particular, the rate of mutations in breast and/ or ovarian
cancer families of Italian origin is controversial and ranges from
8 to 37%. A number of major genes that confer an increased
susceptibility to breast cancer when inherited in a mutated form
have been identified (51). Studies
have suggested that the relative proportion of breast/ovarian
cancer families with mutations in BRCA1 or BRCA2 varies in
different populations. For example, the percentage of familial
breast/ovarian cancer explained by BRCA1 mutations is estimated to
be 29% in Italy, 21% in Britain and 9% in Iceland (52). In addition, in the majority of
populations, BRCA1 mutations are more common than their BRCA2
counterparts in breast/ovarian cancer families, although in Iceland
BRCA2 mutations are more common than BRCA1 ones (52). Specific mutations identified in
BRCA1 or BRCA2 also differ by ethnic group. In Israel, three
specific mutations were reported to account for 36% of familial
breast/ovarian cancer (53). A
specific BRCA1 mutation, 185delAG, was observed primarily in the
Ashkenazi Jewish population (54).
Since specific mutations appear to confer different
breast cancer risks, the variation in breast cancer risk in
different populations may be attributed, in part, to underlying
differences in genetic and molecular factors (55,56).
These genetic differences may appear as ethnic-specific differences
in breast cancer risk associated with a family history of the
disease. Diet patterns and breast cancer risk in Hispanic and
non-Hispanic white women indicate that the rates of breast cancer
(57) and obesity (58) differ among ethnic groups. The rate
of breast cancer among Hispanic women is 2/3 of the rate noted
among non-Hispanic white women (57), while the rate of obesity is higher
among Hispanic women (58). An
increase in the prevalence of obesity with higher intakes of animal
protein and fat composition has previously been shown in
non-Hispanic white women, but not in Hispanic control participants
(59). Ethnic differences were
noted in the association of obesity with risk for breast cancer in
postmenopausal Hispanic and non-Hispanic white women. However, no
differences were noted in these same associations in pre-menopausal
women (60). Fig. 2D shows the percentage per 100,000
women with a breast cancer family history. The data indicated that
breast cancer reached 42.4% for individuals who had a sister with a
history of cancer.
5. Results and conclusions
The present review reports an increase in the
incidence of breast cancer in Arica, Chile, from 1997 to 2007. A
greater percentage of breast cancer was found in individuals
between 46 and 65 years of age when the population was distributed
by age. The Aymara group had only a 13.9% incidence of the disease.
The incidence of breast cancer in patients with no family
background reached approximately 88%, either with or without Indian
ethnicity. There was an increase in the progression of the disease
from stage I to stage III in women that had 1–3 pregnancies, and
20.9 and 33.2% who had received tamoxifen treatment were in stages
I and IIA, respectively. The incidence of breast cancer reached
42.4% when the patient had a sister with the disease. Therefore, it
can be concluded that important differences in breast cancer risk
factors should be identified in the future for comparison with
other biological factors such as genetic and molecular factors.
This may provide greater insight into breast cancer aetiology in
different populations.
Acknowledgements
Thanks are given to FONDECYT 1080482 (G.M.C.) and to
the Convenio de Desempeño Universidad de Tarapacá-Mineduc (G.M.C.,
F.R.). The secretarial assistance of Danissa Barahona is also
greatly appreciated.
References
|
1
|
Breen N, Kessler LG and Brown ML: Breast
cancer control among the underserved – an overview. Breast Cancer
Res Treat. 40:105–115. 1996.
|
|
2
|
Elledge RM, Clark GM, Chamness GC and
Osborne CK: Tumor biologic factors and breast cancer prognosis
among white, Hispanic and black women in the United States. J Natl
Cancer Inst. 86:705–712. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burns RB, McCarthy EP, Freund KM, Marwill
SL, Shwartz M, Ash A and Moskiwitz MA: Black women receive less
mammography even with similar use of primary care. Ann Intern Med.
125:173–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang SW, Kerlikowske K, Napoles-Springer
A, Posner SF, Sickles EA and Perez-Stable EJ: Racial differences in
timeliness of follow-up after abnormal screening mammography.
Cancer. 78:1395–1402. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Facione NC: Delay versus help seeking for
breast cancer symptoms: a critical review of the literature on
patient and provider delay. Soc Sci Med. 36:1521–1534. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gordon NH, Crowe JP, Brumberg DJ and
Berger NA: Socioeconomic factors and race in breast cancer
recurrence and survival. Am J Epidemiol. 135:609–618.
1992.PubMed/NCBI
|
|
7
|
Singh GK, Miller BA and Hankey BF:
Changing area socioeconomic patterns in US cancer mortality,
1950–1998: part II – lung and colorectal cancers. J Natl Cancer
Inst. 94:916–925. 2002.
|
|
8
|
Wells BL and Horm JW: Stage at diagnosis
in breast cancer: race and socioeconomic factors. Am J Public
Health. 82:1383–1385. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edwards B, Howe HL, Ries L, Thun M,
Rosenberg H, Wingo P, Jemal A and Feigal E: Annual report to the
nation on the status of cancer, 1973–1999, featuring implications
of age and aging on US cancer burden. Cancer. 94:2766–2792.
2000.
|
|
10
|
Smigal C, Jemal A, Ward E, Cokkinides V,
Smith R, Howe HL and Thun M: Trends in breast cancer by race and
ethnicity: update 2006. CA Cancer J Clin. 56:168–183. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benz CC, Campisi J, Cohen HJ, Ershler WB,
Haubein L and Irminger-Finger I: Meeting report: translational
research at the aging and cancer interface. Cancer Res.
67:4560–4563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geigl JB, Langer S, Barwisch S, Pfleghaar
K, Lederer G and Speicher MR: Analysis of gene expression patterns
and chromosomal changes associated with aging. Cancer Res.
64:8550–8557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Issa JP: Aging, DNA methylation and
cancer. Crit Rev Oncol Hematol. 32:31–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richardson B: Impact of aging on DNA
methylation. Ageing Res Rev. 2:245–261. 2003. View Article : Google Scholar
|
|
15
|
Ershler WB and Longo DL: Aging and cancer:
issues of basic and clinical science. J Natl Cancer Inst.
89:1489–1497. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balducci L and Ershler WB: Cancer and
ageing: a nexus at several levels. Nat Rev Cancer. 5:655–662. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DePinho RA: The age of cancer. Nature.
408:248–254. 2000. View
Article : Google Scholar
|
|
18
|
Eppenberger-Castori S, Moore DH Jr, Thor
AD, Edgerton SM, Kueng W, Eppenberger U and Benz CC: Age-associated
biomarker profiles of human breast cancer. Int J Biochem Cell Biol.
34:1318–1330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rudolph P, Olsson H, Bonatz G, Ratjen V,
Bolte H, Baldetorp B, Fernö M, Parwaresch R and Alm P: Correlation
between p53, c-erbB-2, and topoisomerase IIa expression, DNA
ploidy, hormonal receptor status and proliferation in 356
node-negative breast carcinomas: prognostic implications. J Pathol.
187:207–216. 1999. View Article : Google Scholar
|
|
20
|
Rapiti E, Fioretta G, Verkooijen HM,
Vlastos G, Schafer P, Sappino AP, Kurtz J, Neyroud-Caspar I and
Bouchardy C: Survival of young and older breast cancer patients in
Geneva from 1990 to 2001. Eur J Cancer. 41:1446–1452. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harvey JM, Clark GM, Osborne CK and Allred
DC: Estrogen receptor status by immunohistochemistry is superior to
the ligand binding assay for predicting response to adjuvant
endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481.
1999.
|
|
22
|
Quong J, Eppenberger-Castori S, Moore D
III, Scott GK, Birrer MJ, Kueng W, Eppenberger U and Benz CC:
Age-dependent changes in breast cancer hormone receptor and oxidant
stress markers. Breast Cancer Res Treat. 76:221–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shoker BS, Jarvis C, Sibson DR, Walker C
and Sloane JP: Oestrogen receptor expression in the normal and
pre-cancerous breast. J Pathol. 188:237–244. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Creighton C, Cordero K, Larios J, Miller
R, Johnson M, Chinnaiyan A, Lippman M and Rae J: Genes regulated by
estrogen in breast tumor cells in vitro are similarly regulated in
vivo in tumor xenografts and human breast tumors. Genome Biol.
7:R282006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Horwitz KB and McGuire WL: Estrogen
control of progesterone receptor in human breast cancer. J Biol
Chem. 253:2223–2228. 1978.PubMed/NCBI
|
|
26
|
Arpino G, Weiss H, Lee AV, Schiff R, De
Placido S, Osborne CK and Elledge RM: Estrogen receptor-positive,
progesterone receptor-negative breast cancer: association with
growth factor receptor expression and tamoxifen resistance. J Natl
Cancer Inst. 97:1254–1261. 2005. View Article : Google Scholar
|
|
27
|
Huang HJ, Neven P, Drijkoningen M, et al:
Association between HER-2/neu and the progesterone receptor in
oestrogen-dependent breast cancer is age-related. Breast Cancer Res
Treat. 91:81–87. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghafoor A, Jemal A, Ward E, Cokkinides V,
Smith P and Thun M: Trends in breast cancer by race and ethnicity.
CA Cancer J Clin. 53:342–355. 2003. View Article : Google Scholar
|
|
29
|
Li CI, Malone KE and Daling JR:
Differences in breast cancer hormone receptor status and histology
by race and ethnicity among women 50 years of age and older. Cancer
Epidemiol Biomarkers Prev. 11:301–307. 2002.PubMed/NCBI
|
|
30
|
Joslyn SA: Hormone receptors in breast
cancer: racial differences in distribution and survival. Breast
Cancer Res Treat. 73:45–59. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newman LA, Mason J, Cote D, Vin Y, Carolin
K, Bouwman D and Colditz GA: African-American ethnicity,
socioeconomic status and breast cancer survival: a meta-analysis of
14 studies involving over 10,000 African-American and 40,000 white
American patients with carcinoma of the breast. Cancer.
94:2844–2854. 2002. View Article : Google Scholar
|
|
32
|
Sweeney C, Giuliano AR, Baumgartner KB,
Byers T, Herrick JS, Edwards SL and Slaterry ML: Oral, injected and
implanted contraceptives and breast cancer risk among US Hispanic
and non-Hispanic white women. Int J Cancer. 21:2517–2523. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsu JL, Glaser SL and West DW:
Racial/ethnic differences in breast cancer survival among San
Francisco Bay Area women. J Natl Cancer Inst. 89:1311–1312. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chevarley F and White E: Recent trends in
breast cancer mortality among white and black US women. Am J Public
Health. 87:775–781. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Frost F, Tollestrup K, Hunt WC, Gilliland
F, Key CR and Urbina CE: Breast cancer survival among New Mexico
Hispanic, American Indian and non-Hispanic white women (1973–1992).
Cancer Epidemiol Biomarkers Prev. 4:861–866. 1996.PubMed/NCBI
|
|
36
|
Sugarman JR, Dennis LK and White E: Cancer
survival among American Indians in western Washington state (United
States). Cancer Causes Control. 5:440–448. 1994. View Article : Google Scholar
|
|
37
|
Meng L, Maskarinec G and Lee J: Ethnicity
and conditional breast cancer survival in Hawaii. J Clin Epidemiol.
50:1289–1296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng L, Maskarinec G and Wilkens L: Ethnic
differences and factors related to breast cancer survival in
Hawaii. Int J Epidemiol. 26:1151–1158. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McGuire WL and Clark GM: Prognostic
factors and treatment decisions in axillary-node-negative breast
cancer. N Engl J Med. 326:1756–1761. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Du Toit RS, Locker AP, Ellis IO, Elston
CW, Nicholson RI, Robertson JFR and Blamey RW: An evaluation of
differences in prognosis, recurrence patterns and receptor status
between invasive lobular and other invasive carcinomas of the
breast. Eur J Surg Oncol. 17:251–257. 1991.PubMed/NCBI
|
|
41
|
Gapstur SM, Dupuis J, Gann P, Collila S
and Winchester DP: Hormone receptor status of breast tumors in
black. Hispanic and non-Hispanic white women: an analysis of 13,239
cases. Cancer. 77:1465–1471. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Joslyn SA and West MM: Racial differences
in breast carcinoma survival. Cancer. 88:114–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pegoraro RJ, Karnan V, Nirmul D and Joubet
SM: Estrogen and progesterone receptors in breast cancer among
women of different racial groups. Cancer Res. 46:2117–2120.
1986.PubMed/NCBI
|
|
44
|
Klonoff-Cohen HS, Schaffroth LB, Edelstein
SL, Molgaard C and Saltzstein SL: Breast cancer histology in
whites, African Americans, Hispanics, Asians and Pacific Islanders.
Ethnicity Health. 3:189–198. 1998. View Article : Google Scholar
|
|
45
|
Li CI, Weiss NS, Stanford JL and Daling
JR: Hormone replacement therapy in relation to risk of lobular and
ductal breast cancer in middle-aged women. Cancer. 88:2570–2577.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lyons TR, Schedin PJ and Borges VFJ:
Pregnancy and breast cancer: when they collide. Mammary Gland Biol
Neoplasia. 14:87–98. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li CI and Daling JR: Changes in breast
cancer incidence rates in the United States by histology subtype
and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev.
16:2773–2780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sellers TA: Genetic factors in the
pathogenesis of breast cancer: their role and relative importance.
J Nutr. 127:S929–S932. 1997.PubMed/NCBI
|
|
49
|
Thomas DB and Karagas MR: Migrant studies.
Cancer Epidemiology and Prevention. 2nd edition. Schottenfeld D and
Fraumeni JF Jr: Oxford University Press; New York: pp. 236–254.
1996
|
|
50
|
Robbins AS, Brescianini S and Kelsey JL:
Regional differences in known risk factors and the higher incidence
of breast cancer in San Francisco. J Natl Cancer Inst. 89:960–965.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cornelisse CJ, Cornelis RS and Devilee P:
Genes responsible for familiar breast cancer. Path Res Pract.
192:684–693. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Szabo CI and King MC: Population genetics
of BRCA1 and BRCA2. Am J Hum Genet. 60:1013–1020. 1997.PubMed/NCBI
|
|
53
|
Levy-Lahad E, Lahad A, Eisenberg S, et al:
Founder BRCA1 and BRCA2 mutations in Ashkenazi Jews in Israel:
frequency and differential penetrance in ovarian cancer and in
breast-ovarian cancer families. Am J Hum Genet. 60:1059–1067.
1997.PubMed/NCBI
|
|
54
|
Berman DB, Wagner-Costalas J, Schultz DC,
Lynch HT, Daly M and Godwin AK: Two distinct origins of a common
BRCA1 mutation in breast-ovarian cancer families: a genetic study
of 15 185delAG mutation kindreds. Am J Hum Genet. 58:1166–1176.
1996.PubMed/NCBI
|
|
55
|
Struewing JP, Abeliovich D, Peretz T,
Avishai N, Kaback MM, Collins FS and Brody LC: The carrier
frequency of the BRCA1 185delAG mutation is approximately 1 percent
in Ashkenazi Jewish individuals. Nat Genet. 11:198–200. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gayther SA, Mazoyer WW, Russell PA,
Harrington PA, Mathias Chiano M and Seal S: Germline mutations of
the BRCA1 gene in breast/ovarian cancer families provide evidence
for a phenotype/genotype correlation. Nat Genet. 11:428–433. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Carrozza SE and Lowe HL: Patterns of
cancer incidence among US Hispanics/Latinos, 1995–2000. Cancer
Causes Control. 17:1067–1075. 2006.PubMed/NCBI
|
|
58
|
Ogden CL, Carroll MD, Curtin LR, McDowell
MA, Tabak CJ and Flegal KM: Prevalence of overweight and obesity in
the United States, 1999–2004. JAMA. 295:1549–1555. 2006.
|
|
59
|
Murtaugh MA, Herrick JS, Sweeney C, et al:
Diet composition and risk of overweight and obesity in women living
in the south western United States. J Am Diet Assoc. 107:1311–1321.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Slattery ML, Sweeney C, Edwards S, et al:
Body size, weight change, fat distribution and breast cancer risk
in Hispanic and non-Hispanic white women. Breast Cancer Res Treat.
38:33–41. 2006.PubMed/NCBI
|