Introduction
Hepatocellular carcinoma (HCC) has the second
highest rate of cancer-associated mortality in men worldwide
(1); in addition, chemotherapeutic
drug resistance is common in HCC patients (2). Previous studies have indicated that the
adjuvant therapies currently available for HCC treatment following
surgery are ineffective and inadequate to prevent recurrence
(3). Large-scale randomized phase III
clinical studies have demonstrated the survival benefits of
sorafenib treatment, a unique first-line drug recommended for
advanced HCC; however, sorafenib is not commonly used due to its
low response rate and drug resistance (4). It is therefore important to elucidate
the underlying mechanisms of sorafenib resistance in order to
identify potential therapeutic strategies to enhance its efficacy
for the treatment of HCC (5–7).
Solid tumors often have a hypoxic internal
microenvironment that includes numerous molecular pathways, which
provide challenges for therapeutic strategies; this
microenvironment is the primary cause of drug resistance to
antitumor therapies (8,9). Previous studies have reported that
sustained sorafenib treatment may promote hypoxia within tumors,
which has been associated with sorafenib-resistance in HCC patients
as well as subcutaneous mice models of HCC (10). In addition, it was reported that
short-term sorafenib therapy reduced vascularization, resulting in
increased tumor hypoxia (11).
Therefore, numerous previous studies have aimed to elucidate the
potential mechanisms of hypoxia-induced sorafenib resistance
mechanisms (5,7,9,10).
Hypoxia-inducible factors (HIFs), which are
heterodimers composed of an α and β subunit, have been implicated
in the regulation of the hypoxic response (12,13). HIF-1
was reported to mediate the expression of hundreds of genes,
including those for vascular endothelial growth factors, glycolytic
enzymes and glucose transporters (14,15). In
addition, HIF-2 was identified to be involved in implementing the
hypoxic response (14,15). HIF-1β was reported to be able to form
a heterodimer complex wither either HIF-1α or HIF-2α, both of which
undergo oxygen-dependent degradation via the von Hippel-Lindau
protein pathway (14,15). In addition, it was reported that
targeting HIF-1α may significantly enhance sorafenib antitumor
sensitivity under hypoxic conditions (10); however, the association between HIF-2α
and sorafenib sensitivity remains to be elucidated.
The present study aimed to investigate the potential
association between HIF-2α and sorafenib sensitivity under hypoxic
conditions and the mechanisms by which this may proceed.
Materials and methods
Cell culture
Human HepG2, Bel-7402 and Huh-7 HCC cell lines were
purchased from the American Type Culture Collection (Rockville, MD,
USA); in addition, SMMC-7402 HCC cells were obtained from the Type
Culture Collection Cell Bank, Chinese Academy of Science (Shanghai,
China). Cells were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco Life Technologies, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (ThermoFisher Scientific, Shanghai,
China), 100 U/ml penicillin and 100 µg/ml streptomycin (Beyotime
Institute of Biotechnology, Shanghai, China) at 37°C in 95% air and
5% CO2 or when hypoxic conditions were required, cells
were stored at 37°C in a sealed MIC-101 hypoxia chamber
(Billups-Rothenberg, Inc., Del Mar, CA, USA) equilibrated with
certified gas containing 1% O2, 5% CO2 and
94% N2.
Antibodies (Abs) and reagents
Abs against HIF-2α, β-catenin, C-Myc and
proliferating cell nuclear antigen (PCNA), were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA); Ab against GAPDH was
purchased from Beyotime Institute of Biotechnology (Jinan, China);
and Abs against Ki-67, HIF-2α small interfering (si)RNA and control
siRNA were purchased from Santa Cruz Biotechnology, Inc. (Dallas,
TX, USA). Lipofectamine 2000 was purchased from Invitrogen Life
Technologies (Beijing, China) and sorafenib tosylate was purchased
from Bayer Pharmaceuticals Co. (West Haven, CT, USA).
HIF-2α/C-Myc silencing using
lentiviral vector-mediated short hairpin (sh)RNA
Silencing of HIF-2α/C-Myc was achieved via
lentivival transduction of the following specific shRNA vectors
(Santa Cruz Biotechnology, Inc.): HIF-2α-specific shRNA,
sc-35316-v; C-Myc-specific shRNA, sc-29226-v; and scramble shRNA
control, sc-108080. The process of transduction was executed
according to the product protocols. Briefly, HepG2 cells
(5×105 cells/well) were seeded in 24-well plates. When
the cell were ~50% confluent, the complete medium in the wells was
replaced by complete medium with Polybrene (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA; sc-134220) at a final
concentration of 3.5 µg/ml. In the control shRNA group, 10 µl
scramble shRNA (sc-108080) was added to each well and in the HIF-2α
shRNA group, 10 µl HIF-2α shRNA (sc-35316-v) was added to each
well. The cells were cultured for 8 h, then the mixture of complete
medium with Polybrene was replaced with complete medium, 12 h
later, the cells were harvested for the following assays.
Cell viability assay
Cell viability was detected using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Beijing,
China). In brief, cells (5×103/well) in the control
(scramble shRNA-treated group) and the group treated with HIF-2
shRNA were seeded onto 96-well plates and cultured for 12, 24, 48
and 72 h. The culture medium was then replaced with fresh medium
containing 10 µl CCK-8 solution. Cells were incubated for a further
2 h at 37°C and the optical density was measured using a PowerWave
HT Microplate Spectrophotometer (BioTek Instruments, Inc.,
Winooski, VT, USA) at 450 nm.
Western blot assay
Cells or tumor tissues were homogenized in protein
lysate buffer and debris was removed by centrifugation. Protein
concentrations in cell/tissue extracts were determined using the
BCA Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Inc.,
Richmond, CA, USA). In brief, equal amounts of protein fractions of
lysates were resolved over SDS-PAGE gels (Beyotime Institute of
Biotechnology) transferred to polyvinylidene fluoride membranes
(Merck Millipore, Darmstadt, Germany) and immunoblotted as
previously described (16,17). The primary antibodies used for western
blotting included, monoclonal rabbit anti-human HIF-2α, (1:1,000
dilution, #7096), polyclonal rabbit anti-human-β-catenin (1:1,000,
#9562), polyclonal rabbit anti-human c-Myc (1:1,000
dilution, #9402), monoclonal mouse anti-human PCNA (1:2,000
dilution, #2586) and monoclonal mouse anti-human GAPDH (1:2,000
dilution, AG019). The secondary antibodies included horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:1,000 dilution,
#A0208; Beyotime Institute of Biotechnology) and (HRP)-conjugated
goat anti-mouse IgG (1:1,000 dilution, A0216; Beyotime Institute of
Biotechnology). Protein bands were developed using ECL reagent
(Tiangen Biotech Co., Ltd., Beijing, China) and visualized using
the AlphaImager HP System (Naturegene Corporation, Medford, NJ,
USA). The protein band intensities were quantified by densitometric
analysis using Image J software, version 2.0 (National Institutes
of Health, Bethesda, MD, USA).
Immunocytochemistry (ICC)
For ICC, the cells (2×104) were plated on
coverslips, fixed with 4% paraformaldehyde for 30 min at room
temperature, and permeabilized with 0.2% Triton X-100 for 20 min at
room temperature. Subsequently, the sections were incubated with
primary anti-β-catenin (1:200 dilution, #9562) overnight at 4°C,
followed by incubation with HRP-conjugated goat anti-rabbit IgG
secondary antibody (1:1,000 dilution, #A0208). Subsequently,
immunoreactivity was developed with 3,3′-diaminobenzidine (DAB;
ZSGB-BIO Biotechnology Co., Ltd., Beijing, China), followed by
counterstaining with hematoxylin.
Subcutaneous HCC experiments
All surgical procedures and care administered to the
animals were in accordance with institutional ethics guidelines.
The study was approved by the ethics committee of Qianfoshan
Hospital, Shandong University (Jinan, China). Male BALB/c mice were
purchased from the Model Animal Research Center (Nanjing, China).
The mice (n=56) were housed in polycarbonate cages (5/cage),
provided with food and water ad libitum and maintained on a
12–12 h light-dark cycle at 22±2°C and 55±20% relative humidity.
Male BALB/c mice were randomly assigned into four groups
(12/group). Eight mice were excluded for different reasons
(mortality or no tumor formed). Male BALB/c (5–6 weeks old) mice
underwent subcutaneous inoculation in the flank with
1.5×106 HepG2 cells/mice suspended in phosphate-buffered
saline (PBS). Sorafenib tosylate was used in vivo
experiments. When tumors of ~100 mm3 were detected (~7
days post inoculation), mice were divided at random into the
following treatment groups (n=12/group): Control, sorafenib-treated
group, HIF-2α siRNA-treated and HIF-2α siRNA + sorafenib-treated.
Mice in each group were administered treatment five times/week
either intratumorally or orally (p.o). In the sorafenib-treated
group, mice were administrated once daily with sorafenib (p.o.; 10
mg/kg; 5 times/week). Equal volumes of 20 nM siRNA and
Lipofectamine 2000 were mixed and this solution was further mixed
with an equal volume of serum-free medium; 50 µl siRNA transfection
solution, containing 250 pmol siRNA, was injected into tumors (5
times/week). Mice in the control group were treated with equal
volume PBS (p.o.) and control siRNA (intratumoral injection). The
tumor volumes were estimated twice a week according to the formula:
π/6xa2xb, where a is the short axis, and b the long axis. All mice
were sacrificed by cervical dislocation on day 35.
Quantification of Ki-67 proliferation
index
As previously described (18), tumor sections were immunostained with
an anti-Ki-67 Ab. Briefly, polyclonal rabbit anti-human Ki-67 (1:50
dilution, SC-15402) was applied overnight at 4°C. After washing,
the sections were incubated with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (1:75 dilution, #A0208;
Beyotime Institute of Biotechnology) for 1 h at 37°C.
Immunoreactivity was developed using DAB and counterstained with
hematoxylin. Ki-67 positive cells were counted under microscopy
with a DM5500B equipped with HCX PL FLVOTAR 5/0.15 and HCX PL
FLVOTAR 10/0.15 dry objective lenses (Leica, Solms, Germany) in ten
randomly selected high-power fields (magnification, x400). The Ki-67
proliferation index was calculated according to the following
formula: Proliferation rate = (number of Ki-67 positive cells/total
cell count) × 100%.
Statistical analysis
Values are presented as the mean ± standard
deviation and statistical analysis was performed using SPSS
software version 16.0 (SPSS Inc., Chicago, IL, USA). Differences
between groups were analyzed using Chi-Square test or Student's
t-test according to the data type. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Targeting HIF-2α enhances the
antiproliferation activity of sorafenib under hypoxic
conditions
Western blot analysis was used to determine HIF-2α
protein expression levels in various HCC cell lines, SNNC-7402,
Bel-7402, HepG2 and Huh7, exposed to normoxia or hypoxia for 24 h.
All of the cell lines demonstrated low expression levels of HIF-2α
under normoxic conditions, while HIF-2α expression was markedly
increased under hypoxic conditions in all the selected HCC cell
lines (Fig. 1A). Based on this data,
these HCC cell lines were further used to detect the inhibitory
effects of sorafenib on the proliferation viability of cells under
hypoxic conditions. Cells were transfected with HIF-2α shRNA or
control shRNA and then treated with sorafenib (10 µM) for 12, 24,
48 and 72 h, following which cell viability was assessed. Compared
with the control shRNA, HIF-2α shRNA further enhanced the
antiproliferation activity of sorafenib in all of the four cell
lines under hypoxic conditions (Fig.
1B).
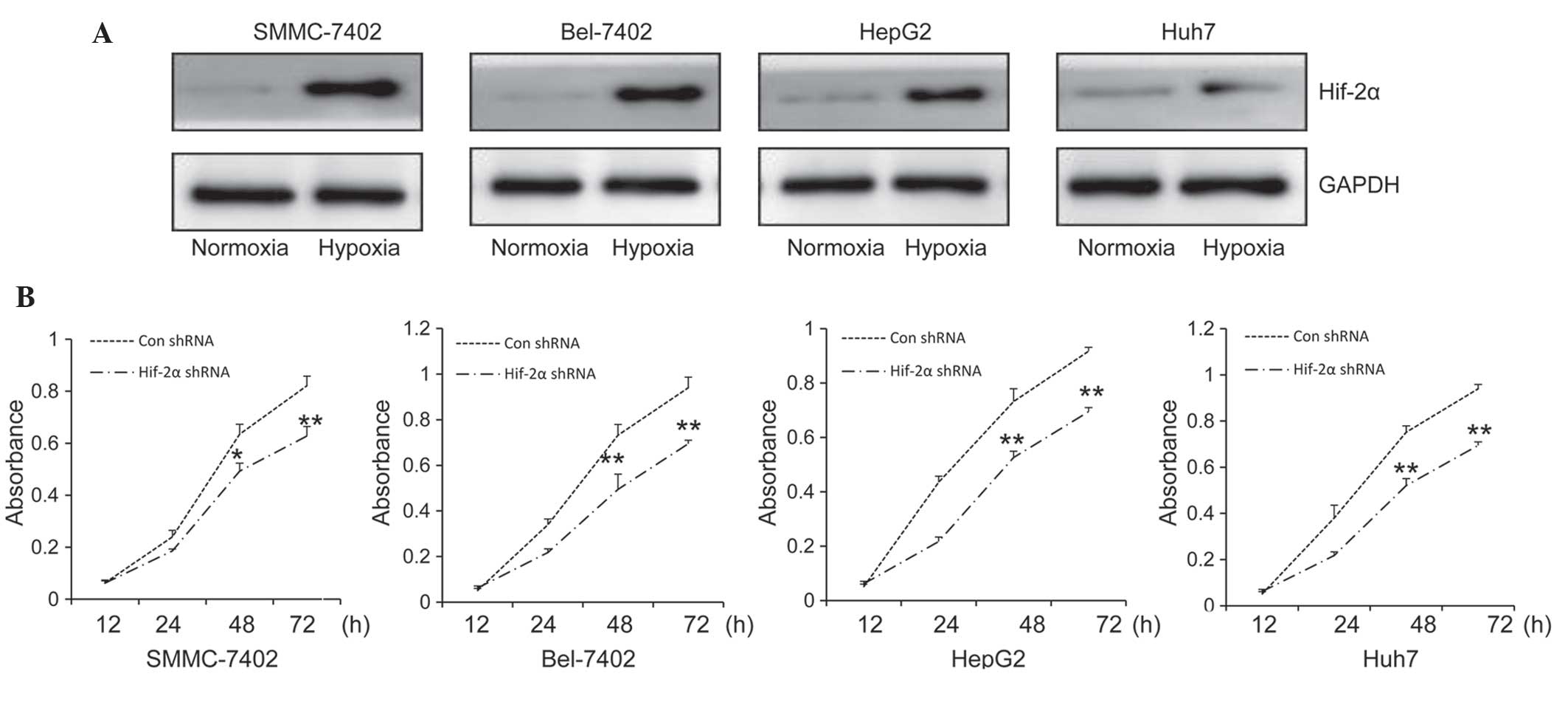 | Figure 1.Targeting HIF-2α enhances the
antiproliferation activity of sorafenib under hypoxic conditions.
(A) Expression of HIF-2α in hepatocellular carcinoma cell lines
SMMC-7402, Bel-7402, HepG2, Huh-7 was detected by western blot
analysis following treatment for 24 h under hypoxic conditions.
GAPDH served as an internal control. (B) SMMC-7402, Bel-7402, HepG2
and Huh-7 cells were treated with Con shRNA or HIF-2α shRNA in
combination with Sorafenib (10 µM) under hypoxic conditions and
cell viability was measured following 12, 24, 48 and 72 h of
treatment. Values are presented as the mean ± standard deviation of
three independent experiments. *P<0.05 and **P<0.001 vs. Con
shRNA. HIF-2α, hypoxia-inducible factor 2α; Con, control; shRNA,
short hairpin RNA. |
Regulation of cell
proliferation-associated protein expression by HIF-2α/C-Myc
shRNA
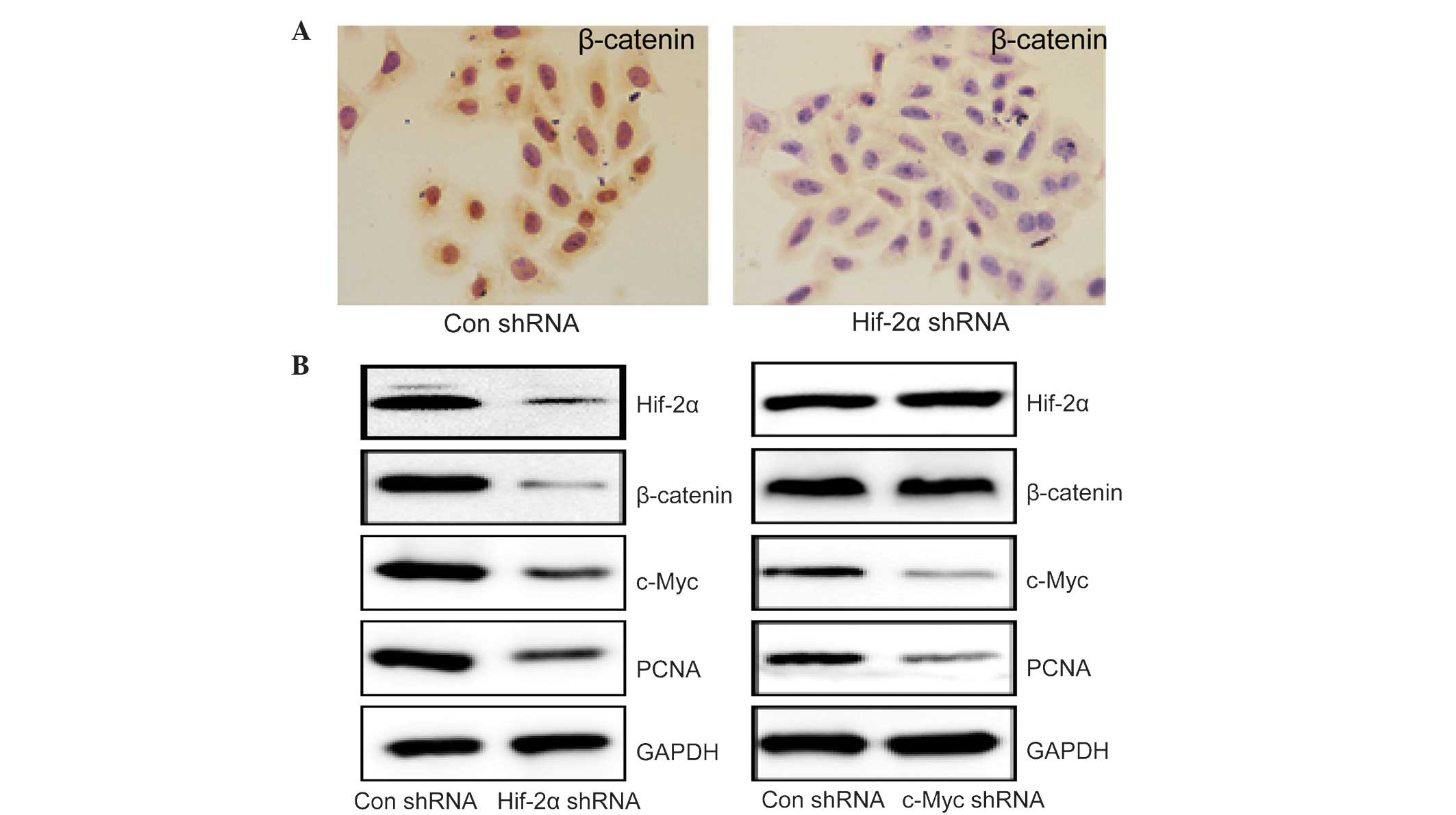
β-catenin is considered to be a key signaling
protein in the regulation of cell proliferation (19–21). HepG2
cells were incubated in a sealed hypoxia chamber for 24 h, which
resulted in significantly higher levels of β-catenin compared with
cells in normoxic conditions (Fig.
2A). Transfection of HIF-2α shRNA markedly reduced the
expression of HIF-2α, β-catenin, C-Myc and PCNA in HepG2 cells
compared with those transfected with control shRNA (Fig. 2B). As C-Myc is a key downstream
protein in β-catenin-mediated cell proliferation (19–21), the
effects of C-Myc shRNA transfection were investigated in HepG2
cells. C-Myc shRNA decreased C-Myc and PCNA expression with no
effect on the expression of HIF-2α and β-catenin (Fig. 2B). These results indicated that HIF-2α
regulated HCC cell proliferation in a β-catenin/C-Myc-dependent
pathway under hypoxic conditions.
Targeting HIF-2α potentiates the
antitumor effects of sorafenib in HepG2 xenograft tumor in mice
through inhibiting cell proliferation
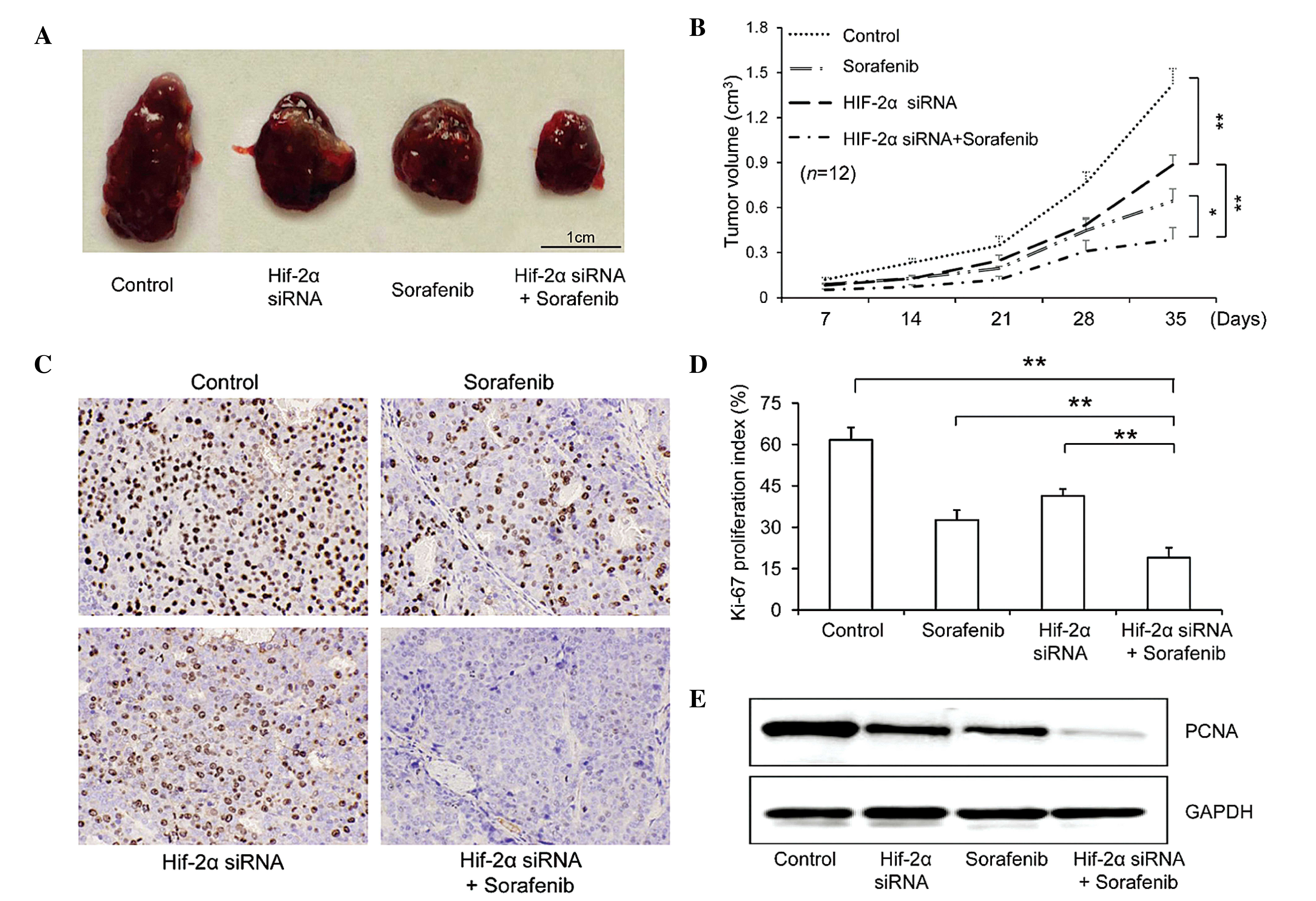
A gradual increase in xenograft HepG2 tumor growth
was observed in the control group compared with the experimental
groups. In addition, mice that underwent combination therapy had
tumors that were significantly smaller than those treated with
sorafenib or HIF-2α siRNA alone (Fig. 3A
and B). Furthermore, a reduced number of Ki-67-positive cells
were present in tumors treated with either sorafenib or HIF-2α
siRNA compared with the control group; while in the combination
therapy group, the number of Ki-67-positive cells was significantly
reduced compared with that of the independent treatment groups
(P<0.01) (Fig. 3C and D). As shown
in Fig. 3E, the reduced PCNA
expression levels in HIF-2α-transfected cells, as determined by
western blot analysis of tumor homogenates, were comparable to the
results observed in vitro (Fig.
3E).
Discussion
In the present study it was demonstrated that HIF-2α
was involved in sorafenib resistance via the regulation of
β-catenin/c-Myc-dependent pathways under hypoxic conditions in HCC.
Although sorafenib is first choice of treatment for advanced HCC,
it has been reported that this systemic drug has limited benefits
and poor response rates (22,23). Solid tumors contain hypoxic cells,
which exhibit resistance to antitumor treatments due to an enhanced
cellular adaptive response to hypoxia, mediated by HIF-1α and
HIF-2α, which promotes survival (14,24–26).
HIF-1α synthesis is suppressed following treatment with sorafenib,
which results in the altered hypoxic response from HIF-1α to
HIF-2α-dependent pathways; this mechanism was reported to result in
more aggressive tumor growth (22,23). In
the present study, it was demonstrated that targeted HIF-2α
knock-down inhibited HCC cell proliferation under hypoxic
conditions in vitro; in addition, this acted synergistically
with sorafenib to further suppress the growth of HCC tumors in
vivo.
β-catenin and C-Myc are important oncogenes, the
overexpression of which were reported to be associated with the
poorer prognosis of HCC patients (27–30). These
oncogenes have numerous biological functions; however, data
regarding the role of β-catenin/C-Myc in tumor proliferation are
limited in HCC cells. Previous studies have indicated that
β-catenin and HIF were frequently co-activated in rapidly growing
tumors (31–34). In addition, it was reported that
HIF-1α expression was closely associated with Wnt/β-catenin
signaling activity (35,36). However, limited studies have
investigated the involvement of HIF-2α in β-catenin regulation and
cell proliferation (34,37). Furthermore, HIF-2α was demonstrated to
interact with β-catenin/T-cell factor (TCF) in order to promote
gene transcription (34). Contrary to
the results of previous studies (38,39), the
present study suggested that HIF-2α may regulate β-catenin/TCF
activity through decreasing β-catenin expression under hypoxic
conditions in HCC cells; this change may be attributed to different
clonal variations.
C-Myc is an important target gene of β-catenin, the
expression of which was reported to be significantly upregulated by
β-catenin in tumor cells (40–42). In
the present study, HIF-2α positively regulated β-catenin/C-Myc
expression and C-Myc directly upregulated PCNA expression under
hypoxic conditions in HCC cells. However, the exact mechanism of
C-Myc-mediated PCNA expression remains to be fully elucidated.
Previous studies have indicated that C-Myc may enhance
transcription factor accumulation in gene promoter regions,
resulting in transcription factor amplification and increased
levels of transcripts within the cells gene expression program
(43–45). Thus, C-Myc may amplify the output of
the PCNA gene expression program in HCC cells under hypoxic
conditions.
In conclusion, the present study demonstrated that
HIF-2α gene transfer and sorafenib effectively inhibited the
proliferation of HCC cells in vivo when administered
independently; however, the combination of HIF-2α shRNA and
sorafenib treatment resulted in a synergistic effect on inhibiting
cell proliferation. The mechanisms underlying these results were
indicated to be primarily attributed to the decreased expression of
PCNA. Given the distinct transcriptional targets of HIF-2α, the
results suggested that downregulating HIF-2α may further enhance
the antitumor activity of sorafenib in HCC via
β-catenin/C-Myc-dependent pathways.
Acknowledgements
The present study was supported by grants from the
National Natural Scientific Foundation of China (nos. 81172331 and
30972890), the Shandong Provincial Science & Technology
Development Program, China (no. 2010GSF10230) and the Youth
Foundation of CSCO-Bayer Schering Pharma for Hepatocellular
Carcinoma (no. Y-B2011-012).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Samuel M, Chow PK, Chan Shih-Yen E, Machin
D and Soo KC: Neoadjuvant and adjuvant therapy for surgical
resection of hepatocellular carcinoma. Cochrane Database Syst Rev.
1:CD0011992009.PubMed/NCBI
|
|
4
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al SHARP Investigators Study Group: Sorafenib in advanced
hepatocellular carcinoma. N Engl J Med. 359:378–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhai B and Sun XY: Mechanisms of
resistance to sorafenib and the corresponding strategies in
hepatocellular carcinoma. World J Hepatol. 5:345–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Malenstein H, Dekervel J, Verslype C,
Van Cutsem E, Windmolders P, Nevens F and van Pelt J: Long-term
exposure to sorafenib of liver cancer cells induces resistance with
epithelial-to-mesenchymal transition, increased invasion and risk
of rebound growth. Cancer Lett. 329:74–83. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berasain C: Hepatocellular carcinoma and
sorafenib: Too many resistance mechanisms? Gut. 62:1674–1675. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rankin EB and Giaccia AJ: The role of
hypoxia-inducible factors in tumorigenesis. Cell Death Differ.
15:678–685. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rohwer N and Cramer T: Hypoxia-mediated
drug resistance: Novel insights on the functional interaction of
HIFs and cell death pathways. Drug Resist Updat. 14:191–201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang Y, Zheng T, Song R, Wang J, Yin D,
Wang L, Liu H, Tian L, Fang X, Meng X, et al: Hypoxia-mediated
sorafenib resistance can be overcome by EF24 through Von
Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in
hepatocellular carcinoma. Hepatology. 57:1847–1857. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YS, Adnane J, Trail PA, Levy J,
Henderson A, Xue D, Bortolon E, Ichetovkin M, Chen C, McNabola A,
et al: Sorafenib (BAY 43-9006) inhibits tumor growth and
vascularization and induces tumor apoptosis and hypoxia in RCC
xenograft models. Cancer Chemother Pharmacol. 59:561–574. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita T, Ohneda K, Nagano M, Miyoshi
C, Kaneko N, Miwa Y, Yamamoto M, Ohneda O and Fujii-Kuriyama Y:
Hypoxia-inducible transcription factor-2alpha in endothelial cells
regulates tumor neovascularization through activation of ephrin A1.
J Biol Chem. 283:18926–18936. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Menrad H, Werno C, Schmid T, Copanaki E,
Deller T, Dehne N and Brüne B: Roles of hypoxia-inducible
factor-1alpha (HIF-1alpha) versus HIF-2alpha in the survival of
hepatocellular tumor spheroids. Hepatology. 51:2183–2192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo D, Wang Z and Wu J, Jiang C and Wu J:
The role of hypoxia inducible factor-1 in hepatocellular carcinoma.
BioMed Res Int. 2014:4092722014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin TY, Chou CF, Chung HY, Chiang CY, Li
CH, Wu JL, Lin HJ, Pai TW, Hu CH and Tzou WS: Hypoxia-inducible
factor 2 alpha is essential for hepatic outgrowth and functions via
the regulation of leg1 transcription in the zebrafish embryo. PLoS
One. 9:e1019802014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong X, Li R, Xiu P, Dong X, Xu Z, Zhai B,
Liu F, Jiang H, Sun X, Li J, et al: Meloxicam executes its
antitumor effects against hepatocellular carcinoma in
COX-2-dependent and -independent pathways. PLoS One. 9:e928642014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiu P, Dong X, Dong X, Xu Z, Zhu H, Liu F,
Wei Z, Zhai B, Kanwar JR, Jiang H, et al: Secretory clusterin
contributes to oxaliplatin resistance by activating Akt pathway in
hepatocellular carcinoma. Cancer Sci. 104:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Sun B, Pan S, Jiang H and Sun X:
Dihydroartemisinin inhibits growth of pancreatic cancer cells in
vitro and in vivo. Anticancer Drugs. 20:131–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie C, Pan Y, Hao F, Gao Y, Liu Z, Zhang
X, Xie L, Jiang G, Li Q and Wang E: C-Myc participates in
β-catenin-mediated drug resistance in A549/DDP lung adenocarcinoma
cells. APMIS. 122:1251–1258. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu H and Li Z, Yang P, Zhang L, Fan Y and
Li Z: PKM2 depletion induces the compensation of glutaminolysis
through β-catenin/c-Myc pathway in tumor cells. Cell Signal.
26:2397–2405. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee KB, Ye S, Park MH, Park BH, Lee JS and
Kim SM: p63-Mediated activation of the β-catenin/c-Myc signaling
pathway stimulates esophageal squamous carcinoma cell invasion and
metastasis. Cancer Lett. 353:124–132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao D, Zhai B, He C, Tan G, Jiang X, Pan
S, Dong X, Wei Z, Ma L, Qiao H, et al: Upregulation of HIF-2α
induced by sorafenib contributes to the resistance by activating
the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell
Signal. 26:1030–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang SL, Liu LP, Jiang JX, Xiong ZF, He QJ
and Wu C: The correlation of expression levels of HIF-1α and HIF-2α
in hepatocellular carcinoma with capsular invasion, portal vein
tumor thrombi and patients’ clinical outcome. Jpn J Clin Oncol.
44:159–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong ZZ, Yao M, Wang L, Wu W, Gu X and Yao
DF: Hypoxia-inducible factor-1alpha: Molecular-targeted therapy for
hepatocellular carcinoma. Mini Rev Med Chem. 13:1295–1304. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wong CC, Kai AK and Ng IO: The impact of
hypoxia in hepatocellular carcinoma metastasis. Front Med. 8:33–41.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He C, Sun XP, Qiao H, Jiang X, Wang D, Jin
X, Dong X, Wang J, Jiang H and Sun X: Downregulating
hypoxia-inducible factor-2α improves the efficacy of doxorubicin in
the treatment of hepatocellular carcinoma. Cancer Sci. 103:528–534.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin CP, Liu CR, Lee CN, Chan TS and Liu
HE: Targeting c-Myc as a novel approach for hepatocellular
carcinoma. World J Hepatol. 2:16–20. 2010.PubMed/NCBI
|
|
28
|
Fang Y, Huang B, Liang Q, Li H and Huang
C: Clinical significance of c-myc oncogene amplification in primary
hepatocellular carcinoma by interphase fluorescence in situ
hybridization. Zhonghua Bing Li Xue Za Zhi. 30:180–182. 2001.(In
Chinese). PubMed/NCBI
|
|
29
|
Li P, Cao Y, Li Y, Zhou L, Liu X and Geng
M: Expression of Wnt-5a and β-catenin in primary hepatocellular
carcinoma. Int J Clin Exp Pathol. 7:3190–3195. 2014.PubMed/NCBI
|
|
30
|
Qu B, Liu BR, Du YJ, Chen J, Cheng YQ, Xu
W and Wang XH: Wnt/β-catenin signaling pathway may regulate the
expression of angiogenic growth factors in hepatocellular
carcinoma. Oncol Lett. 7:1175–1178. 2014.PubMed/NCBI
|
|
31
|
Yoshida T, Hashimura M, Mastumoto T, Tazo
Y, Inoue H, Kuwata T and Saegusa M: Transcriptional upregulation of
HIF-1α by NF-κB/p65 and its associations with β-catenin/p300
complexes in endometrial carcinoma cells. Lab Invest. 93:1184–1193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jeong JK and Park SY: HIF-1α-induced
β-catenin activation prevents prion-mediated neurotoxicity. Int J
Mol Med. 32:931–937. 2013.PubMed/NCBI
|
|
33
|
Yang YY, Lee PC, Huang YT, Lee WP, Kuo YJ,
Lee KC, Hsieh YC, Lee TY and Lin HC: Involvement of the HIF-1α and
Wnt/β-catenin pathways in the protective effects of losartan on
fatty liver graft with ischaemia/reperfusion injury. Clin Sci
(Lond). 126:163–174. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi H, Chun YS, Kim TY and Park JW:
HIF-2alpha enhances beta-catenin/TCF-driven transcription by
interacting with beta-catenin. Cancer Res. 70:10101–10111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang
C, Xie S, Chen C, Hu L, Xu S, et al: Wnt/β-catenin signaling
enhances hypoxia-induced epithelial-mesenchymal transition in
hepatocellular carcinoma via crosstalk with hif-1α signaling.
Carcinogenesis. 34:962–973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lee SH, Kim MH and Han HJ: Arachidonic
acid potentiates hypoxia-induced VEGF expression in mouse embryonic
stem cells: Involvement of Notch, Wnt, and HIF-1alpha. Am J Physiol
Cell Physiol. 297:C207–C216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu L, Huang X, Li L, Huang H, Xu R and
Luyten W: Insights on biology and pathology of HIF-1α/-2α,
TGFβ/BMP, Wnt/β-catenin, and NF-κB pathways in osteoarthritis. Curr
Pharm Des. 18:3293–3312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi H, Chun YS, Kim TY and Park JW:
HIF-2alpha enhances beta-catenin/TCF-driven transcription by
interacting with beta-catenin. Cancer Res. 70:10101–10111. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Park YK, Park B, Lee S, Choi K, Moon Y and
Park H: Hypoxia-inducible factor-2α-dependent hypoxic induction of
Wnt10b expression in adipogenic cells. J Biol Chem.
288:26311–26322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li YJ, Wei ZM, Meng YX and Ji XR:
Beta-catenin up-regulates the expression of cyclinD1, c-myc and
MMP-7 in human pancreatic cancer: Relationships with carcinogenesis
and metastasis. World J Gastroenterol. 11:2117–2123. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Anna CH, Iida M, Sills RC and Devereux TR:
Expression of potential beta-catenin targets, cyclin D1, c-Jun,
c-Myc, E-cadherin, and EGFR in chemically induced hepatocellular
neoplasms from B6C3F1 mice. Toxicol Appl Pharmacol. 190:135–145.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shiina H, Igawa M, Shigeno K, Terashima M,
Deguchi M, Yamanaka M, Ribeiro-Filho L, Kane CJ and Dahiya R:
Beta-catenin mutations correlate with over expression of C-myc and
cyclin D1 Genes in bladder cancer. J Urol. 168:2220–2226. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch
W, Wang R, Green DR, Tessarollo L, Casellas R, et al: c-Myc is a
universal amplifier of expressed genes in lymphocytes and embryonic
stem cells. Cell. 151:68–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin CY, Lovén J, Rahl PB, Paranal RM,
Burge CB, Bradner JE, Lee TI and Young RA: Transcriptional
amplification in tumor cells with elevated c-Myc. Cell. 151:56–67.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahl PB, Lin CY, Seila AC, Flynn RA,
McCuine S, Burge CB, Sharp PA and Young RA: c-Myc regulates
transcriptional pause release. Cell. 141:432–445. 2010. View Article : Google Scholar : PubMed/NCBI
|