Introduction
Narrow band imaging (NBI) is an optics technology
that uses the contrast between two wavelengths of light: Cerulean
narrow band light at 390–445 nm and green narrow band light at
530–550 nm (1). The relative
intensities of the cerulean and green bands are increased, while
the intensity of red light is minimized under NBI. The contrast of
images of capillaries in the surface layers of mucosal membranes is
enhanced and detailed pattern can be visualized. The vasculature
appears dark green or black against an almost white normal
urothelium, whereas similar lesions appear red on a background of
pink normal urothelium under white light imaging (WLI). Thus, the
contrast between urothelial carcinomas (UCs) and normal urothelium
is enhanced by NBI, allowing for the detection of UCs more easily.
This feature is particularly noticeable for small or flat early UCs,
which conventional WLI struggles to detect (2).
NBI is useful for the identification and evaluation
of early-stage cancer in gastrointestinal endoscopy (3), and the utility of NBI for increasing
tumor detection and decreasing recurrence compared with WLI has
been shown in non-muscle invasive bladder cancer (NMIBC) (4–9). However,
it is unclear if transurethral resection (TUR) using NBI (NBI-TUR)
reduces the tumor recurrence rate compared with TUR under
conventional WLI (WLI-TUR). Therefore, the aim of the present study
was to compare the utility of NBI-TUR with WLI-TUR in patients with
NMIBC treated at Hiroshima City Asa Hospital (Hiroshima,
Japan).
Patients and methods
Patients
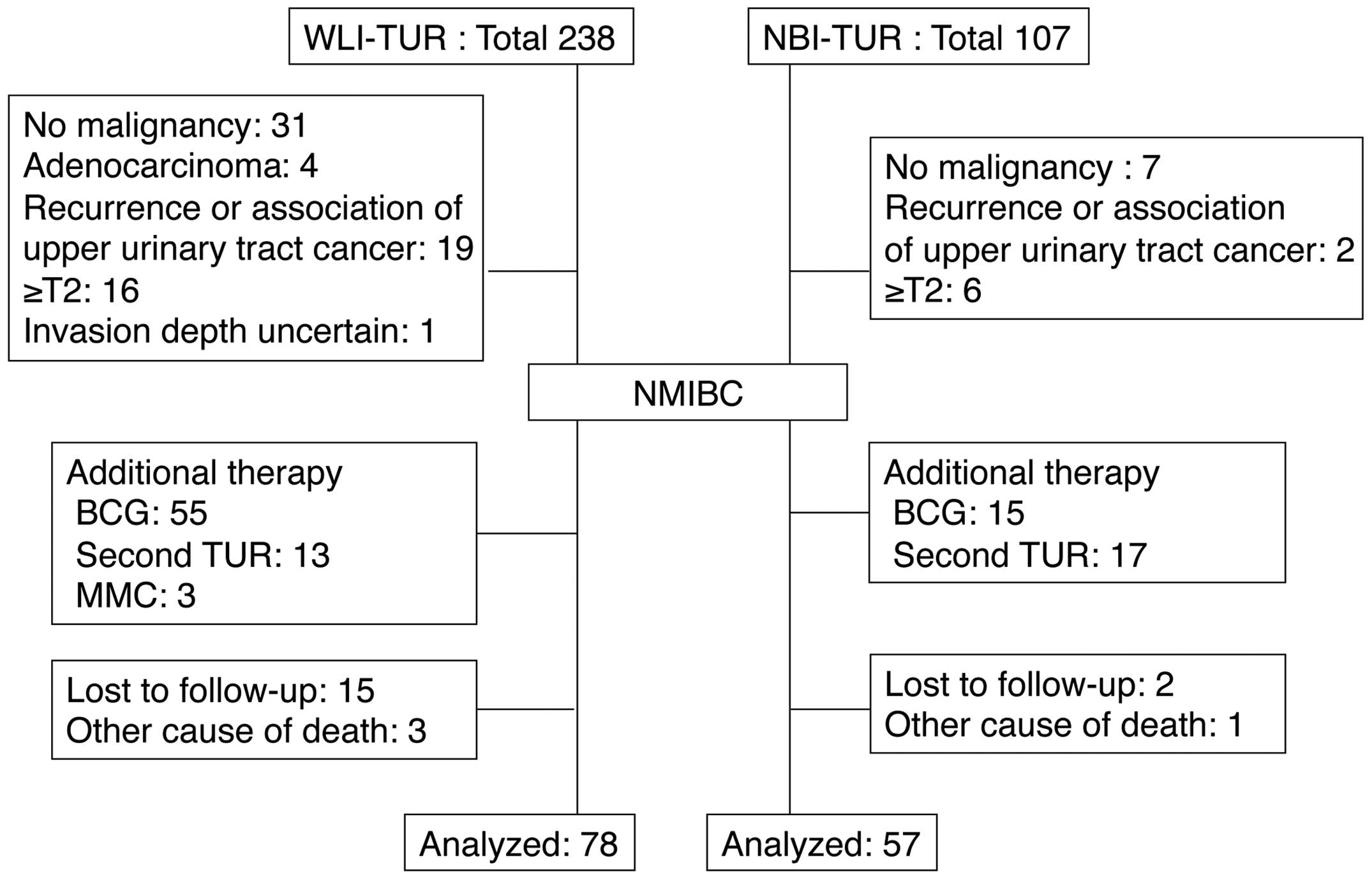
The study cohort consisted of 135 patients with
NMIBC who were followed up for ≥1 year after treatment with TUR at
Hiroshima City Asa Hospital between January 2010 and April 2014,
and who received no post-operative treatment. NBI-TUR has been
performed at this hospital since February 2012. All NBI
observations were made using a Visera Elite system (Olympus, Tokyo,
Japan). Cases that received post-operative intravesical injections
of agents such as Bacillus Calmette-Guérin or anticancer agents,
those who underwent a second TUR and those with a history of
treatment for upper urinary tract cancer were excluded from the
study (Fig. 1). TUR was performed
under general anesthesia.
Treatment
In the WLI-TUR group, systematic intravesical
observation was performed under WLI, and then a multiple site
biopsy (MSB) was performed for the detected tumor lesions and other
lesions in at least five areas: The bilateral outer ureteral
orifice, the bilateral sidewalls and the posterior wall. Lesions
with positive findings (abnormal mucosa) were resected completely
under WLI.
In the NBI-TUR group, systematic intravesical
observation under conventional WLI was conducted using the same
procedure as that in the WLI-TUR group and positive findings were
recorded. Systematic intravesical observation was then conducted
under NBI and positive findings were identified under NBI. MSB as
aforementioned was performed under NBI, and TUR was performed
completely for all the positive findings under NBI.
Cases with an isolated carcinoma in situ
(CIS) lesion were followed up without additional treatment if the
lesion was small with positive findings and was resected
completely. Other cases, including those difficult to distinguish
from normal mucosa or possessing multiple or large lesions,
received additional treatment immediately and were not included in
this study. The post-operative course was followed in the
Outpatient Department by WLI cystoscopy and urinary cytology every
three months for all patients. If tumor recurrence was suspected, a
pathological examination was performed by biopsy or TUR, and cases
with recurrence received immediate additional treatment.
Post-operative tumor recurrence was defined as pathological
confirmation of UC.
Tumor detection
The tumor detection rates under WLI and NBI were
analyzed to determine the sensitivity, specificity,
positive-predictive value (PPV), negative-predictive value (NPV)
and accuracy of the methods (6). In
the NBI-TUR group, these values were calculated separately and
compared with the endoscopic findings and the pathological results
of MSB under WLI and NBI.
Follow-up
The tumor recurrence rates at 3 months and 1 year
after TUR, and the recurrence free-survival rate were analyzed in
the NBI-TUR and WLI-TUR groups. To identify independent risk
factors for the 1-year recurrence of NBI-TUR, the following
background data were analyzed in univariate and multivariate
analyses: Age (<75 or ≥75 years old), gender (male or female),
tumor history (first or recurrence), tumor number (single or
multiple), tumor size (<3 or ≥3 cm), villous lesion (yes or no),
pre-operative urine cytology (negative or positive), pathological
stage [non-pathological tumor stage 1 (pT1) or pT1], concomitant
CIS (yes or no) and pathological grade (grades 1–2 or 3).
Statistical analysis
Statistical analysis was performed using the
χ2 test, unpaired t-test, and Mann-Whitney U test
for the patient background factors, the 3-month and 1-year
recurrence rates, and the tumor detection rate. Recurrence-free
survival was compared using a generalized Wilcoxon test. Logistic
multiple regression analysis was used to evaluate items found to be
significant in univariate analysis. All statistical analyses were
performed using Exel-Toukei 2012 version 1.11 (Social Survey
Research Information Co., Ltd., Tokyo, Japan). P<0.05 was
considered to indicate a statistically significant difference.
Results
Background of patients
The backgrounds of the patients in the WLI-TUR
(n=78) and NBI-TUR (n=57) groups are shown in Table I. Background factors did not differ
significantly between the two groups, with the exception of the
observation period (31.0 vs. 15.0 months; P<0.01).
 | Table I.Patient background. |
Table I.
Patient background.
| Parameter | WLI-TUR (n=78) | NBI-TUR (n=57) | P-value |
|---|
| Median observation
period, months | 31.0 | 15.0 | <0.001 |
| Median age,
years | 73 | 75 | 0.704 |
| Gender, n |
|
| 0.485 |
| Male | 62 | 48 |
|
|
Female | 16 | 9 |
|
| Clinical status,
n |
|
| 0.999 |
| Newly
diagnosed | 33 | 24 |
|
| Recurrent
(<1 year) | 22 | 16 |
|
| Recurrent
(≥1 year) | 23 | 17 |
|
| Number of tumors |
|
| 0.749 |
|
Single | 35 | 24 |
|
|
Multiple | 43 | 33 |
|
| Associated villous
lesions, n | 36 | 31 | 0.345 |
| Tumor size, n |
|
| 0.379 |
| ≥3
cm | 9 | 4 |
|
| <3
cm | 69 | 53 |
|
| TNM Stage, n |
|
| 0.961 |
| pTa | 61 | 45 |
|
| pT1 | 12 | 9 |
|
| pTis | 5 | 3 |
|
| Concomitant CIS,
n | 11 | 13 | 0.165 |
| Grade, n |
|
| 0.436 |
| 1 | 8 | 3 |
|
| 2 | 51 | 36 |
|
| 3 | 19 | 18 |
|
| Urinary cytology
(pre-TUR), n |
|
| 0.780 |
|
Negative | 60 | 45 |
|
|
Positive | 18 | 12 |
|
Tumor detection rate under WLI and
NBI
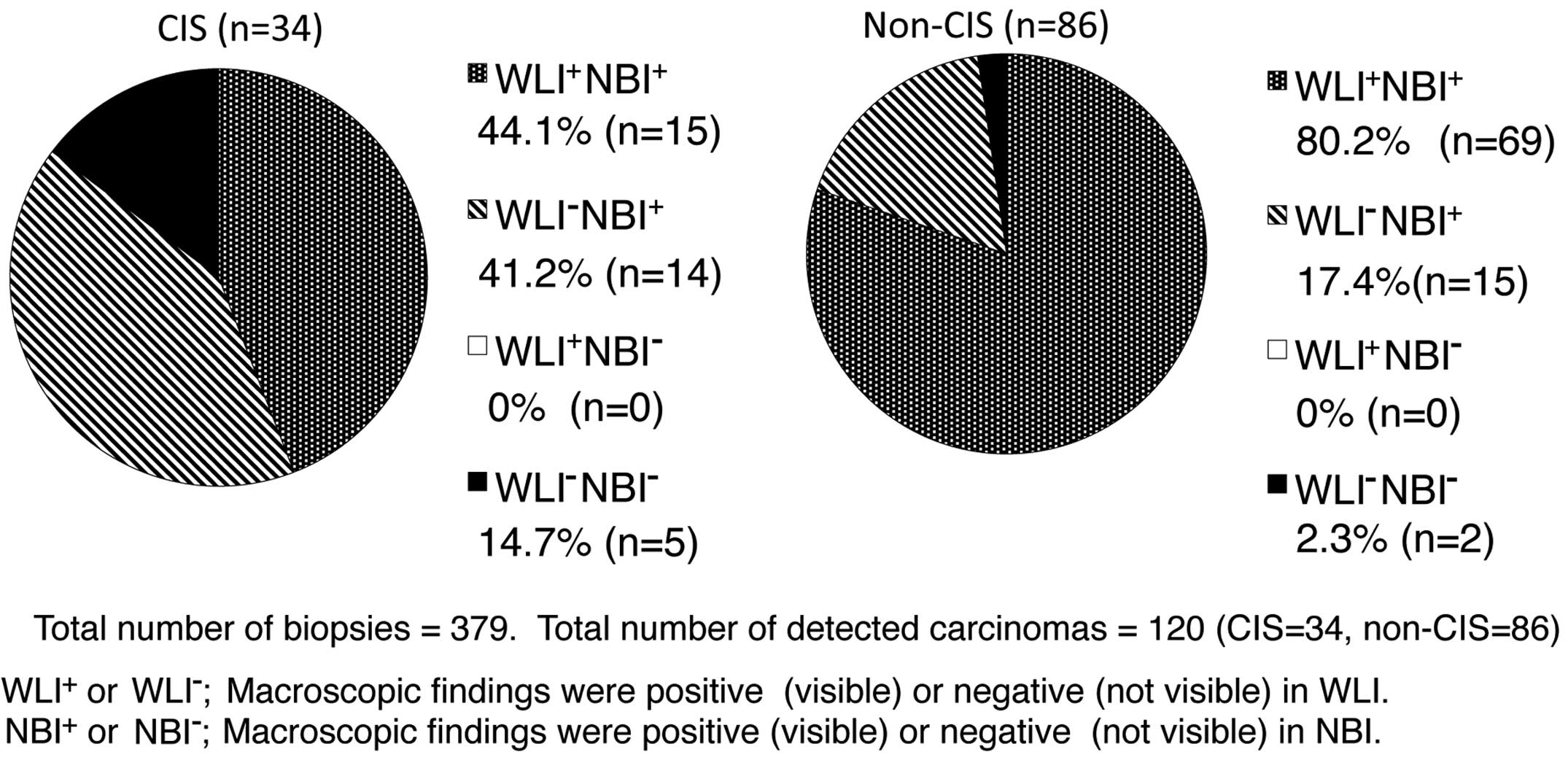
In the NBI-TUR group, out of 379 biopsies, CIS was
detected in 34 and non-CIS (pT1 or pTa) in 86. The procedure under
NBI exhibited significantly higher sensitivity (95.0 vs. 70.0%;
P<0.01) and NPV (97.1 vs. 86.8%; P<0.01) compared with the
rates under WLI, but the specificity was significantly lower (77.2
vs. 89.7%; P<0.01) under NBI. There was no significant
difference in PPV and accuracy between NBI and WLI groups (Table II).
 | Table II.Tumor detection rate under WLI and
NBI.a |
Table II.
Tumor detection rate under WLI and
NBI.a
| Parameter | NBI | WLI | P-value |
|---|
| Sensitivity | 95.0% (114/120) | 70.0% (84/120) | <0.001 |
| Specificity | 77.2% (203/263) | 89.7% (236/263) | <0.001 |
| PPV | 65.5% (114/174) | 75.7% (84/111) |
0.069 |
| NPV | 97.1% (203/209) | 86.8% (236/272) | <0.001 |
| Accuracy | 83.6% (317/379) | 84.4% (320/379) |
0.766 |
There were no cancer lesions identified under WLI
that could not be identified under NBI; whereas 41.2% of CIS and
17.4% of non-CIS lesions were identified with NBI, but not with
WLI; and 14.7% of CIS and 2.3% of non-CIS lesions could not be
identified under NBI or WLI (Fig. 2).
The rate of detection of CIS lesions in the 12 patients with
positive urinary cytology was higher than that in the 45 patients
with negative urinary cytology [14.8% (12/81) vs. 7.5% (22/293)
biopsies; P=0.043].
To evaluate the surgeon's skill in the detection of
UCs in NBI, the patients in the NBI-TUR group were divided into
chronological groups: The first half (n=29) that underwent the
procedure and the second half (n=28). Specificity (68.6 vs. 84.5%;
P<0.01) and accuracy (80.3 vs. 87.1%; P=0.022) were
significantly higher in the second group, but sensitivity did not
differ significantly between the two groups.
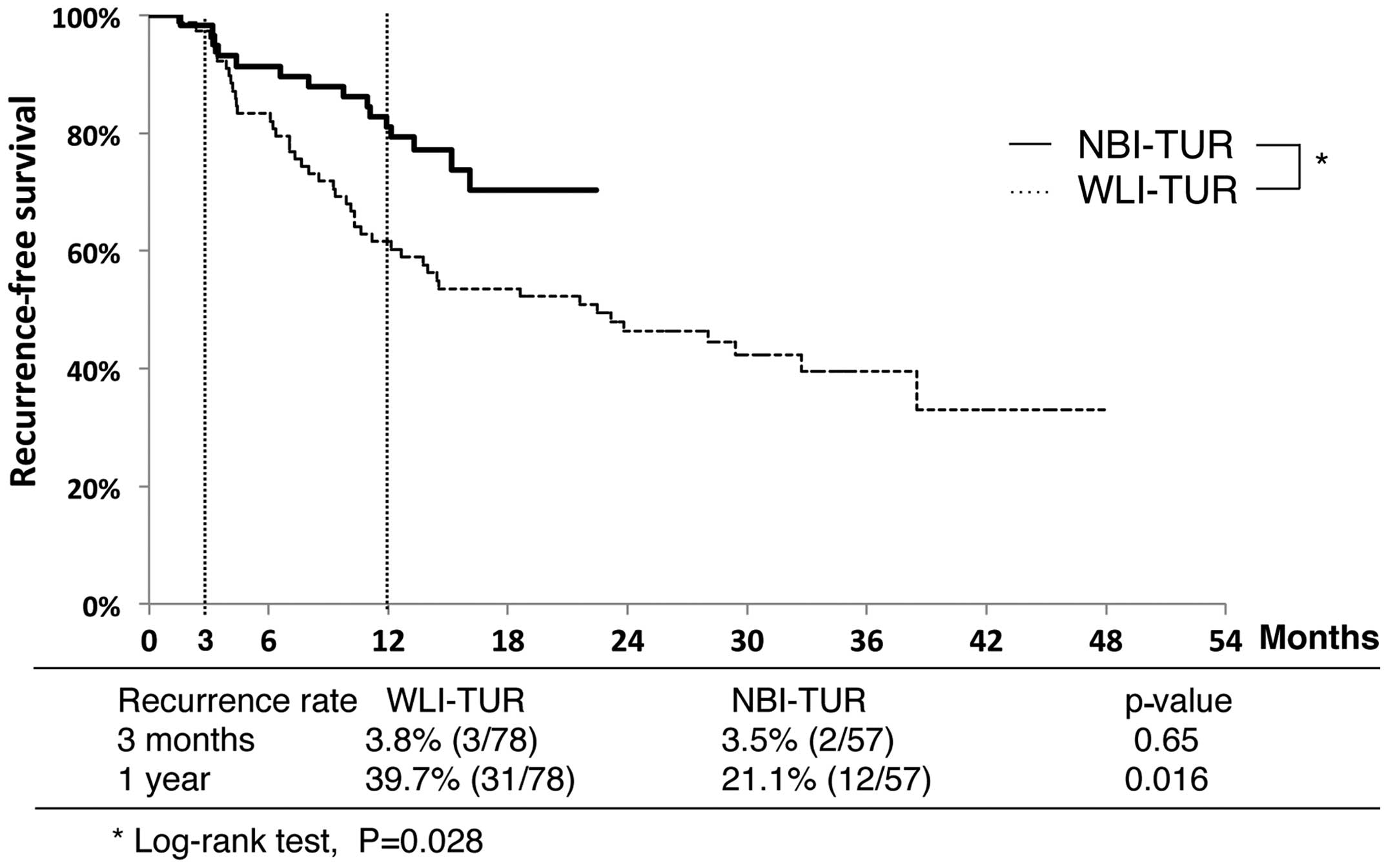
Recurrence rate following TUR
Recurrence-free survival curves for the NBI-TUR and
WLI-TUR groups are shown in Fig. 3.
The 3-month recurrence rates did not differ between the NBI-TUR
(3.5%) and WLI-TUR (3.8%) groups, but the 1-year recurrence rate
was significantly lower in the NBI-TUR group (21.1 vs. 39.7%;
P=0.016). The recurrence-free survival rate was significantly
higher in the NBI-TUR group (P=0.028).
Univariate and multivariate analyses were performed
to identify factors associated with 1-year recurrence in the
NBI-TUR group. Multiple tumors, an associated villous lesion and
positive urinary cytology were significant in univariate analysis,
while positive urinary cytology was the only significant predictor
in multivariate analysis (Table
III).
 | Table III.Predictors of 1-year recurrence in the
transurethral resection under narrow band imaging group. |
Table III.
Predictors of 1-year recurrence in the
transurethral resection under narrow band imaging group.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Possible
predictor | P-value | P-value | HR (95% CI) |
|---|
| Recurrent tumor | 0.489 |
|
|
| Multiple tumors | 0.015 | 0.120 | 6.17
(0.62–61.01) |
| Tumor size (≥3
cm) | 0.840 |
|
|
| Associated villous
lesion | 0.023 | 0.313 | 2.54
(0.42–15.50) |
| pT1 | 0.925 |
|
|
| CIS | 0.238 |
|
|
| Grade 3 | 0.122 |
|
|
| Urinary cytology
(positive) | 0.005 | 0.037 | 5.24
(1.11–24.87) |
| Age (≥75 years) | 0.273 |
|
|
| Gender (male) | 0.925 |
|
|
Discussion
To the best of our knowledge, the present study is
the first to show that NBI-TUR significantly reduces the tumor
recurrence rate compared with WLI-TUR in Japanese patients with
NMIBC. Moreover, the results suggested that NBI was effective for
the detection of CIS lesions, as well as for non-CIS lesions
(Fig. 2). There have been few
previous comparisons of recurrence in NMIBC following NBI-TUR and
WLI-TUR (4). The significantly lower
recurrence rate with NBI-TUR compared to WLI-TUR may be due to the
significantly higher tumor detection rate with NBI-TUR found in the
NMIBC patients in the present study and in previous studies
(4–9).
When performing NBI-TUR for all positive findings
under NBI, the potential for unnecessary injury should be
considered for a non-carcinoma lesion due to the higher
false-positive rate found with NBI in the present study. However,
based on the present results, such injury can be decreased by the
experience of the surgeon, as the specificity was significantly
improved in later cases in the NBI-TUR group. Specificity and
accuracy in the NBI-TUR group were significantly improved by
experience, but the specificity only reached 84.5% in the later
cases and certain lesions, including CIS, could not be detected
under NBI. Furthermore, bleeding or active inflammation of the
bladder means that the detection of abnormal lesions using NBI is
difficult, as enhanced images are generated by light emission from
target areas in two narrow wavebands that are strongly absorbed by
hemoglobin (7). These results
indicate the current technical limitations of NBI.
The present study included cases of small CIS
lesions with positive findings that were resected completely. A CIS
lesion was not found to be a predictor of 1-year recurrence in the
NBI group, but positive urinary cytology was a significant
predictor of 1-year recurrence. Positive urinary cytology is
important in the diagnosis and follow-up of CIS as it has high
sensitivity and specificity for CIS (>90%) (10). Based on these findings, additional
treatment should be administered in all cases with positive urinary
cytology and CIS lesions, even if the lesion is small under NBI
observation.
The tumor recurrence rate at 3 months after TUR is
dependent on the quality of the initial TUR and is an important
prognostic factor for recurrence, progression and survival. The
tumor recurrence rate at 3 months after TUR in NMIBC may also
reflect the skill of the surgeon (4,11,12). Without additional post-operative
treatment, recurrence rates of 3.4 to 20.6% and 7.4 to 45.8% have
been found in patients with single and multiple tumors,
respectively (11). Thus, the 3-month
recurrence rate after TUR in the present study was not inferior to
those of previous studies. These results indicate that the skills
of the surgeons in TUR at Hiroshima City Asa Hospital are
sufficient to provide confidence in the overall findings of the
study.
This study has several limitations, including the
retrospective design and the relatively small number of subjects.
The treatment strategy for patients with NMIBC basically used the
policy in the European Organization for Research and Treatment of
Cancer recurrent risk classification (10,13),
however, certain high-risk patients in the present study were not
willing to undergo additional post-operative treatment, or
non-treatment follow-up was recommended due to their physical
status.
The detection rate of bladder cancer may be improved
by fluorescence cystoscopy using 5-aminolevulinic acid (5-ALA) or
hexaminolevulinate, in addition to NBI. In a randomized prospective
study of treatment outcome, the tumor-free recurrence rate at
1-year after TUR using 5-ALA was 20% lower than that after WLI-TUR
(14). By contrast, no difference was
found in the long-term recurrence- and progression-free rates
following TUR using 5-ALA and WLI-TUR (15).
An international multicenter cooperative study
comparing the treatment outcomes of NBI-TUR and WLI-TUR is
currently in progress (16). The
medical costs from diagnosis to mortality for a patient with
bladder cancer are high, and any intervention that reduces these
costs would be hugely beneficial (17). However, the cost benefit of NBI-TUR
compared with WLI-TUR remains unclear (12). If the surgical outcome after NBI-TUR
includes reduced post-operative treatment, this procedure will be
beneficial to mitigate the burden on patients with NMIBC (5). Thus, it is noteworthy that the present
study showed a significantly lower short-term recurrence rate after
NBI-TUR than after WLI-TUR for patients with NMIBC. Further
analysis is required to examine the longer term effects of NBI-TUR
on the recurrence rate. However, the short-term results of the
present study suggest that NBI-TUR is advantageous over
conventional WLI-TUR in patients with NMIBC.
Acknowledgements
The present work was presented at the 32nd World
Congress of Endourology.
References
|
1
|
Olympus, . News release. December 26–2006
Endoscopic videoscope system. http://www.olympus-europa.com/medical/en/medical_systems/applications/urology/bladder/narrow_band_imaging__nbi_/narrow_band_imaging__nbi_.htmlAccessed.
April 22–2015
|
|
2
|
Hirata I, Nakagawa Y, Ohkubo M, Yahagi N
and Yao K: Usefulness of magnifying narrow-band imaging endoscopy
for the diagnosis of gastric and colorectal lesions. Digestion.
85:74–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bryan RT, Billingham LJ and Wallace DM:
Narrow-band imaging flexible cystoscopy in the detection of
recurrent urothelial cancer of the bladder. BJU Int. 101:702–706.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Naselli A, Introini C, Timossi L, et al: A
randomized prospective trial to assess the impact of transurethral
resection in narrow band imaging modality on non-muscle-invasive
bladder cancer recurrence. Eur Urol. 61:908–913. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cauberg EC, Mamoulakis C, de la Rosette JJ
and de Reijke TM: Narrow band imaging-assisted transurethral
resection for non-muscle invasive bladder cancer significantly
reduces residual tumor rate. World J Urol. 29:503–509. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Naselli A, Introini C, Bertolotto F, Spina
B and Puppo P: Narrow band imaging for detecting residual/recurrent
cancerous tissue during second transurethral resection of newly
diagnosed non-muscle-invasive high-grade bladder cancer. BJU Int.
105:208–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tatsugami K, Kuroiwa K, Kamoto T, et al:
Evaluation of narrow-band imaging as a complementary method for the
detection of bladder cancer. J Endourol. 24:1807–1811. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cauberg EC, Kloen S, Visser M, et al:
Narrow band imaging cystoscopy improves the detection of
non-muscle-invasive bladder cancer. Urology. 76:658–663. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herr HW and Donat SM: A comparison of
white-light cystoscopy and narrow-band imaging cystoscopy to detect
bladder tumour recurrences. BJU Int. 102:1111–1114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A and Palou-Redorta JEuropean Association of
Urology (EAU): EAU guidelines on non-muscle-invasive urothelial
carcinoma of the bladder. Eur Urol. 54:303–314. 2008.
|
|
11
|
Brausi M, Collette L, Kurth K, van der
Meijden AP, et al EORTC Genito-Urinary Tract Cancer Collaborative
Group: Variability in the recurrence rate at first follow-up
cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of
the bladder: A combined analysis of seven EORTC studies. Eur Urol.
41:523–531. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brausi M: Are new technologies for
detection and treatment of non-muscle-invasive bladder cancer
(NMIBC) so important? A plea for adoption by teaching programs to
improve results after standard white light transurethral resection
of NMIBC. Eur Urol. 61:914–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sylvester RJ, van der Meijden AP,
Oosterlinck W, et al: Predicting recurrence and progression in
individual patients with stage Ta T1 bladder cancer using EORTC
risk tables: a combined analysis of 2596 patients from seven EORTC
trials. Eur Urol. 49:466–477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Daniltchenko DI, Riedl CR, Sachs MD, et
al: Long-term benefit of 5-aminolevulinic acid fluorescence
assisted transurethral resection of superficial bladder cancer:
5-year results of a prospective randomized study. J Urol.
174:2129–2133. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schumacher MC, Holmäng S, Davidsson T,
Friedrich B, Pedersen J and Wiklund NP: Transurethral resection of
non-muscle-invasive bladder transitional cell cancers with or
without 5-aminolevulinic acid under visible and fluorescent light:
results of a prospective, randomised, multicentre study. Eur Urol.
57:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clinical Research Office of the
Endourological society, . The Global Randomized NBI Bladder Cancer
study. http://www.croesoffice.org/OngoingProjects/NBIStudy.aspx
|
|
17
|
Botteman MF, Pashos CL, Radaelli A, Laskin
B and Hauser R: The health economics of bladder cancer: A
comprehensive review of the published literature.
Pharmacoeconomics. 21:1315–1330. 2003. View Article : Google Scholar : PubMed/NCBI
|