Introduction
Hepatocellular carcinoma (HCC) is the third leading
cause of cancer-related mortalities worldwide. Approximately 80% of
HCCs develop in fibrotic or cirrhotic livers (1). Conventional treatment strategies include
surgery, chemotherapy and radiotherapy. For the majority of
patients with advanced cancer, chemotherapy must be administered
following surgery. However, the prognoses of HCC patients,
particularly those with high stage or chemoresistant tumors, are
still unsatisfactory (2), and the
range for radiation therapy is relatively narrow. Other optional
treatments include immunotherapy and traditional Chinese medicine.
Therefore, the current study investigated the role and mechanisms
of bilobol, a traditional Chinese medicine ingredient, in order to
understand its antitumor effects and to assess its potential as an
antitumor agent or adjuvant agent.
RhoA is a small G protein, inactive when bound to
guanosine diphosphate (GDP) and activated following guanosine
triphosphate (GTP) binding. GDP/GTP exchange or GTPase reactions
catalyze the conversion between the two forms (3). Rho, a small GTPase, is associated with
various cellular functions, including cell adhesion, cell motility
and migration, growth control, cell contraction and cytokinesis.
Previous studies by our group (4,5) and others
(6,7)
have indicated that RhoA is located in the nucleus, as well as in
the cell membrane and the cytoplasm. In addition, previous
investigations conducted by the present research group demonstrated
that the nuclear localization of RhoA was affected by numerous
factors, including inflammation, damage and certain pharmaceutical
agents (5). Furthermore, these
previous studies revealed increased nuclear translocation of the
RhoA protein when the cell in question is damaged or cancerous.
Bacterial lipopolysaccharide (LPS) is localized to
the outer membrane of Gram-negative bacteria, and is capable of
activating multiple mammalian cell types and intracellular
signaling pathways (8). LPS is a
major inflammatory molecule, which induces the production of
pro-inflammatory toxins and cytokines, including inducible nitric
oxide synthase, cyclooxygenase-2, tumor necrosis factor-α and
interleukin-1 (IL-1), IL-6 and IL-8, in various cell types
(9). These pro-inflammatory cytokines
and bacterial endotoxins induce activation of the Rho GTPase
signaling pathway, which mediates pro-inflammatory responses and
aggressive carcinogenesis (10,11).
Ginkgo biloba L. is a tree native to China,
which has been cultivated for over a millennium. In Asia, the
Ginkgo biloba (Gb) tree has been used medicinally for
centuries. Similarly, Gb leaf extracts are commonly used for a
variety of traditional folk remedies in the West (12). Currently, Gb extracts are one of the
most commonly administered phytomedicines worldwide, applied for
the treatment of neuropsychiatric disorders, asthma, tinnitus,
vertigo and cognitive issues (13,14).
The application of ginkgolic acids as anticancer
agents has previously been reported (15,16).
However, ginkgolic acids are unstable, with bilobol/ginkgo phenol
forming easily following heat, acid or base catalysis (17). The cytotoxic evaluation of bilobol as
an antitumor agent is in the preliminary stages. Furthermore, an
in-depth investigation into the mechanism and effects of bilobol on
its targets has commenced (18–20). In
consideration of previous studies regarding RhoA by our group, the
present study aimed to conduct a broader investigation of the
anticancer efficacy of bilobol by analyzing its effect on the
distribution of RhoA in HepG2 human hepatocellular carcinoma
cells.
Materials and methods
Cell line
The HepG2 human hepatocellular carcinoma cell line
was obtained from the Institution of Cell Biology of the Chinese
Academy of Sciences (Shanghai, China).
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) and Trypsin-EDTA solution were purchased from
Gibco Life Technologies (Grand Island, NY, USA). Fluorescein
isothiocyanate (FITC), tetrarhodamine isothiocyanate (TRITC) and
horseradish peroxidase (HRP)-conjugated secondary antibodies [goat
anti-mouse polyclonal IgG (cat no. 115-005-209) and rabbit
anti-goat polyclonal IgG (cat no. 305-005-003)] were purchased from
Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Furthermore, electrochemiluminescence (ECL) reagents were purchased
from GE Healthcare Life Sciences (Chalfont, UK). A Nuclear and
Cytoplasmic Extract kit (cat no. KC-435) and antibodies against
GAPDH were purchased from Kangchen Biotech, Inc. (Hangzhou,
Zhejiang, China). Mouse monoclonal immunoglobulin G
(IgG)1 anti-RhoA (cat no. sc-418) and goat polyclonal
IgG anti-Rho-associated protein kinase 2 (ROCK2; cat no. sc-1851)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). In addition, the ELISA kit was obtained from
R&D Systems, Inc. (Minneapolis, MN, USA), LPS was from
Sigma-Aldrich (St. Louis, MO, USA) and bilobol (high-performance
liquid chromatography purity, >96.5%) was obtained from the
Laboratory of Food and Biological Engineering School, Jiangsu
University (Zhenjiang, Jiangsu, China).
Cell culture
HepG2 cells were maintained in DMEM supplemented
with 10% FBS at 37°C in a humidified atmosphere containing 5%
CO2. The medium was changed every two days and the cells
were maintained at subconfluence.
Bilobol preparation and cell
treatment
Bilobol was dissolved in 0.1% dimethyl sulfoxide
(Bio-Link, York, UK) to obtain a concentration of 15 mg/ml. A total
of 2.5×106 HepG2 cells were seeded in 6 well plates for
use in the Western blot analysis and IL-8/IL-6 expression assays
and a total of 5×105 HepG2 cells were seeded in 24 well
culture plates for the immunofluorescence assays, and incubated in
a humidified atmosphere with 5% CO2 at 37°C. When the
cells had reached ~80% confluence, DMEM medium with free serum was
added to the wells. Next, 1 µM bilobol was added to the 6 and
24-well plates and the cells were cultured for 12 h. Untreated
cells were used as the blank control.
Preparation of cytoplasmic and nuclear
protein samples
The cytoplasmic and nuclear proteins were extracted
from the cultured HepG2 cells according to protocol outlined in the
Nuclear and Cytoplasmic Extract kit (Zhejiang Kangchen Biotech Co.,
Ltd., (Hangzhou, China) manufacturer's instructions. Briefly, the
cells were lysed in lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM
EDTA and 1% Triton X-100; pH 7.4), collected in an Eppendorf tube
(Genetimes Technology, Inc., Shanghai, China), centrifuged at 3,000
× g for 15 sec and suspended in 300 µl Buffer A.
Phenylmethylsulfonyl fluoride (1 µl; Shanghai Bogoo Biotechnology
Co. Ltd., Shanghai, China), a phosphatase inhibitor (aprotinin;
Beijing Leagene Biotech Co., Ltd., Beijing, China), a protease
inhibitor mixture, containing leupeptin (Beijing Leagene Biotech
Co., Ltd.) and 12 µl NP-40 solution were added and the cells were
placed on ice for 10 min, then vortexed for 10 sec. Next, the cells
were placed on ice for 10 min and centrifuged at 12,000 × g for 15
sec. The supernatant, which contained the cytoplasmic protein, was
removed and transferred to an Eppendorf tube. The cells were then
resuspended in 30 µl Buffer B containing protease inhibitors and
placed on ice for 30 min. The cells were then centrifuged at 12,000
× g for 15 sec at 4°C and the supernatant was removed. This
supernatant contained the nuclear protein. Equal volumes of 2X SDS
loading buffer were added to the cytoplasmic and nuclear proteins,
respectively. The proteins were then incubated in a Dry Bath
Incubator (BG-themoRT; Bay Gene, Inc., Burlingame, CA, USA) at
100°C for 5 min.
Immunofluorescence microscopy
HepG2 cells cultured on cover slips were fixed with
freshly prepared paraformaldehyde (40 g/l in phosphate-buffered
saline; Nantong Jiangtian Chemical Co., Ltd., Nantong, Jiangsu,
China) for 30 min. Following penetration with 30 ml/l Triton X-100
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) and blocking with 30 g/l bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA), the cells were incubated with
primary antibodies (dilution, 1:200) at 4°C overnight (o/n).
Subsequently, the cells were incubated with TRITC-conjugated
polyclonal goat anti-mouse IgG secondary antibodies (dilution,
1:1,000) for 1 h at room temperature (RT). The cells were washed
three times following each incubation. The cellular distribution of
the target protein was analyzed under a fluorescence microscope
(IX51; Olympus Corporation, Tokyo, Japan).
Western blotting
The sample proteins were run on 10.0 or 12% SDS
polyacrylamide gels. Subsequently, the proteins were transferred
onto polyvinyl difluoride (PVDF) membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The PVDF membranes were initially blocked
with 5% milk in Tris-buffered saline and Tween 20 (80 g/l NaCl, 2
g/l KCl, 30 g/l Tris and 0.1% Tween-20; pH 7.4) for 1 h at RT and
then incubated with the primary antibodies (dilution, 1:1,000) at
4°C o/n. Following subsequent incubation of the membranes with
HRP-conjugated secondary antibodies (dilution, 1:10,000) for 1 h at
RT, ECL reagents were applied, according to the manufacturer'
instructions, to reveal the positive bands on the membrane. The
bands were detected using a Typhoon 9400 imager (GE Healthcare Life
Sciences, Piscataway, NJ, USA).
Determination of IL-8 and IL-6
expression
HepG2 cells were treated with or without LPS (1
µg/ml) for 12 h. The supernatants were then harvested and measured
for IL-8 and IL-6 production using an ELISA kit, according to the
manufacturer's instructions.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean (n=6). The independent samples t-test was used to
compare the expression of IL-6 and IL-8. The results were analyzed
using SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Bilobol suppresses LPS-induced
inflammation
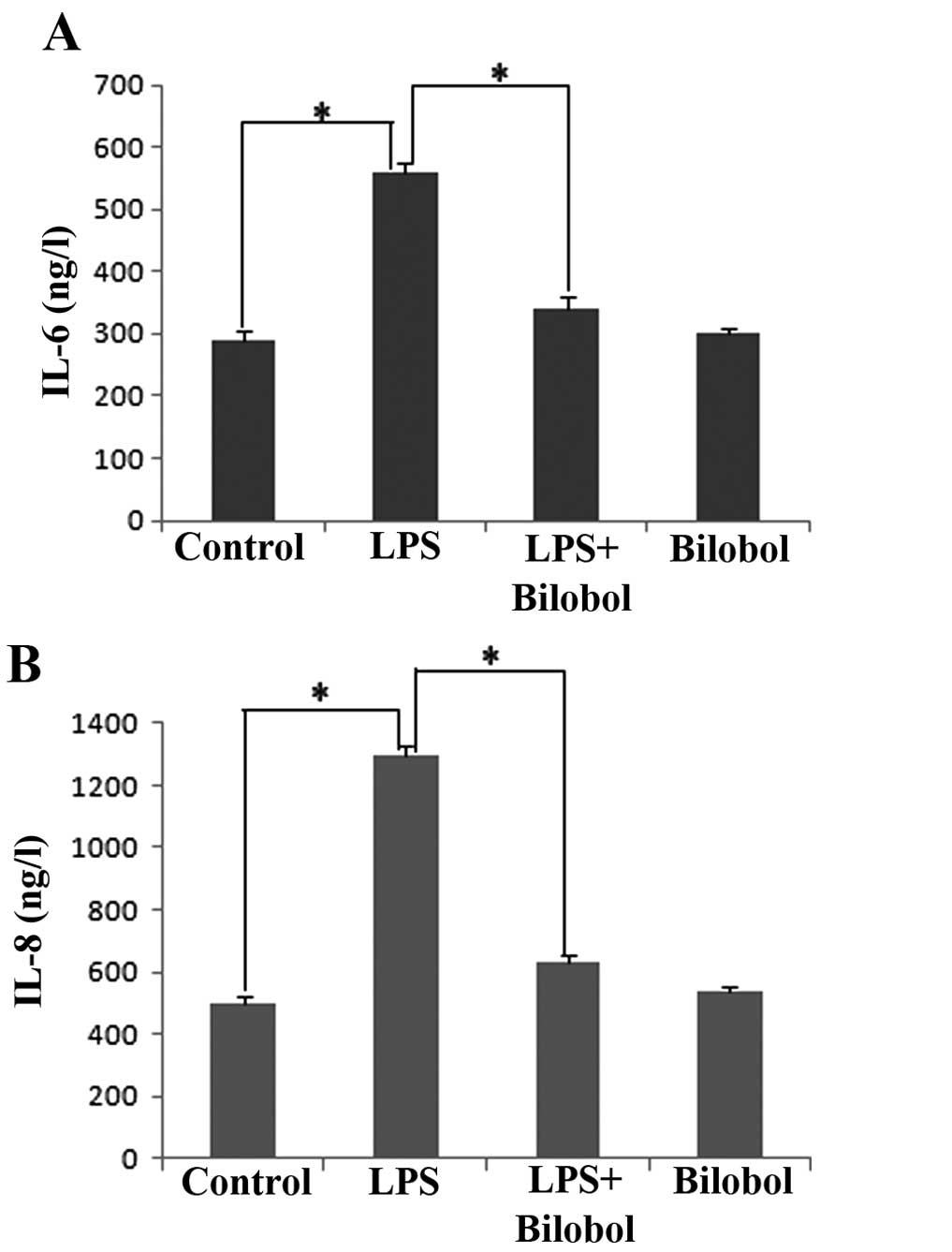
The present study investigated whether LPS was able
to mediate the transcriptional activation of the pro-inflammatory
genes IL-6 and IL-8, and whether bilobol was able to suppress this
LPS-induced inflammatory reaction. HepG2 human hepatocellular
carcinoma cells were treated with 1 µg/ml LPS and/or 15 µg/ml
bilobol for 12 h, and the concentration of IL-6 and IL-8 in the
supernatants was measured by ELISA. The results indicated that LPS
significantly increased the transcriptional activation of IL-6
(Fig. 1A) and IL-8 (Fig. 1B; P<0.05). Furthermore, bilobol was
identified to significantly suppress the LPS-induced release of
IL-6 (Fig. 1A) and IL-8 (Fig. 1B; P<0.05). Thus, bilobol exhibited
an anti-inflammatory effect.
Bilobol inhibits the expression and
nuclear translocation of RhoA
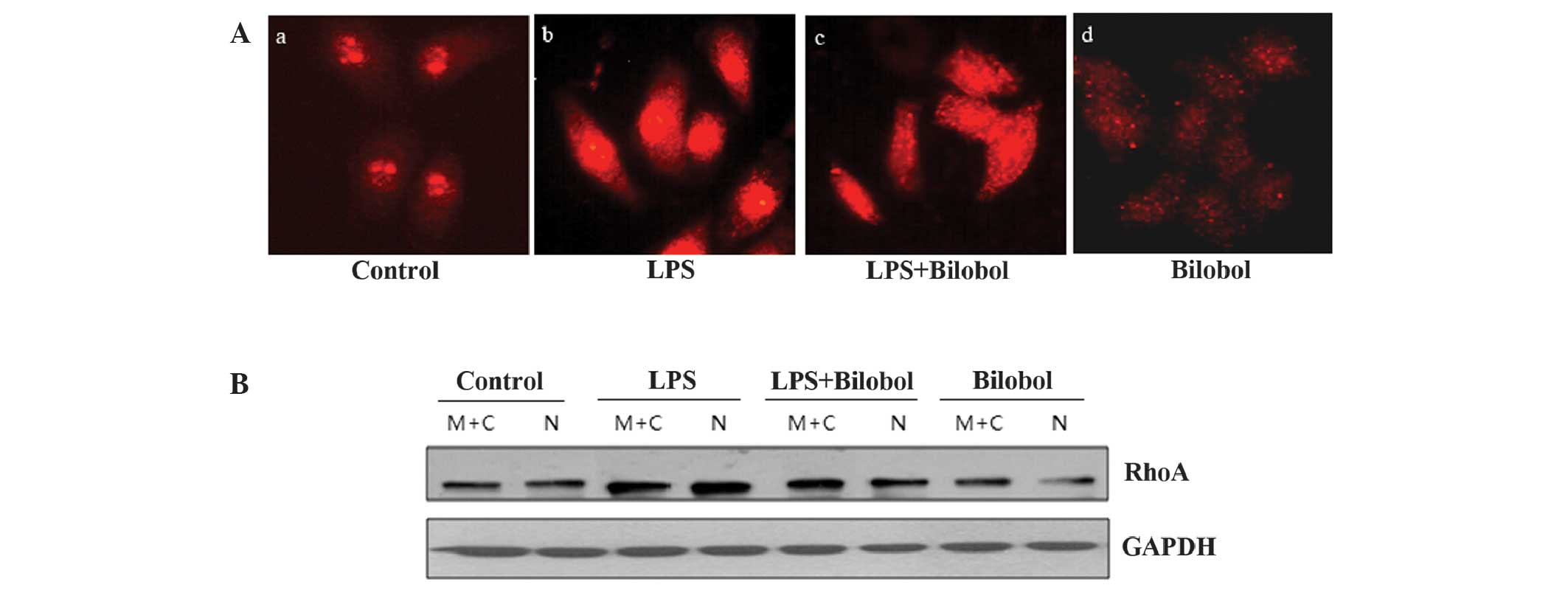
An immunofluorescence assay was performed, in order
to determine the level of RhoA expression in the nucleus using an
antibody against RhoA. Comparison with control cells revealed that
LPS activated RhoA and triggered its translocation to the nucleus
in HepG2 human hepatocellular carcinoma cells (Fig. 2Aa and Ab). Furthermore, treatment with
bilobol appeared to suppress this effect (Fig. 2Ac and Ad). Thus, the expression of
RhoA protein and its localization within the nucleus was increased
when the cells were treated with LPS. Bilobol had the opposite
effect; it was able to inhibit the two aforementioned phenomena.
Subsequent to the immunofluorescent analysis, nuclear extracts were
prepared and analyzed by western blotting, using antibodies against
RhoA. The western blot analysis results were in agreement with the
immunofluorescence data (Fig. 2B),
indicating that bilobol inhibits the expression and nuclear
translocation of RhoA.
Bilobol inhibits the expression of
ROCK2
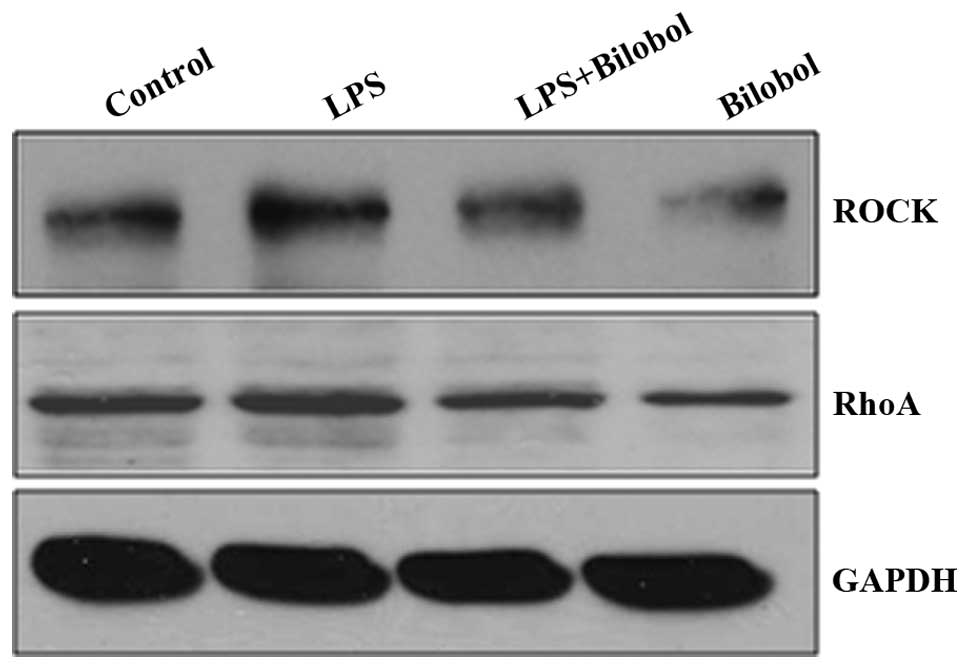
Western blotting was used to determine the
expression of ROCK2 and RhoA, when HepG2 cells were treated with or
without 1 µg/ml LPS and/or 15 µg/ml bilobol for 12 h (Fig. 3). The expression of ROCK2 was
increased following LPS stimulation, however, treatment with
bilobol was able to suppress its enhanced expression. ROCK2
appeared to follow a similar expression pattern to that of RhoA
following treatment with LPS and/or bilobol.
Discussion
RhoA has a molecular mass of 21 kDa and is the most
extensively studied member of the Rho GTPase family, which is part
of the Ras superfamily of small G proteins. RhoA has been reported
to regulate various biological activities, for example gene
transcription (21) and tumor
progression (22). Furthermore, RhoA
activation is typically associated with invasive growth and
metastasis, and therefore functions as an oncogene. RhoA is
associated with the regulation of numerous biological processes,
including stress fiber formation, membrane transport, gene
transcription, focal adhesion and tumor progression (23,24).
Ginkgo biloba L. is the last surviving member
of one of the oldest living seed plant groups, Gymnospermae, and is
of medicinal, spiritual and horticultural importance worldwide. Gb
extracts (EGb) are one of the most commonly administered
therapeutic agents, and are often prescribed in Europe for use as
nootropic agents in old age and dementia (25).
Investigation into the antitumor efficacy of the EGb
alkylphenol, ginkgolic acid, has focused on ginkgo acid,
specifically. Park et al (16)
evaluated the anticancer effects of EGb in estrogen-independent
breast cancer. It has been proposed that the chemopreventive
effects exhibited by EGb in estrogen receptor-independent breast
cancer occur via antiproliferative and apoptosis-inducing
activities. Previously, Liu and Zeng (19) identified that HepG2 cells were more
sensitive to the cytotoxicity of ginkgolic acid than primary rat
hepatocytes. For example, ginkgolic acid appeared to significantly
inhibit growth, halt G0/G1 phase progression and decrease B cell
lymphoma-2 (Bcl-2)/Bcl-2-associated × protein expression in HepG2
cells (20), as well as induce the
apoptosis of pituitary gland tumor cells by increasing the
sensitivity of the cells to radiation (26).
However, alkylphenol acid obtained from EGb is
unstable, rapidly forming bilobol/ginkgo phenol under heat, acid or
base catalysis. Bilobol is considered to exhibit stronger
anticancer properties.
In the present study, LPS appeared to stimulate the
nuclear translocation of RhoA protein, while bilobol treatment
blocked the subcellular distribution of RhoA under LPS-induced
inflammatory conditions. Therefore, in consideration of the results
of previous studies by our group, it was proposed that bilobol may
exhibit anti-inflammatory and anticancer effects on HepG2 cells.
Similarly, standardized EGb 761 and bilobalide treatment have been
demonstrated to inhibit inflammation in rats; however, the
mechanism of action has remained elusive (27,28).
Furthermore, only a small number of studies have been conducted
investigating the anti-inflammatory and anticancer effects of
bilobol.
The best-known effector of RhoA is ROCK. ROCK
appears to be crucial in modulating the force and velocity of
actomyosin crossbridging in smooth muscle and non-muscle cells by
inhibiting myosin phosphatase-mediated dephosphorylation of the
regulatory chain of myosin II (29).
In the present study, RhoA/ROCK expression and activity were
increased following treatment with LPS, and reduced following the
application of bilobol. In addition, bilobol appeared to inhibit
the RhoA/ROCK signaling pathway during the anti-inflammatory
response and exhibited an anticancer effect. However, the mechanism
involved requires further clarification.
In conclusion, the present study identified that the
administration of bilobol blocked cancer progression in HepG2
cells. This is in agreement with the results of previous studies by
our group, which indicated that the nuclear translocation of RhoA
promoted cancer progression. To further assess the antitumor effect
of bilobol and develop it into a candidate novel antitumor agent,
the antitumor mechanism of bilobol requires additional
investigation.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81372404), the
Specialized Research Fund for Senior Personnel Program of Jiangsu
University (grant no. 11JDG129), the Postdoctoral Foundation of
China (grant no. 2012M521018) and the Postdoctoral Foundation of
Jiangsu Province (grant no. 1201025B). These grants were awarded to
Ms. Yueying Li.
References
|
1
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: From genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall A: The cellular functions of small
GTP-binding proteins. Science. 249:635–640. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Chen Y, Tao Y, Xu J and Chen M: RhoA
protein is generally distributed in the nuclei of cancer cells.
Oncol Rep. 24:1005–1009. 2010.PubMed/NCBI
|
|
5
|
Li Y, Chen Y and Xu J: Factors influencing
RhoA protein distribution in the nucleus. Mol Med Rep. 4:1115–1119.
2011.PubMed/NCBI
|
|
6
|
Dubash AD, Guilluy C, Srougi MC, Boulter
E, Burridge K and García-Mata R: The small GTPase RhoA localizes to
the nucleus and is activated by Net1 and DNA damage signals. PLoS
One. 6:e173802011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guilluy C, Dubash AD and García-Mata R:
Analysis of RhoA and Rho GEF activity in whole cells and the cell
nucleus. Nat Protoc. 6:2050–2060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Tu Q, Yan W, et al: CXC195
suppresses proliferation and inflammatory response in LPS-induced
human hepatocellular carcinoma cells via regulating
TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem Biophys
Res Commun. 456:373–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
MacMicking J, Xie QW and Nathan C: Nitric
oxide and macrophage function. Annu Rev Immunol. 15:323–350. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knaus UG: Rho GTPase signaling in
inflammation and transformation. Immunol Res. 21:103–109. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu M, Wu ZF, Rosenthal DT, Rhee EM and
Merajver SD: Characterization of the roles of RHOC and RHOA GTPases
in invasion, motility and matrix adhesion in inflammatory and
aggressive breast cancers. Cancer. 116 (Suppl):S2768–S2782. 2010.
View Article : Google Scholar
|
|
12
|
Hori T, Ridge RW, Tulecke W, Del Tredici
P, Tremouillaux-Guiller J and Tobe H: Ginkgo biloba - A
Global TreasureFrom Biology to Medicine. 1st. Springer; Tokyo:
1997
|
|
13
|
Brondino N, De Silvestri A, Re S, Lanati
N, Thiemann P, Verna A, Emanuele E and Politi P: A systematic
review and meta-analysis of Ginkgo biloba in
neuropsychiatric disorders: From ancient tradition to modern-day
medicine. Evid Based Complement Alternat Med. 2013:9156912013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
von Boetticher A: Ginkgo biloba
extract in the treatment of tinnitus: A systematic review.
Neuropsychiatr Dis Treat. 7:441–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu Y, He L, Zhang L, Chen J, Yi Z, Zhang
J, Liu M and Pang X: Anacardic acid (6-pentadecylsalicylic acid)
inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases
signaling pathway. J Pharmacol Exp Ther. 339:403–411. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park YJ, Kim MJ, Kim HR, Yi MS, Chung KH
and Oh SM: Chemopreventive effects of Ginkgo biloba extract
in estrogen-negative human breast cancer cells. Arch Pharm Res.
36:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang XM, Wang YF, Li YY and Ma HL: Thermal
stability of ginkgolic acids from Ginkgo biloba and the
effects of ginkgol C17:1 on the apoptosis and migration of SMMC7721
cells. Fitoterapia. 98:66–76. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jaggy H and Koch E: Chemistry and biology
of alkylphenols from Ginkgo biloba L. Pharmazie. 52:735–738.
1997.PubMed/NCBI
|
|
19
|
Liu ZH and Zeng S: Cytotoxicity of
ginkgolic acid in HepG2 cells and primary rat hepatocytes. Toxicol
Lett. 187:131–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou C, Li X, Du W, Feng Y, Kong X, Li Y,
Xiao L and Zhang P: Antitumor effects of ginkgolic acid in human
cancer cell occur via cell cycle arrest and decrease the Bcl-2/Bax
ratio to induce apoptosis. Chemotherapy. 56:393–402. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sotiropoulos A, Gineitis D, Copeland J and
Treisman R: Signal-regulated activation of serum response factor is
mediated by changes in actin dynamics. Cell. 98:159–169. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kamai T, Yamanishi T, Shirataki H, Takagi
K, Asami H, Ito Y and Yoshida K: Overexpression of RhoA, Rac1 and
Cdc42 GTPases is associated with progression in testicular cancer.
Clin Cancer Res. 10:4799–4805. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Price LS and Collard JG: Regulation of the
cytoskeleton by Rho-family GTPases: Implications for tumour cell
invasion. Semin Cancer Biol. 11:167–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Narumiya S: The small GTPase Rho: Cellular
functions and signal transduction. J Biochem. 120:215–228. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kennedy DO and Wightman EL: Herbal
extracts and phytochemicals: Plant secondary metabolites and the
enhancement of human brain function. Adv Nutr. 2:32–50. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sukumari-Ramesh S, Singh N, Jensen MA,
Dhandapani KM and Vender JR: Anacardic acid induces
caspase-independent apoptosis and radiosensitizes pituitary adenoma
cells. J Neurosurg. 114:1681–1690. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee CY, Yang JJ, Lee SS, Chen CJ, Huang
YC, Huang KH and Kuan YH: Protective effect of Ginkgo biloba
leaves extract, EGb761, on endotoxin-induced acute lung injury via
a JNK- and Akt-dependent NFκB pathway. J Agric Food Chem.
62:6337–6344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goldie M and Dolan S: Bilobalide, a unique
constituent of Ginkgo biloba, inhibits inflammatory pain in
rats. Behav Pharmacol. 24:298–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wettschureck N and Offermanns S:
Rho/Rho-kinase mediated signaling in physiology and
pathophysiology. J Mol Med Berl. 80:629–638. 2002. View Article : Google Scholar : PubMed/NCBI
|